Abstract
A recombinant DNA strategy was applied to analyze and screen the shotgun expression library from a clinically confirmed local virulent isolate of Mycobacterium tuberculosis with sera from tuberculosis patients, which led to expression and purification of highly immunoreactive and specific mycobacterial antigens expressed during the course of active disease which could be of diagnostic significance. An enzyme-linked immunoassay for diagnosis of tuberculosis was devised by using a shotgun immunoexpression library in the λgt11 vector. DNA from a virulent M. tuberculosis patient isolate (TBW-33) confirmed with the BACTEC 460 system was sheared and expressed to generate shotgun polypeptides. β-Galactosidase fusion proteins capable of demarcating active tuberculosis infections from Mycobacterium bovis BCG-vaccinated healthy subjects or people harboring environmental mycobacteria were selected by comparative immunoreactivity studies. Promising mycobacterial DNA cassettes were subcloned and expressed into the glutathione S-transferase (GST) fusion vector pGEX-5X-1 with a strong tac promoter and were expressed in Escherichia coli BL21. These fusion proteins were severed at a built-in factor Xa recognition site to separate the GST tags and were utilized in an indirect enzyme-linked immunoassay for serodiagnosis of patients with active tuberculosis. The system offered a clear demarcation between BCG-vaccinated healthy subjects and patients with active tuberculosis and proved to be effective in detecting pulmonary as well as extrapulmonary tuberculosis, with an overall sensitivity of 84.33% and an overall specificity of 93.62%.
Tuberculosis (TB) is the main cause of mortality due to a single-pathogen infection, killing nearly 2 million people every year and developing into active disease in 8.7 million others. At the same time, the rising incidence both of multidrug-resistant TB and of TB and human immunodeficiency virus (HIV) coinfection poses serious new challenges to the response effort (34). The success of mycobacteria as pathogens resides in their ability to replicate or persist in a dormant state within macrophages for long periods of time. Such a state of dormancy may reflect metabolic shutdown resulting from the action of a cell-mediated immune response that can contain but not eradicate the infection. As immunity decreases through aging or immune suppression, the dormant bacteria reactivate, causing an outbreak of disease often many decades after the initial infection.
The major goal of biomedical TB research is to lessen the public health burden of this epidemic by developing improved, affordable diagnostic, therapeutic, and intervention strategies. Because of low-sensitivity (direct staining of acid-fast bacilli) and time-consuming (culture) diagnostic techniques, a fast etiological confirmation is essential for further management of patients. In this context, approaches based on serological diagnosis have been developed in the past (6, 7, 9, 22, 23, 25, 29, 36, 37, 39), but differentiation of patients with active disease from Mycobacterium bovis BCG-vaccinated individuals or those with Mantoux-positive testing and clinical signs of disease still remains a major problem (10, 30). The efforts to explore candidate antigens have received a great boost with the complete sequencing of the Mycobacterium tuberculosis H37Rv genome, comprising 4,411,529 bp with 3,924 open reading frames (ORFs), accounting for ∼91% of the potential coding capacity. However, the functions of a considerable number of ORFs are still not known (5). At the same time, a number of proteins, such as MPT 63 (20), MPT 83 (11), and MTC 28 (21), are yet to be tested for the stringent requirements of an effective serodiagnosis regimen.
The present study aimed to assess the diagnostic potential of the shotgun polypeptides generated from genomic fragmentation of a BACTEC 460-confirmed virulent isolate of M. tuberculosis in differentiating BCG-vaccinated healthy subjects from those with active TB.
MATERIALS AND METHODS
Mycobacterial strains.
M. tuberculosis strains were isolated from sputa of patients with pulmonary TB by culture on Lowenstein-Jensen egg-based solid medium (16). Isolate TBW-33 was identified as an M. tuberculosis strain at Choithram Research Center, Indore, India, on the basis of the criteria set by Centers for Disease Control and Prevention protocols (14). In addition, the identity of the isolate was confirmed by (i) an inability to grow in the presence of para-nitrobenzoic acid and thiosemicarbazone and (ii) a lack of growth in synthetic N medium. Individual colonies were suspended in gel saline (0.5% gelatin, 0.9% NaCl) at a concentration of ∼108/ml and stored at −70°C in aliquots. Besides these criteria, M. tuberculosis was confirmed with BACTEC 460 TB vials by the measuring growth index.
Determination of virulence.
The virulence of each isolate was determined in duplicate in the guinea pig model. Bacilli (107 in 0.5 ml of gel saline) from each isolate were injected intramuscularly into the thigh muscles of guinea pigs. Animals were sacrificed on 35th day, and the extent of dissemination of the bacilli in the spleen, liver, and lung was used as a measure of virulence (24).
Patient enrollment and serum collection. (i) In-house study.
A total of 811 blood samples for in-house study were collected from following groups of subjects.
(a) Subjects with chronic infection (n = 76; smear, culture, and X-ray positive).
The first group consisted of individuals who had active disease for about a year and had bacilli in their sputa. The sera of these subjects were pooled and used for primary screening of the library.
(b) Subjects in the very early stages of TB (n = 250; smear negative and culture and X-ray positive).
The second group consisted of individuals who had symptoms of TB for the first time and who were clinically judged to be in very early stages of disease.
(c) Healthy BCG-vaccinated subjects without TB (n = 220; smear, culture, and X-ray negative).
The third group consisted of individuals who had no clinical symptoms of TB and were smear, culture, and X-ray negative.
(d) Subjects with non-TB lung diseases (n = 220; smear, culture, and X-ray negative).
The fourth group included subjects suffering from non-TB lung diseases such as lung abscess, chronic bronchitis, and bronchial asthma and without any clinical or laboratory evidence of TB.
(e) Subjects with extrapulmonary TB (n = 45; smear negative and culture and biopsy positive).
The fifth group consisted of subjects suffering from extrapulmonary TB as evident from culture and biopsy analysis.
(ii) External studies.
