Abstract
Rotavirus is the most common cause of severe gastroenteritis in young children, but the pathogenesis and immunity of this disease are not completely understood. To examine the host response to acute infection, we collected paired serum specimens from 30 children with rotavirus diarrhea and measured the levels of nine cytokines (interleukin-1β [IL-1β], IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, gamma interferon [IFN-γ], and tumor necrosis factor alpha [TNF-α]) using a microsphere-based Luminex Flowmetrix system. Patients with acute rotavirus infection had elevated median levels of seven cytokines in serum, and of these, the levels of three (IL-6, IL-10, and IFN-γ) were significantly (P < 0.05) higher than those in serum from control children without diarrhea. Patients with fever had significantly (P < 0.05) higher levels of IL-6 in serum than control children, and those with fever and more episodes of diarrhea had significantly (P < 0.05) higher levels of TNF-α than those without fever and with fewer episodes of diarrhea. We further demonstrated a negative association (P < 0.05) between the levels of IL-2 and the number of stools on the day on which the first blood sample was collected. Finally, patients with vomiting had significantly (P < 0.05) lower levels of IFN-γ than those without vomiting. Our pilot study provides evidence that the types and magnitudes of cytokine responses to rotavirus infection in children influence or reflect the clinical outcome of disease. These findings suggest that certain cytokines may play an important role in the pathogenesis of and the protection against rotavirus disease in children and, consequently, may provide directions and insights that could prove critical to the prevention or treatment of this important disease.
Group A rotavirus is the single most important cause of severe dehydrating diarrhea in young children worldwide, and in developing countries, it causes over 500,000 deaths each year (20). Rotavirus is a nonenveloped, double-stranded RNA virus that infects mature epithelial cells of the small intestine. Children with rotavirus infections often develop symptoms of fever, vomiting, and diarrhea. Several mechanisms have been proposed to describe the pathogenesis of rotavirus diarrhea in animals (18), but it is unclear whether these apply to humans as well. Furthermore, the mechanisms involved in the induction of fever and vomiting are poorly understood. This incomplete understanding of the pathogenesis of disease in natural rotavirus infection has hampered our ability to design the most effective strategies to prevent or treat rotavirus diarrhea.
A host typically responds to a viral infection by developing both innate immunity and specific adaptive immunity (35). Innate immunity occurs in the early days of infection and plays an important role in either the pathogenesis of or immunity to the disease by an array of mechanisms, including activation of proinflammatory signaling pathways and the rapid production of cytokines (34, 35, 45). Cytokines, which also include chemokines, can exhibit both pro- and anti-inflammatory activities and mediate disease or protect the host against viral infection or disease (15, 16, 38-40, 46). In contrast, specific adaptive immunity appears later and protects the host from viral infection by antigen-specific T-cell-mediated killing of infected cells and antibody and effector cytokine responses (34, 35).
Rotavirus infection results in both cellular and humoral responses that protect against reinfection or severe diarrhea in young children or animals when they are reinfected (5, 6, 29). However, children with a typical rotavirus infection begin recovering from disease in the first week of illness, before rotavirus-specific T cells or antibodies are fully developed in the small intestine (18, 28). This observation suggests that innate immunity, such as that provided by cytokines, participates in aiding in early recovery from disease.
Only a few recent studies have demonstrated elevated levels of several cytokines—alpha interferon (IFN-α), IFN-γ, interleukin-10 (IL-10), and tumor necrosis factor alpha (TNF-α)—in the serum or plasma of children with rotavirus diarrhea (2, 7). Little is known, however, about the role of these cytokines in the pathogenesis of or immunity to rotavirus disease. In order to further our understanding of the cytokine profiles in children with rotavirus infections, we collected serum specimens from children with acute rotavirus diarrhea and from controls without gastroenteritis and measured the concentrations of nine cytokines. We further identified several cytokines that might be involved in innate immunity to rotavirus and the pathogenesis of rotavirus disease.
MATERIALS AND METHODS
Study population and specimen collection.
