Abstract
CCR5, a β-chemokine receptor, plays an important role in human immunodeficiency virus (HIV) infection of human immune cells, as it is a primary coreceptor for HIV entry into macrophages. We have applied a newly developed real-time reverse transcriptase PCR (RT-PCR) assay for the quantification of CCR5 mRNA in human blood immune cells. The CCR5 real-time RT-PCR assay has a sensitivity of 100 mRNA copies, with a dynamic range of detection between 102 and 106 copies of the CCR5 mRNA transcripts per reaction. The assay is highly reproducible, with an intra-assay coefficient of variation of the threshold cycle of less than 2.07%. When used for quantification of CCR5 mRNA in human monocyte-derived macrophages (MDM) and peripheral blood lymphocytes (PBL), the assay has precision and reproducibility. MDM expressed higher levels of CCR5 mRNA than did PBL. Thus, this assay has the potential and a wide application for the investigation of the role of CCR5 in inflammatory diseases and viral infections, including HIV disease.
There are two major families of chemokine receptors: CC chemokine receptors (CCR) and CXC chemokine receptors. CCR5 is a CCR for β-chemokines, including macrophage inflammation proteins 1α and 1β and RANTES. The expression and regulation of CCR5 in human immune cells are not only implicated in inflammatory diseases but also involved in viral infections such as human immunodeficiency virus (HIV). CCR5 is a primary coreceptor for HIV entry into macrophages (2). Individuals with homozygous deletion of the 32 bp in the CCR5 gene are highly resistant to HIV infection (1, 5, 9, 12). Thus, the precise measurement of CCR5 mRNA expression in human immune cells is essential for studies on the cellular and molecular role of CCR5 signaling in inflammation and HIV infection.
Although reverse transcriptase PCR (RT-PCR) and/or competitive RT-PCR assays have been used to semiquantitatively determine CCR5 mRNA transcripts, they not only are laborious and time-consuming but also cause variation and contamination due to post-PCR manipulation. Since PCR amplification is an exponential assay, a very small change in the amplification efficiency produces a dramatic difference in the amount of final products (11). The monitoring of the entire process of the PCR (real-time) rather than merely the end product (such as in RT-PCR and competitive PCR) permits precise quantification. More importantly, real-time PCR which uses both primer pairs and a probe (such as the molecular beacon [MB]) significantly increases the specificity of the assay. The MB utilized for the construction of probes is critical for real-time detection of nucleic acid hybridization events (14). MB is a single-stranded nucleic acid molecule that possesses a stem-loop structure. Studies by our group and others have successfully employed MB for a variety of real-time PCR applications (3, 7, 10, 13-16).
In the present study, we describe a simple, sensitive, rapid, and reproducible real-time RT-PCR assay which uses both primers and probe (MB) for the quantification of CCR5 mRNA levels in human blood monocyte-derived macrophages (MDM) and peripheral blood lymphocytes (PBL).
MATERIALS AND METHODS
Human immune cells.
Peripheral blood was obtained from three healthy adult donors recruited from our hospital workers. The blood samples were identified as HIV type 1 antibody negative by anonymous testing by enzyme-linked immunosorbent assay (Coulter Immunology, Hialeah, Fla.). Informed consent was obtained, and the Institutional Research Board of our institution approved this study. Blood was obtained and used within 4 h. Following phlebotomy, monocytes were purified according to previously described techniques (4). In brief, heparinized blood was separated by centrifugation over lymphocyte separation medium (Oreganon Teknika, Durham, N.C.) at 400 to 500 × g for 45 min. The peripheral blood mononuclear cells (PBMC) were then collected. A portion of PBMC was used as the RNA standard for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) real-time RT-PCR. The other portion of PBMC was incubated with Dulbecco's modified Eagle's medium (GIBCO, Grand Island, N.Y.) in a 2% gelatin-coated flask for 45 min at 37°C. Nonadherent PBL were then removed from the flasks and washed three times with phosphate-buffered saline. Monocytes were detached by EDTA and plated in 48-well culture plates at a density of 0.5 × 106 cells/well in Dulbecco's modified Eagle's medium containing 10% fetal calf serum. The total length of time in culture for MDM was 7 to 10 days. These immune cells (MDM and PBL) were then subjected to real-time RT-PCR to determine CCR5 and GAPDH mRNA expression as described below. The limulus amoebocyte lysate assay demonstrated that all media and reagents were endotoxin free.