A total of 334 serum samples for external studies (conducted at Gandhi Medical College, Bhopal, India) were collected from the following different groups.
(a) Subjects with active pulmonary and extrapulmonary TB (n = 52).
The first group included patients who had pulmonary TB with extrapulmonary dissemination of tubercle bacilli.
(b) Subjects with pulmonary TB (n = 78).
The second group consisted of chronically infected individuals, as determined from smear, culture, and X-ray results.
(c) Subjects with extrapulmonary TB (n = 48).
The third group included patients with extrapulmonary TB, as determined from culture and biopsy results.
(d) Subjects with other infectious diseases (n = 60).
The fourth group consisted of subjects with leprosy (n = 22), hepatitis (n = 18), or HIV disease (n = 20).
(e) Healthy normal subjects (n = 96).
The fifth group consisted of normal healthy controls who had a normal chest radiogram and no past personal or family history of TB.
Serum samples were collected, pooled, preabsorbed against Escherichia coli Y1090 sonicates, and stored frozen in the presence of 0.05% NaN3 at −20°C until use.
M. tuberculosis DNA isolation.
The M. tuberculosis isolate was streaked and grown on the surface of solid Lowenstein-Jensen medium (1). Bacilli were harvested, heat inactivated at 85°C for 30 min, and washed in 5 ml of TEN buffer (10 mM Tris-HCl, 1 mM EDTA, 100 mM NaCl [pH 8.0]). The bacillus pellet was resuspended in 2 ml of TEN buffer containing 15% sucrose and 10 μg of lysozyme per ml and was incubated at 37°C for 3 h with occasional shaking. Sodium dodecyl sulfate (SDS) was added at a final concentration of 2% (wt/vol), and the suspension was incubated at 50°C for 30 min, at which time lysis resulting in an increase in viscosity was observed. The suspension was diluted to 5 ml with TEN buffer. Chloroform-phenol (25:24, vol/vol) extraction of the aqueous phase was performed, followed by chloroform-isoamyl alcohol (24:1, vol/vol) extraction with gentle agitation for 10 min. DNA was precipitated from 0.1 M sodium acetate (pH 5.0) with 2.5 volumes of chilled ethanol after treatment with DNase-free RNase. Finally, the pellet was resuspended in 20 mM Tris (pH 7.5)-100 mM NaCl-1 mM EDTA at a concentration of 0.35 mg/ml (ratio of optical density at 260 nm [OD260] to OD280, 1.8).
Construction of genomic expression library.
The genomic expression library was constructed as described by Young et al. (38). Ten micrograms of M. tuberculosis DNA was randomly sheared by repeated passaging through a 26-gauge needle. Fragments ranging in size from 1 to 7 kb were recovered from low-melting-point agarose gels following electrophoresis. One microgram of this sheared DNA was methylated with EcoRI methylase and made blunt ended with T4 DNA polymerase, followed by ligation and digestion of the EcoRI linker with T4 DNA ligase and EcoRI restriction enzymes, respectively. DNA fragments were purified from excess linkers by passage over a gel filtration column (Biogel P-60; 100 to 200 mesh; 5 ml) followed by agarose gel electrophoresis. DNA in size range of 1 to 7 kb was electroeluted into an NA 45 membrane (Schleicher & Schuell). A piece of NA 45 was inserted in a slit cut just in front of the desired DNA. The gel was run at 100 mA until DNA of the appropriate size range had completely stacked up on the NA 45. The paper was quickly rinsed in TE buffer (10 mM Tris [pH 8.0], 1 mM EDTA) and placed in an Eppendorf tube with 0.4 ml of 1 M NaCl containing 0.05 M arginine (free base). The filter was incubated at 70°C for 2 h to elute the DNA. The NA 45 was removed, ethanol was added to fill the Eppendorf tube, and DNAs were precipitated at −20°C for 2 h. The DNA was resuspended in TE buffer and ligated with 1 μg of EcoRI-digested and dephosphorylated λgt11 DNA at a vector/insert ratio of 20:1. In vitro packaging of the phage DNA was accomplished with Gigapack II Gold packaging extract as according to the instructions of the manufacturer (Stratagene, La Jolla, Calif.). The library was amplified on lawns of E. coli Y1088. In brief, E. coli Y1088 was grown in Luria-Bertani (LB) broth supplemented with 60 μg of ampicillin per ml, 10 mM MgSO4, and 0.2% (wt/vol) maltose. The bacteria were pelleted, washed, and resuspended in sterile 10 mM MgSO4 to an OD600 of 0.5. Two hundred microliters of this bacterial suspension was mixed at 37°C for 20 min with 0.5 μl of the bacteriophage expression library. Molten top agar (3 ml) was added and mixed thoroughly, and the whole contents were immediately poured onto 1-day-predried LB agar plates. Phages as clear visible plaques on the plates were obtained in SM buffer (50 mM Tris [pH 7.5], 100 mM NaCl, 0.2% MgSO4, 2.5% gelatin) and were stored at 4°C with 0.6% CHCl3 till further use. The library was passaged through E. coli Y1090 once and amplified by the method of Young et al. (38).
Screening of the recombinant λgt11 library.
Immunological screening of the immunoexpression library was carried out with E. coli Y1090. Infection of E. coli Y1090 with the recombinant phage library to obtain visible plaques was done as described above. The plaques obtained were transferred to a nitrocellulose membrane impregnated with 10 mM IPTG (isopropyl-β-d-thiogalactopyranoside), washed, blocked, probed with pooled sera (preabsorbed against E. coli lysate) from 20 patients with chronic TB at 1:100 dilution in blocking buffer for 2 h at 37°C, washed, and incubated with anti-human immunoglobulin G (IgG)-horseradish peroxidase (HRP) conjugate (1:5,000) at 37°C for 2 h. Positive plaques were visualized with 0.8 mg of 3,3′-diaminobenzidine per ml, 0.4 mg of NiCl2 per ml, and 0.1% H2O2 in 100 mM Tris (pH 7.5) in the dark. Reactive positive plaques were rescreened by probing with sera from patients with chronic TB (n = 20) and sera from normal human subjects (n = 20), and clones differentiating patients from healthy individuals were reselected by performing a complementation assay. A panel of sera form patients with early chronic TB, treated patients, patients with relapse, contacts, and BCG-vaccinated individuals were included with each selected clone.