From February 1999 to June 2000, a prospective study was conducted to collect blood and fecal specimens from children <2 years of age seen at Children's Healthcare of Atlanta in Atlanta, Ga., and Hasbro Children's Hospital in Providence, R.I. Thirty patients who were admitted for treatment of acute gastroenteritis in the winter or spring but who were otherwise in good health were enrolled in the study if their stools tested positive for rotavirus by enzyme immunoassay (EIA). Nurses constantly observed the patients. Demographic and clinical data, including fever, vomiting, diarrhea, dehydration, and total severity scores during the entire period of illness and on the day on which the first blood sample was collected, were obtained from 25 patients enrolled in Hasbro Children's Hospital. Each patient's temperature was measured rectally approximately every 4 h. Total severity scores were based on symptom scores for diarrhea, vomiting, fever, dehydration, and hospital stay (13). Two blood samples were obtained from each patient; the first sample was collected within 72 h of enrollment and the second one was collected 21 to 28 days later. The sera were aliquoted and stored at −70°C. Fecal specimens were collected from all patients and stored at −20°C.
Blood and fecal specimens were also obtained from nine children who did not have gastroenteritis. Like most of the patients, the control children were enrolled in the spring (April to June). Single serum specimens were obtained from three children, and paired serum specimens were obtained 5 weeks apart from the other six children. Finally, individual serum specimens were obtained from healthy adults and screened for cytokines, and if they were negative, they were pooled for use in the assay as a diluent (blank) for the standard curves. Written consent was obtained from all parents or guardians, and the protocol was approved by the institutional review boards of the Centers for Disease Control and Prevention and each of the hospitals.
Characterization of rotavirus strains.
The G and P genotypes and serotypes of the rotavirus strains were determined by reverse transcriptase PCR and serotyping EIA, respectively, by procedures described previously (9, 14).
Detection of cytokines.
A standard capture sandwich assay was developed with the Luminex Flowmetrix system (Luminex, Austin, Tex.) to determine the levels of cytokines in sera. Each capture antibody was coupled to a different bead set (microsphere beads; Luminex) (Table 1). Standards (recombinant cytokines) diluted in pooled blank serum collected from healthy adults and test sera from patients or controls were examined by one of the two multiplex assays: the 4-plex assay (with four bead sets) and the 5-plex assay (with five beads sets). The individual bead sets in the 4-plex assay were coupled with monoclonal antibody to IL-1β, IL-8, IL-10, or IL-12; and the individual bead sets in the 5-plex assay were coupled with antibody to IL-2, IL-4, IL-6, IFN-γ, or TNF-α. The beads were incubated first with diluted standards or sera and then with detector antibodies for 30 min each at room temperature; washed twice in phosphate-buffered saline supplemented with 0.02% Tween 20, 0.1% bovine serum albumin, and 0.02% NaN3; and incubated for 15 min with fluorescent dye-conjugated streptavidin (Table 1). Cytokine levels were measured by using a flow cytometer and were analyzed with Flowmetrix software (Luminex).
TABLE 1.
Reagents used for measurement of cytokine levels with the Luminex Flowmetrix system
| Assay | Standard
|
Capture antibody
|
Detector antibody
|
|||||
|---|---|---|---|---|---|---|---|---|
| Cytokine | Source | Clone | Type | Bead set | Clone | Type | Concn (μg/ml) | |
| 4-Plex | IL-1β | R&D Systems | 2805.31 | Mouse IgG1a | 6947 | Polyclonal | Goat IgG | 20, 40 (cold)b |
| IL-8 | R&D Systems | 6217.111 | Mouse IgG1 | 6969 | Polyclonal | Goat IgG | 20, 80 (cold) | |
| IL-10 | R&D Systems | 23738.111 | Mouse IgG2b | 8047 | Polyclonal | Goat IgG | 120 | |
| IL-12 | R&D Systems | 24945.11 | Mouse IgG1 | 8053 | Polyclonal | Goat IgG | 80 | |
| 5-Plex | IL-2 | Pharmingen | 5344.111 | Mouse IgG1 | 6953 | B33-2 | Mouse IgG1 | 40 |
| IL-4 | Pharmingen | 8D4-8 | Mouse IgG1 | 6958 | MP4-25D2 | Rat IgG1 | 80 | |
| IL-6 | Pharmingen | MQZ-13A5 | Rat IgG1 | 6964 | MQZ-39C3 | Rat IgG2a | 120 | |
| IFN-γ | Pharmingen | NIB42 | Mouse IgG1 | 8058 | 4S.B3 | Mouse IgG1 | 120 | |
| TNF-α | Pharmingen | MAb1 | Mouse IgG1 | 8064 | MAb11 | Mouse IgG1 | 40 | |
IgG1, immunoglobulin G1.