Preparation of CCR5 RNA standard.
We used a plasmid (pCCR5) as the template to synthesize the CCR5 RNA standard by in vitro transcription. The pCCR5 was obtained from the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program. The transformed bacteria containing pCCR5 were cultured in Luria-Bertani broth complemented with 100 μg of ampicillin/ml, and the plasmid was then purified with the Wizard Plus Miniprep DNA purification system (Promega, Madison, Wis.). The purified pCCR5 plasmid was linearized by XbaI digestion and transcribed into specific CCR5 RNA with the MEGAshortscript kit (Ambion, Austin, Tex.). The resulting CCR5 RNA solution was treated with RNase-free DNase I to remove residual pCCR5 DNA template. The CCR5 RNA was then purified by phenol-chloroform extraction and alcohol precipitation as previously reported (6, 8). The concentration of purified CCR5 RNA was determined with a spectrophotometer. The copy numbers of the RNA transcript were calculated based on the concentration and the molecular weight of the CCR5 RNA transcript. Tenfold serial dilutions of known amounts of CCR5 RNA transcripts were used as standards to quantify CCR5 mRNA levels in human immune cells by real-time RT-PCR.
Preparation of total RNA standard for GAPDH real-time RT-PCR.
To control for the integrity of RNA and normalize CCR5 mRNA copy numbers in MDM and PBL, GAPDH was also amplified by real-time RT-PCR. Since GAPDH is a housekeeping gene, total RNA extracted from the pooled PBMC was used to generate a GAPDH standard curve by real-time RT-PCR to quantify GAPDH mRNA levels. Total RNA was extracted from pooled PBMC (4 × 107 cells) of the three donors by using Tri-reagent (Molecular Research Center, Cincinnati, Ohio), and the concentration of total RNA was measured with a spectrophotometer. Serial dilutions of known amounts of total RNA, ranging from 8 to 1,000 ng, were used as standards to quantify GAPDH mRNA levels in MDM and PBL by real-time RT-PCR (as described below). These RNAs were also used for intra- and interassay experiments.
Design of MB and primers.
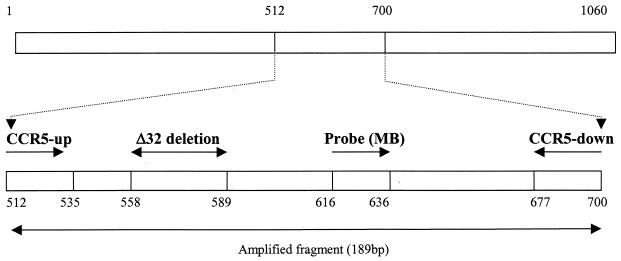
The PCR primers and MB were designed based on the sequence of the CCR5 gene (GenBank accession no. AF011539) by using Primer Express software (PE Biosystems) and were synthesized by Integrated DNA Technologies, Inc. (Coralville, Iowa). The primer pair CCR5-up-CCR5-down was designed to specifically amplify a 189-bp fragment of the CCR5 gene (Fig. 1). Their positions and sequences were as follows: CCR5-up, 5′-CAAAAAGAAGGTCTTCATTACACC-3′ (position, 512 to 535); CCR5-down, 5′-CCTGTGCCTCTTCTTCTCATTTCG-3′ (position, 677 to 700). The MB for CCR5 was located between CCR5-up-CCR5-down and its sequence was 5′-FAM- GCGAGTCCTGCCGCTGCTTGTCATGGTCCTCGC-DABCYL-3′ (Fig. 1). To control for the integrity and amount of RNA used for the real-time RT-PCR, the mRNA of a housekeeping gene, GAPDH, was amplified. The primers for GAPDH were 5′-GGTGGTCTCCTCTGACTTCAACA-3′ (sense) and 5′-GTTGCTGTAGCCAAATTCGTTGT-3′ (antisense). The stem sequence (underlined in the MB sequence) was selected such that they would not complement the sequences within the loop region. The length of the MB was designed such that the annealing temperature was slightly higher than that of the PCR primers. The MB was labeled at the 5′ end with 6-carboxyfluorescein (FAM) and at the 3′ end with quencher 4-(4′-dimethylaminophenylaso) benzoic acid (DABCYL). The primers and MB were resuspended in Tris-EDTA buffer and stored at −30°C.