Insert DNA preparation and subcloning.
After subsequent clonal propagation and PFU determination, recombinant phage DNA was isolated and purified from selected positive clones by using the Qiagen lambda DNA purification kit according to the manufacturer's instructions. Purified recombinant DNA (4 μg) was restricted with EcoRI to release the inserts and analyzed on a 0.8% agarose gel. The insert DNA band was extracted from the gel by using the Clean Gene kit (Bangalore Genei) according to the manufacturer's recommendations. The resulting insert DNA was ligated to predigested (with EcoRI) and dephosphorylated (with calf intestinal alkaline phosphatase) linearized plasmid pGEX-5X-1 DNA by using T4 DNA ligase. The E. coli DH5α strain (transformation efficiency of 106 to 108) was transformed with 100 ng of ligated DNA and screened by a two-pronged strategy incorporating the rapid lysis method for molecular weight analysis and Southern hybridization (1). In brief, individual colonies were grown separately, and DNA was prepared, digested with EcoRI, and analyzed on a 0.8% agarose gel along with a control plasmid and a DNA molecular weight ladder. Later, the DNA inserts was transferred to a nylon membrane and hybridized with total M. tuberculosis (isolate TBW-33) DNA labeled with digoxigenin (DIG) in a solution consisting of 6× SSC (1× SSC is 150 mM NaCl plus 15 mM sodium citrate), 15× Denhardt's solution, and 0.1% SDS for 14 h at 65°C. The membranes were washed thrice with 0.3× SSC containing 0.1% SDS at 65°C for 30 min and developed with 5-bromo-4-chloro-3-indolylphosphate (BCIP)-nitroblue tetrazolium by the protocol described in the DIG nucleic acid detection kit (Boehringer, Mannheim, Germany). All recombinant DNA techniques and immunoscreening of the λgt11 library were carried out by standard protocols (27). Restriction and modification enzymes were from Promega. The confirmed positive colonies were selected, grown in LB medium, and screened for fusion protein expression.
Protein analysis.
Recombinant plasmids for each clone were transformed into E. coli strain BL21 for overexpression of the desired proteins. Transformed colonies were grown to an A260 of 0.4, induced with 10 mM IPTG, and grown for 16 h at 37°C in a shaking incubator. Cells were harvested and lysed, and the fusion proteins were purified with the use of a glutathione-Sepharose 4B slurry (Pharmacia Biotech) and analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) (17). The protein of interest was retrieve from its fusion partner (glutathione S-transferase [GST]) by incubation with factor Xa enzyme at 16°C for 12 h. The GST partner was removed from such preparations by rebinding the digested fusion protein with glutathione-Sepharose beads. For Western blot analysis (32), proteins electrophoresed by SDS-15% PAGE were transferred to nitrocellulose membranes. The membranes were blocked and probed with pooled patient sera at a dilution of 1:50 in blocking buffer, followed by incubation with anti-human IgG-HRP (Dako) at a final dilution of 1:4,000 for 1 h at 37°C. The blots were developed with 0.8 mM 3,3′-diaminobenzidine in the presence of 0.045% H2O2 and 0.4 mM NiCl2.
Enzyme-linked immunosorbent assay (ELISA).
Each well of Nunc Maxisorp C8 plates was coated with a total of 500 ng of expressed purified peptide antigens, mixed in the same amount in 200 μl of 100 mM phosphate-buffered saline (PBS) (pH 7.4). The uncoated sites were blocked with 350 μl of blocking solution (100 mM PBS [pH 7.4] plus 1.0% normal rabbit serum) at 37°C for 2 h. The plates were then washed once with 10% sucrose solution for stability. At this juncture, the plates were emptied and vacuum dried in a desiccator containing silica gel (activation criteria, 120°C for 1 h) at room temperature and stored at 4°C. The positive control (pooled sera from 20 patients with chronic TB), negative control (sera from 10 individuals known to be TB negative), and TB and normal human sera to be tested were diluted 1:40 in dilution buffer (100 mM PBS [pH 7.4], 1.0% [vol/vol] normal rabbit serum, 0.1% [vol/vol] Tween 20), and 200-μl portions of these diluted sera were applied in duplicate to microplate wells, incubated at 37°C for 30 min, and washed with wash buffer (100 mM PBS [pH 7.4] plus 0.1% [vol/vol] Tween 20). The plates were incubated with 200 μl of goat anti-human IgG-HRP (Dako) at a dilution of 1:40,000 at 37°C for 30 min and then washed. Finally, the OD450 was read after addition of the substrate 3,3′,5,5′-tetramethylbenzidine.
Statistical analysis.
The sensitivity, specificity, positive predictive value, and negative predictive value of the assay were calculated (31). In comparisons with results and patient clinical data, statistical analysis was done by using the chi-square test, and the cutoff value for positivity was set on the basis of positive control and negative control OD values by using the formula cutoff = (mean positive control OD + mean negative control OD)/7.
RESULTS
Genomic library construction and screening with antibody probes.