The cold antibodies used came from the same source and clone but lacked the reporter dye.
Analysis of cytokine data.
Standard curves for each cytokine were generated on a log-log plot for each assay, and the cytokine concentrations in each serum sample were calculated from the corresponding curve-fitting equations (8). Each specimen was tested at least twice, and the average values were reported and analyzed. Nonparametric statistics were used for analysis because the data were not normally distributed (25). The cytokine levels in patients with rotavirus diarrhea and control children were compared by the Wilcoxon rank-sum test. These comparisons were adjusted for sex and age by using the blocked Wilcoxon rank-sum test. Cytokine levels in acute- and convalescent-phase sera were compared by the Wilcoxon signed-rank test.
The Wilcoxon rank-sum test was used to examine the association of cytokine levels with the presence of symptoms (fever, vomiting, and diarrhea) (25). Spearman's rank correlation coefficients were used to measure the strength of the associations between cytokine levels and symptom severity.
RESULTS
Characteristics of patients and characterization of rotavirus strains.
A total of 30 patients (10 girls, 20 boys) were enrolled in the study. These patients ranged in age from 1 to 20 months, with a mean age of 8.0 ± 1.2 months and a median age of 6.5 months. Most patients had typical clinical symptoms of rotavirus gastroenteritis: fever, vomiting, and diarrhea. Twenty-five of the 30 patients were enrolled in Hasbro Children's Hospital, and detailed symptom scores during the course of illness and on the day on which the first blood sample was collected were available for these patients; these included temperature, the number of vomiting episodes per day and the duration of vomiting, the severity of diarrhea (numbers of stools per day, duration of diarrhea, and percent dehydration), length of stay in hospital, and total symptom scores. Such detailed symptom scores were not available for the other five patients enrolled at Children's Healthcare of Atlanta. The nine control children (six girls, three boys) ranged in age from 1 to 7 months, with a mean age of 4.9 ± 0.5 months and a median age of 5 months. This mean age was not significantly (P > 0.05) different from that for the patients.
Of the 30 rotavirus strains examined, 23 (77%) were P[8],G1; 4 (13%) were P[4],G2; and 1 (3%) each was P[6],G9, P[8],G9, and P[6],G1. Of the strains from 25 patients whose symptom scores were available and whose sera were examined for cytokine responses, 20 (80%) were P [8],G1, 4 (17%) were P[4],G2, and 1 (4%) was P[8],G9.
Development of multiplex Luminex assays.
To simultaneously measure the levels of multiple cytokines in individual serum specimens, we developed 4-plex and 5-plex assays. The standard curves for the cytokines in multiplex assays were comparable to those for cytokines in single assays (data not shown). These results indicate a lack of steric hindrance and cross-reactivity between cytokines and antibodies in our multiplex assays.
Elevation of cytokine levels in sera of children with rotavirus diarrhea.