FIG. 1.
Location and orientation (arrows) of the primers and probe (MB) for the real-time RT-PCR assay of CCR5 mRNA. The amplified PCR product has a size of 189 bp, and the probe sequence is located downstream of the Δ32 deletion as indicated.
RNA extraction.
Total RNA was extracted from PBMC, MDM, and PBL by using Tri-reagent (Molecular Research Center) as instructed by the manufacturer. In brief, total RNA was extracted by a single step, guanidinium thiocyanate-phenol-chloroform extraction. After centrifugation at 13,000 × g for 15 min, RNA-containing aqueous phase material was precipitated in isopropanol. RNA precipitates were then washed once in 75% ethanol and resuspended in 50 μl of RNase-free water.
Reverse transcription.
Total RNA and the CCR5 RNA standard were subjected to reverse transcription with the reverse transcription system (Promega) with modification. Both the random primers and the specific CCR5 primer (antisense) were used in the same reaction. The random primers were used to prime GAPDH. The final reaction mixture (20 μl) contained the following components: 5 mM MgCl2, 1× reverse transcription buffer, 500 μM concentrations of each deoxynucleoside triphosphate, 1 U of recombinant RNasin/μl, 10 to 15 U of avian myeloblastosis virus RT, 50 ng of random primers, and 0.1 μM CCR5-specific antisense primer. The reverse transcription was performed at 42°C for 1 h. The reaction was terminated by holding the reaction mixture at 99°C for 5 min. One-tenth (2 μl) of the resulting cDNA was used as a template for real-time PCR amplification.
Real-time PCR.
The ABI Prism 7000 sequence detection system (ABI 7000 SDS) was used for real-time PCR analysis. Thermal cycling conditions were designed as follows: initial denaturation at 95°C for 10 min followed by 40 to 45 cycles of 95°C for 15 s and 60°C for 1 min. Fluorescent measurements were recorded during each annealing step. At the end of each PCR run, data were automatically analyzed by the system and amplification plots were obtained. For each PCR, 2 μl of cDNA template was added to 48 μl of PCR master mixture (5 μl of 1× PCR buffer II, 5 mM MgCl2, 250 μM concentrations of deoxynucleoside triphosphates, 400 nM concentrations of each primer, 1.5 U of AmpliTaq Gold DNA polymerase, 400 nM concentrations of MB, and 24.7 μl of water). The PCR buffer contained 5-carboxy-X-rhodamine (500 nM) as the reference dye for normalization of the reactions. Any possible fluctuations in 5-carboxy-X-rhodamine signals are used to correct the sample signal. The master mixtures were prepared freshly for each of the real-time PCR assays. To generate a CCR5 RNA standard curve to quantify CCR5 mRNA in human immune cells, known amounts of the CCR5 RNA standard were 10-fold serially diluted and then amplified in the same plate under identical conditions. The quantity of CCR5 mRNA in the samples was automatically calculated by the ABI 7000 SDS based on the data obtained from the standard curve. All amplification reactions were performed in duplicate, and average copy numbers of the duplicates are presented in this report, unless otherwise specified. To control for the integrity of RNA and normalize CCR5 mRNA levels in MDM and PBL, GAPDH RNA isolated from MDM and PBL was amplified by real-time RT-PCR with brilliant SYBR green QPCR master mix (Stratagene, La Jolla, Calif.) with the ABI 7000 SDS. For each PCR, duplicates of 2 μl of cDNA template were added to 48 μl of the QPCR master mix that contained 200 nM of each of the two primers and the mixture was amplified. The cycle conditions were the same as that for CCR5, except amplification continued for 40 cycles. The master mix was used as instructed by Stratagene. To generate a total RNA standard curve with GAPDH primers, known amounts of total RNA standard extracted from pooled PBMC were serially diluted (ranging from 8 to 1,000 ng) and amplified for 40 cycles. The MDM and PBL samples were amplified in the same plate with the GADPH standard under identical conditions. The quantity (in nanograms) of total RNA in the samples was also automatically calculated by the ABI 7000 SDS based on the data obtained from the total RNA standard curve. All amplification reactions were performed in duplicate, and an average RNA quantity (in nanograms) of the duplicates was used to normalize the CCR5 mRNA levels in MDM and PBL. To quantify the CCR5 mRNA levels, the CCR5 mRNA copy numbers in MDM and PBL samples were divided by the amount of total RNA (in nanograms) determined by the GAPDH real-time RT-PCR in the same sample and then multiplied by 1,000 to convert the units to CCR5 mRNA copy numbers per microgram of total RNA. Thus, the CCR5 mRNA levels in these cells are expressed as the mean copy number of CCR5 mRNA per microgram of total RNA. To study the dynamic range, accuracy, and reproducibility, 10-fold serial dilutions of the CCR5 RNA standard, ranging from 102 to 106 copies, were amplified and used as a standard curve in each assay.