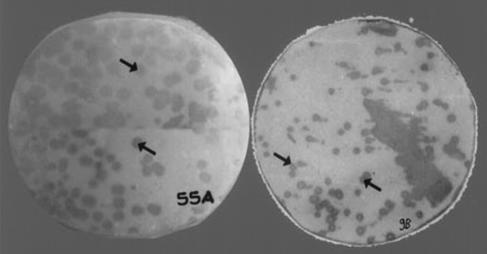
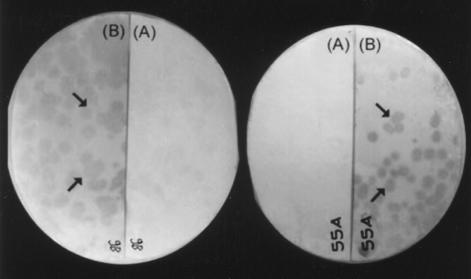
We obtained 17 plaques per μg of DNA, of which 2% were blue and were screened for complementation assay. Amplification of the library in E. coli Y1089 resulted in a titer of 109 PFU/ml. Repeated amplification of the library was avoided to prevent overgrowth of nonrecombinant phages. A total of 2 × 103 colonies plated on each plate were screened with pooled sera from 20 patients with chronic TB. The reactive plaques were selected and plaque purified until a homogenously reactive plaque progeny was obtained, by carrying out repeated infection and propagating the progeny from a single reactive plaque (Fig. 1). Most of the plaques that reacted with sera from TB patients as well as healthy controls were discarded. Forty-six selected reactive phages clones were again hybridized with sera from individual TB patients as well as healthy contacts. The clones reactive only with patient sera were reprobed with sera from BCG-vaccinated individuals to check cross-reactivity, and we finally narrowed the panel to around 14 recombinants. The intensities of the signals with sera from patients with active TB were very strong compared to those with sera from BCG-vaccinated subjects, where the reactivity was negligible (Fig. 2). Most of the selected clones had very faint signals with sera from the close contacts (Fig. 3).
FIG. 1.
Analysis of λgt11. M. tuberculosis recombinant 9B and 55A clones with pooled TB patient sera are shown. Arrows indicate reactive recombinants.
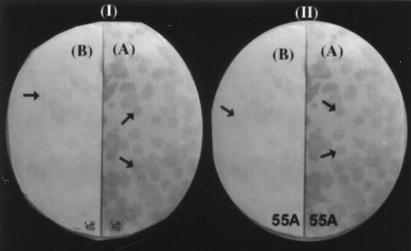
FIG. 2.
Analysis of serum-reactive λgt11 recombinant clones 9B and 55A with pooled sera from BCG-vaccinated healthy human subjects and sera from patients with chronic TB. Recombinant plaque progeny infecting lawn of E. coli Y1090 were transferred to a nitrocellulose membrane after fusion protein induction. The membrane filter was cut in half and probed with pooled TB sera (A) or pooled sera from BCG-vaccinated healthy human subjects (B).
FIG. 3.
Immunoreactivity analysis of recombinant phage clones 9B (I) and 55A (II) with sera from TB patients (A) and active contacts (B). Arrows indicate the reactive plaques.
Subcloning and expression of GST fusion proteins.
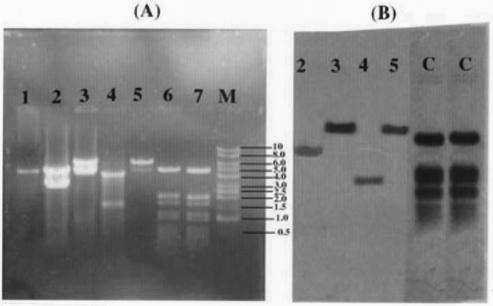
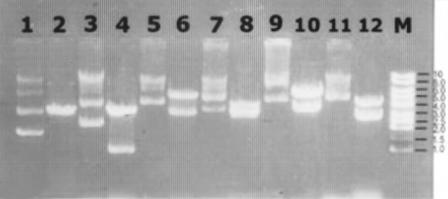
Ligated plasmid DNA were introduced into competent E. coli DH5α. Four recombinant clones were selected on the basis of hybridization and analyzed for the presence of insert DNA. All four clones carried inserts ranging in size from 1 to 7 kb (1.5, 3.5, 6.0, and 6.5 kb) (Fig. 4A, lanes 2 to 5) that hybridized to M. tuberculosis genomic DNA (Fig. 4B). The strong signal intensity in two clones, namely, 2D/16 and XX/30, was likely due to multiple copies of this fragment in the M. tuberculosis genome. DNA inserts of the clones were hybridized to obtain clones with single TB gene inserts. Selected recombinant plasmids from each clone were purified and compared with nonrecombinant plasmids. All recombinant clones showed higher molecular sizes (Fig. 5) than control plasmids. On restriction of these plasmids with EcoRI, the electrophoresis results (Fig. 5) indicate that in three of the clones (Fig. 5, lanes 6, 8, and 10), the mycobacterial cassettes were larger than pGEX-5X-1 itself. Aside from these, all other positive clones yielded fragments smaller than pGEX (Fig. 5, lanes 4 and 12).
FIG. 4.
Southern blot analysis of four patient serum-reactive λgt11 clones restricted with EcoRI, transblotted, and probed with virulent M. tuberculosis H37Rv whole DNA labeled with DIG. (A) Recombinant plasmid DNAs restricted with EcoRI. Lane 1, pGEX-5X-1 plasmid DNA restricted with EcoRI; lanes 2 to 5, recombinant plasmid clones 9B/30, 2D/16, B-55A/2, and XX/30, respectively, digested with EcoRI;. lanes 6 and 7, unlabeled control PBR328 DNA digested separately with BamHI, BglI, and HinfI; lane 8, 1-kb DNA ladder from Promega. (B) Southern analysis of insert DNA as shown in panel A. Lanes 2 to 5, recombinant plasmid clones 9B/30, 2D/16, B-55A/2, and XX/30, respectively. digested with EcoRI and hybridized to total M. tuberculosis DNA labeled with DIG. Lanes C, controls.
FIG. 5.