Cytokine levels were measured in 30 paired serum specimens from patients with rotavirus diarrhea and 15 serum specimens from control children, with sera from healthy adults used as the blank. Patients in the acute phase generally had higher median levels of seven of the nine cytokines (i.e., all cytokines except IL-4 and IL-12) than control children (Table 2); but the levels of IL-6, IL-10, and IFN-γ were significantly elevated (P < 0.05). In comparison, patients in the convalescent phase generally had higher median levels of six of the nine cytokines, with significant (P < 0.05) elevations in the levels of IL-6 and TNF-α. When the concentrations of cytokines in acute- and convalescent-phase sera were compared, lower median levels of five cytokines (IL-2, IL-6, IL-8, IFN-γ, and TNF-α) were detected in convalescent-phase sera, but only the level of IL-6 was significantly (P < 0.05) lower. The levels of the other four cytokines in convalescent-phase sera were little changed (IL-4 and IL-10) or elevated (IL-1β and IL-12). Because the levels of IL-1β and IL-8 in the sera of patients with rotavirus diarrhea were generally high and were outside the linear ranges of the standard curves, the concentrations of these two cytokines were measured in the presence of nonlabeled detector antibodies (Table 1).
TABLE 2.
Concentrations of nine cytokines in sera of rotavirus-infected patients and control childrena
| Cytokine | Concn (pg/ml)
|
|||||
|---|---|---|---|---|---|---|
| Acute-phase serum (n = 29)
|
Convalescent-phase serum (n = 29)
|
Control serum (n = 15)
|
||||
| Median | Range | Median | Range | Median | Range | |
| IL-1β | 0.6 | 0-35.8 | 1.5 | 0-50.8 | 0.3 | 0-6.5 |
| IL-2 | 6.0 | 0-93.2 | 3.2 | 0-211.8 | 4.5 | 0.5-39.8 |
| IL-4 | 10.7 | 4.1-158.6 | 12.7 | 1.0-77.5 | 15.1 | 6.4-59.9 |
| IL-6 | 174.9b | 27.2-1089.3 | 125.2bc | 8.7-460.6 | 109.5 | 29.3-252.1 |
| IL-8 | 1.4 | 0-40.1 | 0.7 | 0-47.5 | 1.1 | 0-18.1 |
| IL-10 | 9.8b | 0.8-198.2 | 15.4 | 0-216.7 | 2.3 | 0-18.0 |
| IL-12 | 0.7 | 0-264.8 | 0.8 | 0-139.2 | 0.7 | 0-5.4 |
| IFN-γ | 19.0b | 0-452.1 | 3.0 | 0-670.7 | 1.4 | 0-112.0 |
| TNF-α | 20.2 | 0-201.8 | 16.4b | 0-216.4 | 14.6 | 0-52.8 |
Thirty paired serum specimens from 30 patients and 15 serum specimens from 9 control children were examined with the Luminex system. Extremely high cytokine values were observed in two specimens, one in the 4-plex assay and another in the 5-plex assay, and are considered outliers (data not shown). The average values from two independent experiments for each serum specimen are presented.
Significantly higher compared with those for control children (P < 0.05).
Significantly lower compared with that for the acute-phase serum sample (P < 0.05).
Cytokines as mediators for symptom expression.
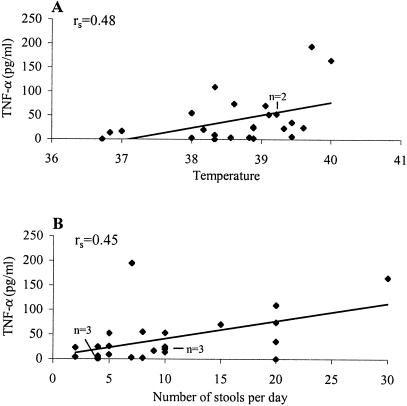
We then examined possible associations between the levels of the nine cytokines in acute-phase sera and scores for the 10 different symptoms for the 24 patients enrolled in Hasbro Children's Hospital by determining Spearman's rank correlation coefficients. Of the 90 potential correlations, we identified weak but significantly (P < 0.05) positive associations between elevated TNF-α levels (median, 23.7 pg/ml; mean, 41.0 ± 10.5 pg/ml) and several symptom scores. For example, three patients had no fever and their TNF-α levels in serum were lower than the median level (23.7 pg/ml). In contrast, 21 patients manifested a temperature above 37°C, and 14 (67%) of them had TNF-α levels that were similar to or higher than the median, with the concentrations ranging from 20.2 to 165.6 pg/ml. There was a weak but significant (P < 0.05) dose-response relationship between elevated TNF-α levels and the highest temperature during the entire period of illness (Fig. 1A). Elevated TNF-α levels were also associated, in a dose-dependent manner, with the numbers of diarrheic stools that patients shed per day (P < 0.05) (Fig. 1B).