RESULTS
Sensitivity of the real-time RT-PCR.
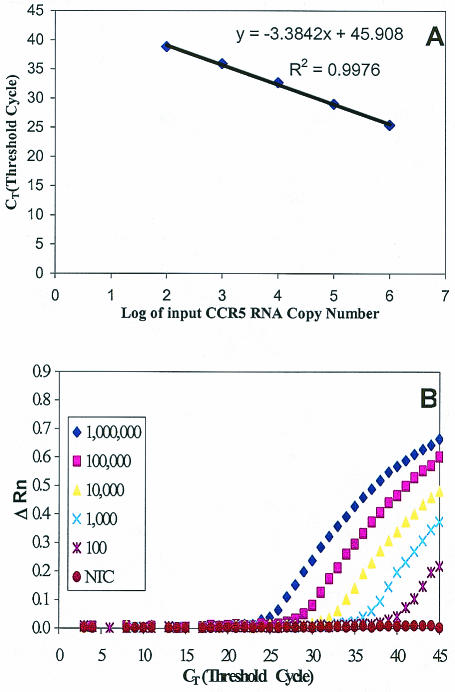
The sensitivity of the real-time RT-PCR was determined by using a dilution series (0, 10, 102, 103, 104, 105, and 106 copies) of the CCR5 RNA standard. The real-time RT-PCR could detect CCR5 mRNA copy numbers as low as 10 molecules with the detection rate at only 50% (5 of 10 replicates of 10 CCR5 mRNA copies added in the reverse transcription reaction) (data not shown). The detection rate, however, was 100% at CCR5 mRNA copy numbers of 102 and higher (10 of 10 replicates). The detection limit, therefore, was 102 RNA molecules per reaction. A representative result is shown in Fig. 2.
FIG. 2.
Real-time RT-PCR sensitivity and linearity analysis with the CCR5 RNA standard derived from pCCR5. Tenfold serial dilutions of the RNA standard starting from 102 to 106 molecules per reaction were amplified by the real-time PCR. (A) Standard curve of serial dilutions of CCR5 standard RNA with a correlation coefficient (R2) of 0.9976. (B) Amplification plot of serial dilutions of CCR5 standard RNA showing the dynamic detection range of 5 orders of magnitude from 102 to 106 molecules. A reading of change in fluorescence (ΔRn) as a function of cycle numbers was demonstrated for a range of known input copy numbers of the CCR5 RNA transcript. The detection sensitivity was 100 copies of CCR5 mRNA per PCR.
Linearity, range of quantification, and precision.
Amplification of the CCR5 RNA standard at different concentrations showed linearity over a range of 5 orders of magnitude (Fig. 2), and the correlation coefficient (R2) was 0.9976. To determine the variation of repetitive measurements of the real-time PCR between assays which were performed separately (2 to 3 days apart), 10-fold serial dilutions of the CCR5 RNA standard (ranging from 102 to 106 copies) were amplified by real-time RT-PCR in five separate experiments. The coefficients of variation (CV) of intra-assay threshold cycle (CT) values ranged from 0.1 to 2.07% (Table 1), and interassay variation is comparable to that of the intra-assay variation (Table 2).