Recombinant plasmids with M. tuberculosis cassettes run on 0.8% agarose gel along with control pGEX-5X-1 plasmid DNA with no insert. Undigested recombinant plasmids and EcoRI-digested recombinant plasmids were loaded alternately. Lane 1, pGEX-5X-1 plasmid (unrestricted); lane 2, pGEX-5X-1 plasmid digested with EcoRI; lanes 3, 5, 7, 9, and 11, recombinant plasmid clones B-55A/2, 102/2, 2D/16, 9B/30, and XX/30, respectively; lanes 4, 6, 8, 10, and 12, recombinant plasmid clones B-55A/2, 102/2, 2D/16, 9B/30, and XX/30, respectively, digested with EcoRI; lane M, 1-kb DNA ladder.
Expression of GST fusion proteins.
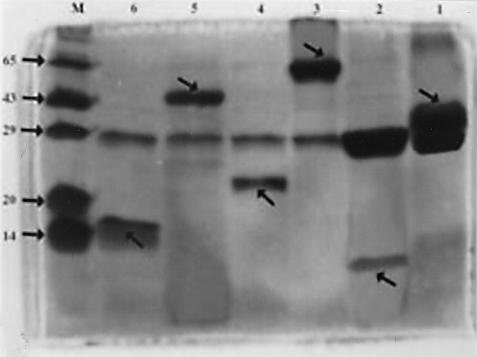
Selected recombinant plasmids for each clone were transformed into E. coli strain BL21 for overexpression of the desired proteins. Fusion proteins (Fig. 6) showing molecular masses of 35, 65, and 41 kDa were obtained for clones 2D/16, B-55A/2, and 9B/30, respectively. The recombinant protein, when extensively dialyzed in PBS and incubated with factor Xa for 16 h at 16°C, yielded recombinant fragments of around 8, 22, and 14 kDa, respectively, and a parent GST band at 27 kDa. The results were visualized by SDS-15% PAGE (Fig. 6), and the reactivity of these positive fusion protein band was confirmed by Western blotting (Fig. 7). Proteins were electrophoresed, transferred to three nitrocellulose membranes each, and hybridized with TB sera, affinity-purified anti-GST antibodies (Sigma), and normal human sera. No reactivity was observed with pooled sera from normal humans, and strong signals were found with patient sera and anti-GST antibodies (Fig. 7). Overall cross-reactivity was observed with GST protein for both patients and normal human subjects (Fig. 7).
FIG. 6.
SDS-PAGE (15%) analysis of recombinant proteins purified from clones 2D/16, B-55A/2, and 9B/30. Lanes 1, 3, and 5, purified fusion proteins (2 μg each) from clones 2D/16, B-55A/2, and 9B/30, respectively; lanes 2, 4, and 6, purified fusion proteins (4 μg each) from clones 2D/16, B-55A/2, and 9B/30, respectively, cut with 0.1 U of factor Xa at 16°C for 16 h; lane 7, Molecular mass marker. Arrows indicate GST, GST fusion protein, and digested expressed peptide bonds.
FIG. 7.
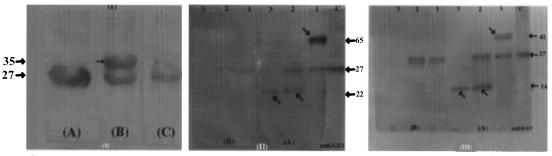
Western blot analysis of recombinant proteins purified from clones 2D/16, B-55A/2, and 9B/30. (I) A, GST protein probed with anti-GST antibodies. B, fusion protein from clone 2D/16 probed with pooled sera from TB patients. C, fusion protein from clone 2D/16 probed with pooled sera from normal human subjects. (II) GST fusion protein from clone B55A/2 (lanes 1), recombinant protein digested with factor Xa (lanes 2), and expressed peptide after removal of GST tag (lanes 3) probed with pooled TB sera (A) or pooled normal human sera (B). (III) GST fusion protein from clone 9B/30 (lanes 1), recombinant protein digested with factor Xa (lanes 2), and expressed peptide after removal of GST tag (lanes 3) probed with pooled TB sera (A) or pooled normal human sera (B). Lanes C in panels II and III, controls. Sizes are indicated in kilodaltons. Arrows indicate the reactive bands.
ELISA.
The cocktail of expressed protein antigens, when assessed by indirect ELISA at a serum dilution of 1:40, gave an overall sensitivity of 84.33% and an overall specificity of 93.62% with control subjects. The frequencies of negative results with the non-TB study groups were 304 of 316 (96.20%) for BCG-vaccinated healthy volunteers with no TB and 254 of 280 (90.71%) for subjects with non-TB lung disease (lung abscess, chronic bronchitis, or bronchial asthma) and other related infectious disease (leprosy, hepatitis C, or HIV disease). The frequencies of positive results were 345 of 404 (85.39%) for subjects with freshly diagnosed pulmonary TB as determined by smear, culture, and X-ray results; 72 of 93 (77.41%) for subjects with extrapulmonary TB (biopsy and culture positive); and 46 of 52 (88.46%) for patients with chronic pulmonary TB with disseminated bacilli in the extrapulmonary region. These results are from data obtained in the internal study (Table 1) and the external study (Table 2), conducted at our center and at Gandhi Medical College and TB Hospital, Bhopal, India, respectively.
TABLE 1.
Diagnostic potential of shotgun polypeptides expressed by the M. tuberculosis genome (internal study)
| Group | No. tested | No. positive | % Positive |
|---|---|---|---|
| Healthy controls (BCG vaccinated) | 220 | 10 | 4.55 |
| TB patients | |||
| Smear, culture, and X-ray positive | 76 | 72 | 94.73 |
| Smear negative, culture and X-ray positive | 250 | 200 | 80.0 |
| Extrapulmonary TB patients | 45 | 33 | 73.33 |
| Non-TB lung disease patients | 220 | 20 | 9.1 |
TABLE 2.
Results obtained with coded serum samples by ELISA (external study)
| Sample group | No. of samples tested | No. of positive results | No. of negative results | % Positivite results |
|---|---|---|---|---|
| TB patients (active), pulmonary + extrapulmonary | 52 | 46 | 6 | 88.46 |
| Pulmonary TB | 78 | 73 | 5 | 93.58 |
| Extrapulmonary TB | 48 | 39 | 9 | 81.25 |
| Other diseases | ||||
| Leprosy | 22 | 3 | 19 | 13.63 |
| Hepatitis | 18 | 1 | 17 | 5.56 |
| HIV disease | 20 | 2 | 18 | 10.00 |
| Healthy normal controls | 96 | 2 | 94 | 2.08 |
DISCUSSION
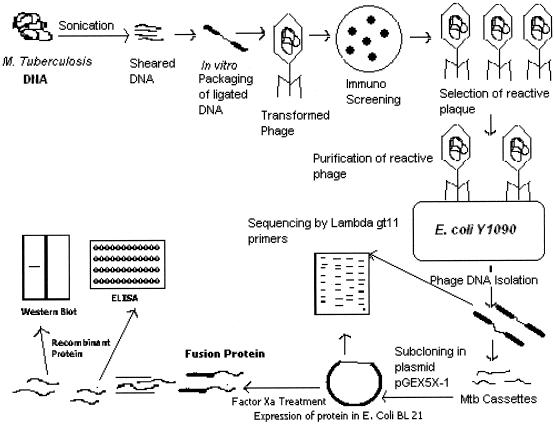
Serological testes for the diagnosis of TB have been described since 1898, employing antigens such as M. tuberculosis extracts, sonicates, and filtrates (8). The most critical problem in using these reagent for TB serodiagnosis was identified as low specificity due to the presence of M. tuberculosis cross-reactive antigens that may be recognized by nonspecific antibodies in healthy subjects (3, 8). The use of purified antigens (including recombinant proteins and peptides) has partially solved this problem by giving high specificity despite reduced sensitivity of each single serological assay. However, the combined use of different purified antigens in single serological tests appeared to improve the sensitivity while keeping the same level of specificity (2, 3, 18, 33). In this context, to devise and develop a sensitive and specific diagnostic tool for TB, a genomic shotgun λgt11 immunoexpression library was constructed from a field isolate of M. tuberculosis as depicted in Fig. 8. Bacilli obtained from sputum were tested for virulence in the guinea pig model and propagated once on Lowenstein-Jensen solid medium before DNA was utilized for library construction. This was considered essential to restore the virulence and original nature of the isolate strain used. Strain H37Rv was avoided as a source of DNA, because the presence of virulence-associated gene products or antigens in such a laboratory strain may be called into question (1). The screening method reported here permitted a survey of antigens expressed by approximately 106 independent recombinant phages in a single experiment. Similar approaches in the past have been instrumental in identifying out important genes and antigens of infectious agents, especially M. tuberculosis (13, 15, 35). DNA expression libraries constructed with cDNA and with DNase-digested genomic DNA were not used, as they may not contain insert DNA that is representative of all of the coding sequences of interest in a pathogen genome, mainly because cDNA is enriched in more abundant or housekeeping mRNAs, and DNase treatment may happen in a nonrandom fashion on genome (38). Moreover, any two genomic libraries previously reported (1, 38) that were prepared by shearing of genomic DNA, even from the same strain, may vary in outcome, depending on the degree of shear and sites of fragmentation. Hence, we created a new shotgun library from an Indian clinical isolate of M. tuberculosis. This approach also abolishes host-specific gene expression, which has already been demonstrated in other pathogenic bacteria, such as Salmonella enterica serovar Typhimurium (19) and Borrelia burgdorferi (4). Presumably, the activation of such differentially expressed genes is brought about in response to signals unique to the host environment. The expression or identification of such genes can hardly be achieved by other approaches (28).
FIG. 8.
Diagrammatic representation of shotgun approach for TB.
Three proteins, having molecular masses of 8, 22, and 14-kDa, from clones 2D/16, B-55A/2, and 9B/30, respectively, were successfully expressed and purified. The first protein was isolated from the supernatant, and the other two were isolated from the inclusion bodies. In the case of the fusion protein obtained from clone B55A/2 and internal factor Xa site was also observed, resulting in smaller fragments of digested expressed protein compared to expressed GST fusion protein. The expressed polypeptides, when pooled in equal quantities, showed a high sensitivity against patient sera in ELISA. For specificity studies, none of the patient serum-reactive clones was reactive with BCG-vaccinated or healthy carriers when assessed separately or in a cocktail. These observations are similar to those reported earlier (1, 15) for work on the same lines but in a different context. The values for close contacts were higher than those for other the control groups, probably due to the constant exposure of these subjects to the TB pathogen. None of the subjects from the close contact control group developed disease even after 1 year of follow-up. We observed phages that showed differential reactivity to pooled sera obtained from recently infected as opposed to chronically diseased TB patients. This suggests alterations either in the expression of these proteins or in the host immune response to these proteins as the disease transforms itself from the initial onset to a chronic disease. Some of these antigens may be elaborated only by live, actively replicating M. tuberculosis. We also found recombinant phage clones which had low reactivity with sera from patients with chronic disease but had very high reactivity to sera from healthy contacts and some BCG-vaccinated subjects. Such proteins may be plausible markers of protection and are the subject of further detailed investigations.
Nonpolypeptide antigenic determinants cannot be identified by these approaches based on expression of mycobacterial genes in an E. coli background, and this could be a minor drawback of our approach. Handling such nonprotein antigens by conventional methods will obviously require their purification and identification. Isolation of antigenic polysaccharides and lipids (6, 7, 25, 26) from virulent strains is time-consuming, cumbersome, and risky, and the use of promising recombinant antigens can be a strong advantage in this direction. With the recently deciphered DNA sequence of the 4.4-Mb circular chromosome of M. tuberculosis H37Rv (5), only 40% of the ORFs have been assigned functions. Our studies may aid in assigning functions to yet-unexplored functional genes and more specifically to antigens produced by them. Also, DNA sequences, if worked out for these antigens, can provide valuable amplification-based targets for TB diagnosis.