TABLE 1.
Intra-assay accuracy of CCR5 mRNA quantification by real-time RT-PCR
| Repetition no. |
CT for no. of input copiesa
|
||||
|---|---|---|---|---|---|
| 1,000,000b | 100,000c | 10,000d | 1,000e | 100f | |
| 1 | 24.59 | 29.22 | 32.16 | 35.64 | 38.73 |
| 2 | 25.24 | 29.13 | 32.18 | 35.51 | 38.09 |
| 3 | 25.65 | 29.13 | 32.71 | 36.74 | 40.42 |
| 4 | 25.91 | 29.16 | 33.17 | 35.21 | 38.02 |
Input copy numbers of CCR5 RNA standard.
Mean ± standard deviation, 25.35 ± 0.43; CV, 1.71%.
Mean ± standard deviation, 29.16 ± 0.03; CV, 0.10%.
Mean ± standard deviation, 32.56 ± 0.39; CV, 1.18%.
Mean ± standard deviation, 35.78 ± 0.48; CV, 1.35%.
Mean ± standard deviation, 38.82 ± 0.80; CV, 2.07%.
TABLE 2.
Interassay reproducibility of CCR5 mRNA quantification by real-time RT-PCR
| Assay no. |
CT for no. of input copiesa
|
||||
|---|---|---|---|---|---|
| 1,000,000b | 100,000c | 10,000d | 1,000e | 100f | |
| 1 | 24.92 | 29.18 | 32.17 | 35.58 | 38.41 |
| 2 | 25.04 | 27.93 | 32.54 | 35.87 | 37.91 |
| 3 | 26.21 | 30.14 | 33.14 | 37.51 | 39.94 |
| 4 | 25.78 | 29.15 | 32.94 | 35.98 | 39.22 |
| 5 | 24.98 | 28.61 | 32.62 | 34.84 | 38.67 |
Input copy numbers of CCR5 RNA standard.
Mean ± standard error of the mean, 25.39 ± 0.49.
Mean ± standard error of the mean, 29.00 ± 0.59.
Mean ± standard error of the mean, 32.68 ± 0.29.
Mean ± standard error of the mean, 35.96 ± 0.63.
Mean ± standard error of the mean, 38.83 ± 0.60.
Linearity and precision of GAPDH real-time RT-PCR.
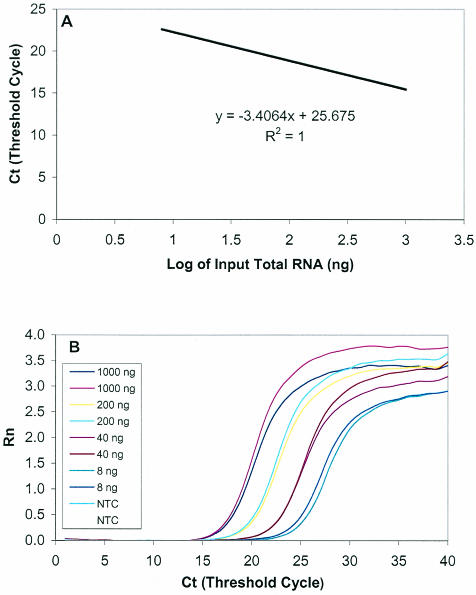
The linearity and precision of the GAPDH real-time RT-PCR was evaluated by amplifying serially diluted total RNA of PBMC, ranging from 8 to 1,000 ng per reaction, as described earlier. For the intra-assay precision, a serially diluted known amount of total RNA was amplified with four repetitions on the same plate. As shown in Table 3, the GAPDH real-time PCR has excellent intra-assay precision, with a CV of 0.44 to 0.68%. To examine the interassay accuracy of the GAPDH real-time RT-PCR, we performed the assay with the same samples five times on different dates. For each amplification, an aliquot of total RNA standard was diluted and amplified in duplicate. As shown in Table 4, the data from each assay were highly reproducible. One of the representative standard curves and amplification efficiency plots is shown in Fig. 3. The linearity and efficiency were excellent as determined by the correlation coefficient (R2 = 1.0) and by the equation for the standard curve (y = −3.4064x + 25.675).