The candidate test for diagnosis of TB needs a high degree of sensitivity, specificity, and reproducibility. In addition, the procedures involved should be simple, not capital intensive, and environmentally friendly and should have a low cost per test, particularly for developing countries. A number of attempts (6, 7, 9, 22, 23, 25, 29, 36, 37, 39) have been made to select antigens relevant for serodiagnosis, but none of them have been reported to be promising (10). Three antigens of M. tuberculosis, viz., Mtb11, Mtb8, and Mtb48, produced as a genetically fused polyprotein were tested together with the previously reported 38-kDa protein in an ELISA to detect antibodies in TB patients. These proteins detected antibodies in >80% of smear-positive individuals and >60% smear-negative individuals, with a specificity of approximately 98% (12), while our cocktail of peptide antigen showed a 94.73% sensitivity with smear-positive individuals and an 80% sensitivity with smear-negative individuals.
Further large multicenter studies are needed to address the real impact of these new reagents on the serodiagnosis of active TB, including the analysis of subjects coming from countries with different TB incidences. However, their ability to elicit a high degree of differential response to TB and BCG vaccination sera, as well as to discriminate subjects with TB from those with other lung diseases, give our antigens great potential to as diagnostic reagents.
Acknowledgments
We thank D. S. Chitnis, Chouthram Research Centre, Indore, India, for providing clinical isolate TBW33. We are indebted to Q. Khan and V. Ramnani, Gandhi Medical College, Bhopal, India, for providing serum samples from TB-infected individuals and for providing us with facilities for evaluation studies. We thank Massimo Amicosante (Department of Internal Medicine, University of Rome Tor-Vergata, Rome, Italy) for critical reading of the manuscript. We acknowledge the excellent technical assistance of Martin Zate and Vikas Joshi.
We thank the University Grants Commission, New Delhi, India, for providing instrumental facilities through COSIST and the DSA program to the Institute of Microbiology and Biotechnology, Barkatullah University, Bhopal, India.
REFERENCES
- 1.Amara, R. R., and V. Satchidanandam. 1996. Analysis of a genomic DNA expression library of Mycobacterium tuberculosis using tuberculosis patient sera: evidence for modulation of host immune response. Infect. Immun. 64:3765-3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amicosante, M., S. Barnini, V. Corsini, G. Paone, C. A. Read, Jr., P. L. Tartoni, M. Singh, C. Albera, A. Bisetti, S. Senesi, M. Campa, and C. Saltini. 1995. Evaluation of novel tuberculosis complex-specific 34 kDa protein in the serological diagnosis of tuberculosis. Eur. Respir. J. 8:2008-2014. [DOI] [PubMed] [Google Scholar]
- 3.Bothamley, G. H. 1995. Serological diagnosis of tuberculosis. Eur. Respir. J. 8(Suppl. 20):676-688. [PubMed] [Google Scholar]
- 4.Champion, C. I., D. R. Blanco, J. T. Skare, D. A. Haake, M. Giladi, D. Foley, J. N. Miller, and M. A. Loveft. 1994. A 9.0-kilobase-pair circular plasmid of Borrelia burgdorferi encodes an exported protein: evidence for expression only during infection. Infect. Immun. 62:2653-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole, S. T., R. Brosch, J. Parkhill, T. Gamier, C. Churcher, D. Harris, S. V. Gordon, K. Eigimeier, S. Gas, C. E. I. Barry, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osbome, M. A. Quail, M. A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 6.Daffe, M., F. Papa, A. Laszlo, and H. L. David. 1989. Glycolipids of recent clinical isolates of Mycobacterium tuberculosis: chemical characterization and immunoreactivity. J. Gen. Microbiol. 135:2759-2766. [DOI] [PubMed] [Google Scholar]
- 7.Daleine, G., and P. H. Lagrange. 1995. Preliminary evaluation of a Mycobacterium tuberculosis lipo-oligosaccharide (LOS) antigen in the serological diagnosis of tuberculosis in HIV seropositive and seronegative patients. Tubercle Lung Dis. 76:234-239. [DOI] [PubMed] [Google Scholar]
- 8.Daniel, T. M., and S. M. Bebanne. 1987. The serodiagnosis of tuberculosis and other mycobacterial diseases by enzyme-linked immunosorbent assay. Am. Rev. Respir. Dis. 135:1137-1151. [DOI] [PubMed] [Google Scholar]
- 9.Engers, H., V. Houba, J. Bennedsen, T. Buchanan, S. Chaparas, G. Kadival, O. Closs, J. Daniel, H. van Embden, T. Godal, S. Mustafa, J. Ivanyi, D. Young, S. Kaufmann, A. Khomenko, A. Kolk, M. Kubin, J. Louis, P. Minden, T. Shinnick, L. Trnka, and R. Young. 1986. Results of a World Health Organization workshop to characterize antigens recognized by mycobacterium-specific monoclonal antibodies. Infect. Immun. 51:718-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garg, S. K., R. P. Tiwari, D. Tiwari, V. Ramnani, R. Singh, D. Malhotra, G. B. K. S. Prasad, R. Chandra, M. Fraziano, V. Colizzi, and P. S. Bisen. 2003. Diagnosis of tuberculosis: available technologies, limitations and possibilities. J. Clin. Lab. Anal. 17:155-163. [DOI] [PMC free article] [PubMed]
- 11.Harboe, M., A. O. Whelan, G. Alvund, M. C. Nair, J. M. Pollock, R. G. Hewinson, and H. G. Wiker. 2002. Generation of antibodies to the signal peptide of the MPT83 lipoprotein of Mycobacterium tuberculosis. Scand. J. Immunol. 55:82-87. [DOI] [PubMed] [Google Scholar]
- 12.Houghton, R. L., M. J. Lodes, D. C. Dillon, L. D. Reynolds, C. H. Day, P. D. McNeill, R. C. Hendrickson, Y. A. Skeiky, D. P. Sampaio, R. Badaro, K. P. Lyashchenko, and S. G. Reed. 2002. Use of multiepitope polyproteins in serodiagnosis of active tuberculosis. Clin. Diagn. Lab. Immunol. 9:883-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Husson, R. N., and R. A. Young. 1987. Genes for the major protein antigens of Mycobacterium tuberculosis: the etiologic agents of tuberculosis and leprosy share an immunodominant antigen. Proc. Natl. Acad. Sci. USA 84:1679-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kent, P. T., and G. P. Kubica. 1985. Public health mycobacteriology: a guide for the level III laboratory. Centers for Disease Control, Atlanta, Ga.