TABLE 3.
Intra-assay accuracy of GAPDH assay by real-time RT-PCR
| Repetition no. |
CT for amt of input RNA (ng)a
|
|||
|---|---|---|---|---|
| 1,000b | 200c | 40d | 8e | |
| 1 | 16.20 | 18.22 | 20.64 | 22.73 |
| 2 | 16.11 | 18.13 | 20.77 | 22.54 |
| 3 | 15.96 | 18.34 | 20.96 | 22.54 |
| 4 | 16.17 | 18.25 | 20.75 | 22.68 |
Input RNA amount.
Mean ± standard deviation, 16.11 ± 0.11; CV, 0.68%.
Mean ± standard deviation, 18.24 ± 0.09; CV, 0.49%.
Mean ± standard deviation, 20.78 ± 0.13; CV, 0.63%.
Mean ± standard deviation, 22.62 ± 0.10; CV, 0.44%.
TABLE 4.
Interassay reproducibility of GAPDH assay by real-time RT-PCR
| Assay no. |
CT for amt of input RNA (ng)a
|
|||
|---|---|---|---|---|
| 1,000b | 200c | 40d | 8e | |
| 1 | 16.16 | 18.18 | 20.71 | 22.64 |
| 2 | 16.01 | 18.30 | 20.30 | 22.61 |
| 3 | 16.08 | 18.10 | 20.24 | 22.67 |
| 4 | 16.09 | 18.10 | 20.86 | 22.67 |
| 5 | 15.96 | 18.01 | 20.47 | 22.64 |
Input total RNA amount.
Mean ± standard error of the mean, 15.96 ± 0.13.
Mean ± standard error of the mean, 18.10 ± 0.08.
Mean ± standard error of the mean, 20.47 ± 0.15.
Mean ± standard error of the mean, 22.64 ± 0.01.
FIG. 3.
Linearity and efficiency of GAPDH real-time RT-PCR. Fivefold serial dilutions of the total RNA standard starting from 8 to 1,000 ng per reaction were amplified in duplicate by real-time PCR. (A) Standard curve of serial dilutions of total RNA with a correlation coefficient (R2) of 1.0; the equation for the line is y = −3.4064x + 25.675. (B) Amplification plot of serial dilutions of total RNA showing the dynamic detection range from 8 to 1,000 ng. A reading of change in fluorescence (ΔRn) as a function of cycle numbers was demonstrated for a range of known input total RNA (in nanograms).
Quantification of CCR5 mRNA in MDM and PBL.
Because human immune cells are the principle cells that express CCR5, the major coreceptor for HIV entry into macrophages, we examined the applicability of real-time RT-PCR to quantification of CCR5 mRNA in MDM and PBL isolated from adult peripheral blood. To compare CCR5 mRNA levels in MDM and PBL from the same donor, the total RNA of the MDM and PBL obtained from 3 donors was amplified by CCR5 real-time RT-PCR and quantified as described above. The CCR5 mRNA levels were expressed as CCR5 mRNA copies per microgram of total RNA. The reproducibility of the assay with four repetitions was excellent, with a variation of less than 10%. As expected, MDM expressed higher levels of CCR5 mRNA than did PBL isolated from the same donor (Table 5).
TABLE 5.
Intra-assay accuracy of real-time RT-PCR for quantification of CCR5 mRNA in MDM and PBL
| Repetition no. | Level of CCR5 mRNA (copies/μg of RNA) in:
|
|||||
|---|---|---|---|---|---|---|
| PBL-1a | MDM-1b | PBL-2c | MDM-2d | PBL-3e | MDM-3f | |
| 1 | 1,304,886 | 10,480,994 | 2,157,276 | 3,392,902 | 1,469,018 | 3,073,700 |
| 2 | 1,222,644 | 9,820,417 | 1,845,234 | 3,305,692 | 1,478,613 | 2,936,775 |
| 3 | 1,383,623 | 10,898,482 | 2,061,175 | 2,921,087 | 1,394,470 | 3,301,880 |
| 4 | 1,313,409 | 11,186,006 | 1,809,547 | 3,437,376 | 1,440,607 | 3,113,981 |
Mean ± standard deviation, 1,306,140 ± 65,930 copies/μg of RNA.
Mean ± standard deviation, 10,596,470 ± 592,834 copies/μg of RNA.
Mean ± standard deviation, 1,968,308 ± 168,013 copies/μg of RNA.
Mean ± standard deviation, 3,264,262 ± 23,523 copies/μg of RNA.
Mean ± standard deviation, 1,445,677 ± 37,760 copies/μg of RNA.
Mean ± standard deviation, 3,106,854 ± 150,678 copies/μg of RNA.
DISCUSSION
The ability to monitor the real-time progress of the amplification process completely revolutionizes the PCR-based quantification of DNA and RNA. In real-time PCR, reactions are characterized by the point in time during cycling when amplification of a PCR product is first detected rather than the amount of PCR product accumulated at the end of the entire PCR process. The CT is defined as the fractional cycle number at which the fluorescence passes the fixed threshold. The higher the starting copy numbers of the nucleic acid target, the sooner a significant increase in fluorescence is observed, and the lower the CT value is. Real-time PCR allows the CT to be observed when PCR amplification is still in the exponential phase. Therefore, the CT is a reliable measurement of starting copy numbers of mRNA in a real-time PCR amplification process.
Since CCR5 real-time RT-PCR has a wide dynamic detection range (102 to 106 copies per reaction), there is no need to dilute or concentrate samples, which was one of the problems encountered in the competitive RT-PCR quantification (8). Since there is no need to run the PCR-amplified products on agarose gel after the real-time RT-PCR, this assay not only eliminates post-PCR processing but also avoids variation and contamination caused by post-PCR manipulation. In addition, the costs associated with the use of this system are similar to those of the conventional RT-PCR, since this system allows processing of multiple samples with minimal labor time and there is no need to run an agarose gel electrophoresis.
In this study, we have successfully utilized the real-time RT-PCR assay for the quantification of CCR5 mRNA levels in human blood immune cells. This assay was highly specific, since the assay utilized both specific primers and a probe (MB) to amplify CCR5 mRNA. The CCR5 real-time RT-PCR was also a highly sensitive assay with a wide detection range and an excellent reproducibility (Fig. 2; Tables 1 and 2). Using CCR5 real-time PCR assay, we showed that MDM had higher levels of CCR5 mRNA transcripts than did PBL (Table 5). Our data have demonstrated that CCR5 real-time RT-PCR is precise, sensitive, and highly reproducible. Thus, this assay has broad application in the studies on the molecular role of CCR5 signaling in inflammation and viral infections, including HIV.
Acknowledgments
This work was supported by NIH grants DA 112815 and 16200 to W.-Z.H. and NIH grants MH 49981, AA 13547, U01 AI 32921, and U01 AI 41089 to S.D.D.