- 15.Kessel, M. D., P. Gilot, M. Missone, M. Coene, and C. Cocito. 1993. Cloning and expression of a portion of the 34-kilodalton protein gene of Mycobacterium paratuberculosis: its application to serological analysis of Johne's disease. J. Clin. Microbiol. 31:947-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubica, G. P. 1984. Clinical microbiology, p. 133-175. In G. P. Kubica and L. G. Wayne (ed.), The mycobacteria. A sourcebook, part A. Marcel Dekker Inc., New York, N.Y.
- 17.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 277:680-685. [DOI] [PubMed] [Google Scholar]
- 18.Lyashchenko, K., R. Colangeli, M. Houde, H. Ai Jahdali, D. Menzies, and M. L. Gennaro. 1998. Heterogenous antibody response in tuberculosis. Infect. Immun. 66:3936-3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahan, M. J., J. M. Slauch, and J. J. Mekalanos. 1993. Selection of bacterial virulence genes that are specifically induced in host tissues. Science 259:686-688. [DOI] [PubMed] [Google Scholar]
- 20.Manca, C., K. Lyashchenko, H. G. Wiker, D. Usai, R. Colangeliand, and M. L. Gennaro. 1997. Molecular cloning, purification, and serological characterization of MPT63, a novel antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 65:16-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manca, C., K. Lyashchenko, R. Colangeliand, and M. L. Gennaro. 1997. MTC-28, a novel 28-kilodalton proline-rich secreted antigen specific for the Mycobacterium tuberculosis complex. Infect. Immun. 65:4951-4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manuel, G., S. Johnson, and M. L. Gennaro. 2000. Identification of secreted proteins of Mycobacterium tuberculosis by a bioinformatic approach. Infect. Immun. 68:2323-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagai, S., H. G. Wiker, M. Harboe, and M. Kinomoto. 1991. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect. Immun. 59:372-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naganathan, N., B. Mahadev, V. K. Challu, R. Rajalakshami, B. Jones, and D. W. Smith. 1986. Virulence of tubercle bacilli isolated from patients with tuberculosis in Bangalore, India. Tubercle 67:262-267. [DOI] [PubMed] [Google Scholar]
- 25.Papa, F., P. Cruaud, M. Luquin, M. F. Thorel, K. S. Gohand, and H. L. David. 1993. Isolation and characterization of serologically reactive lipo-oligosaccharides from Mycobacterium tuberculosis. Res. Microbiol. 144:91-99. [DOI] [PubMed] [Google Scholar]
- 26.Sada, E., P. J. Brennan, T. Herrera, and M. Torres. 1990. Evaluation of lipoarabinomannan for the serological diagnosis of tuberculosis. J. Clin. Microbiol. 28:2587-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 28.Sathish, M., R. E. Esser, J. E. R. Thole, and J. E. Clark-Curtiss. 1990. Identification and characterization of antigenic determinants of Mycobacterium leprae that react with antibodies in sera of leprosy patients. Infect. Immun. 58:1327-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savage, C., P. Vincet, and H. Leclerc. 1993. Serodiagnosis of tuberculosis: evaluation of a sulpholipid antigen. Int. J. Med. Microbiol. Virol. Parasitol. Infect. Dis. 278:49-57. [DOI] [PubMed] [Google Scholar]
- 30.Ten Dam, H. G. 1993. BCG vaccination, p. 251-269. In L. B. Reichman and E. S. Hershfield (ed.), Tuberculosis. Marcel Dekker Inc., New York, N.Y.
- 31.Toman, K. 1981. Sensitivity, specificity and predictive value of diagnostic test. Bull. Int. Union Tuberc. 56:18-28. [PubMed] [Google Scholar]
- 32.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verbon, A., G. J. Weverling, S. Kuijper, P. Speelman, H. M. Jansen, and A. H. J. Kolk. 1993. Evaluation of different tests for the serodiagnosis of tuberculosis and the use of likelihood ratios in serology. Am. Rev. Respir. Dis. 148:378-384. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. 2001. W. H. O. annual reports. Stop TB. World Health Organization, Geneva, Switzerland.
- 35.Young, D. B., L. Kent, and R. A. Young. 1987. Screening of a recombinant mycobacterial DNA library with polyclonal antiserum and molecular weight analysis of expressed antigens. Infect. Immun. 55:1421-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young, D. B., and T. R. Garbe. 1991. Heat shock proteins and antigens of Mycobacterium tuberculosis. Infect. Immun. 59:3086-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young, D. B., S. H. E. Kaufmann, P. W. M. Hermans, and J. E. R. Thole. 1992. Mycobacterial protein antigens: a compilation. Mol. Microbiol. 6:133-145. [DOI] [PubMed] [Google Scholar]
- 38.Young, R. A., B. R. Bloom, C. M. Grosskinsky, J. Ivanyi, D. Thomas, and R. W. Davis. 1985. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc. Natl. Acad. Sci. USA 82:2583-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young, R. A., V. Mehra, D. Sweetser, T. Buchnan, J. C. Curtiss, R. W. Davis, and B. R. Bloom. 1985. Genes for the major protein antigens of the leprosy parasite. Nature 316:45. [DOI] [PubMed] [Google Scholar]