REFERENCES
- 1.Dean, M., M. Carrington, C. Winkler, G. A. Huttley, M. W. Smith, R. Allikmets, J. J. Goedert, S. P. Buchbinder, E. Vittinghoff, E. Gomperts, S. Donfield, D. Vlahov, R. Kaslow, A. Saah, C. Rinaldo, R. Detels, and S. J. O'Brien. 1996. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science 273:1856-1862. (Erratum, 274:1069.) [DOI] [PubMed] [Google Scholar]
- 2.Dragic, T., V. Litwin, G. P. Allaway, S. R. Martin, Y. Huang, K. A. Nagashima, C. Cayanan, P. J. Maddon, R. A. Koup, J. P. Moore, and W. A. Paxton. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667-673. [DOI] [PubMed] [Google Scholar]
- 3.Giesendorf, B. A., J. A. Vet, S. Tyagi, E. J. Mensink, F. J. Trijbels, and H. J. Blom. 1998. Molecular beacons: a new approach for semiautomated mutation analysis. Clin. Chem. 44:482-486. [PubMed] [Google Scholar]
- 4.Hassan, N. F., J. R. Cutilli, and S. D. Douglas. 1990. Isolation of highly purified human blood monocytes for in vitro HIV-1 infection studies of monocyte/macrophages. J. Immunol. Methods 130:283-285. [DOI] [PubMed] [Google Scholar]
- 5.Huang, Y., W. A. Paxton, S. M. Wolinsky, A. U. Neumann, L. Zhang, T. He, S. Kang, D. Ceradini, Z. Jin, K. Yazdanbakhsh, K. Kunstman, D. Erickson, E. Dragon, N. R. Landau, J. Phair, D. D. Ho, and R. A. Koup. 1996. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat. Med. 2:1240-1243. [DOI] [PubMed] [Google Scholar]
- 6.Lai, J. P., S. D. Douglas, E. Rappaport, J. M. Wu, and W. Z. Ho. 1998. Identification of a delta isoform of preprotachykinin mRNA in human mononuclear phagocytes and lymphocytes. J. Neuroimmunol. 91:121-128. [DOI] [PubMed] [Google Scholar]
- 7.Lai, J. P., S. D. Douglas, F. Shaheen, D. E. Pleasure, and W. Z. Ho. 2002. Quantification of substance P mRNA in human immune cells by real-time reverse transcriptase PCR assay. Clin. Diagn. Lab. Immunol. 9:138-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai, J.-P., S. D. Douglas, M. Zhao, and W.-Z. Ho. 1999. Quantification of substance P mRNA in human mononuclear phagocytes and lymphocytes using a mimic-based RT-PCR. J. Immunol. Methods 230:149-157. [DOI] [PubMed] [Google Scholar]
- 9.Liu, R., W. A. Paxton, S. Choe, D. Ceradini, S. R. Martin, R. Horuk, M. E. MacDonald, H. Stuhlmann, R. A. Koup, and N. R. Landau. 1996. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86:367-377. [DOI] [PubMed] [Google Scholar]
- 10.Piatek, A. S., S. Tyagi, A. C. Pol, A. Telenti, L. P. Miller, F. R. Kramer, and D. Alland. 1998. Molecular beacon sequence analysis for detecting drug resistance in Mycobacterium tuberculosis. Nat. Biotechnol. 16:359-363. [DOI] [PubMed] [Google Scholar]
- 11.Raeymaekers, L. 1993. Quantitative PCR: theoretical considerations with practical implications. Anal. Biochem. 214:582-585. [DOI] [PubMed] [Google Scholar]
- 12.Samson, M., F. Libert, B. J. Doranz, J. Rucker, C. Liesnard, C. M. Farber, S. Saragosti, C. Lapoumeroulie, J. Cognaux, C. Forceille, G. Muyldermans, C. Verhofstede, G. Burtonboy, M. Georges, T. Imai, S. Rana, Y. Yi, R. J. Smyth, R. G. Collman, R. W. Doms, G. Vassart, and M. Parmentier. 1996. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382:722-725. [DOI] [PubMed] [Google Scholar]
- 13.Tyagi, S., D. P. Bratu, and F. R. Kramer. 1998. Multicolor molecular beacons for allele discrimination. Nat. Biotechnol. 16:49-53. [DOI] [PubMed] [Google Scholar]
- 14.Tyagi, S., and F. R. Kramer. 1996. Molecular beacons: probes that fluoresce upon hybridization. Nat. Biotechnol. 14:303-308. [DOI] [PubMed] [Google Scholar]
- 15.Vet, J. A., A. R. Majithia, S. A. Marras, S. Tyagi, S. Dube, B. J. Poiesz, and F. R. Kramer. 1999. Multiplex detection of four pathogenic retroviruses using molecular beacons. Proc. Natl. Acad. Sci. USA 96:6394-6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang, J. H., J. P. Lai, S. D. Douglas, D. Metzger, X. H. Zhu, and W. Z. Ho. 2002. Real-time RT-PCR for quantitation of hepatitis C virus RNA. J. Virol. Methods 102:119-128. [DOI] [PubMed] [Google Scholar]