FIG. 1.
Associations of serum TNF-α levels with severity of fever (A) and diarrhea (B). TNF-α levels and symptom scores for 24 patients were analyzed as described in the text. Spearman's rank correlation coefficient (rs) is presented (P < 0.05).
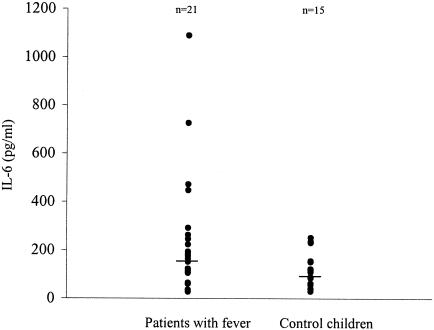
Patients with fever also had elevated levels of IL-6 in the acute-phase serum (median, 178.3 pg/ml; mean, 241.3 ± 48.9 pg/ml). This association, however, was not in a dose-response manner, as the severity of fever did not clearly correlate with more elevated levels. Nevertheless, 21 patients with fever (temperature, >37°C) had IL-6 levels (median, 174.9 pg/ml; mean, 251.2 ± 55.4 pg/ml) significantly (P < 0.05) higher than those in 15 serum specimens from control children (median, 109.5 pg/ml; mean, 121.7 ± 18.4 pg/ml) (Fig. 2).
FIG. 2.
Comparison of IL-6 levels in sera of patients with fever and control children. The concentrations of IL-6 in 21 serum specimens from patients with fever and 15 specimens from controls are shown. Patients had significantly higher levels of IL-6 than control children. The statistical significance of the differences was determined by the two-tailed t test (P < 0.05).
Cytokines as effectors against symptoms.
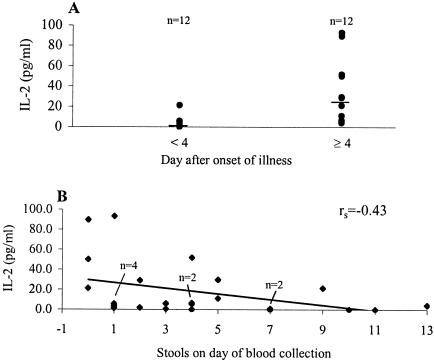
Of the nine cytokines, two (IL-2 and IFN-γ) were identified to be significantly associated with less severe symptom scores on the day on which the first blood sample was collected. IL-2 was detected in sera from 20 of the 24 patients, and the levels were significantly (P < 0.01) higher in sera collected 4 days after the onset of illness (median, 25.2 pg/ml; mean, 33.0 ± 9.2 pg/ml) than in sera collected in the first 3 days (median, 1.1 pg/ml; mean, 3.4 ± 1.7 pg/ml) (Fig. 3A). There was a weak but significant (P < 0.05) association between elevated IL-2 levels and fewer diarrheic episodes in patients with rotavirus infection (Fig. 3B).
FIG. 3.
Concentrations of IL-2 in sera of patients with diarrhea (A) and association of serum IL-2 levels with severity of diarrhea (B). Of the 24 serum specimens from patients, 12 were collected 4 days after the onset of illness and had significantly higher levels of IL-2 than those collected in the first 3 days of illness. The statistical significance of the difference was determined by the two-tailed t test (P < 0.01). Elevated levels of IL-2 were significantly associated with fewer stools on the day of blood collection. Spearman's rank correlation coefficient (rs) is presented (P < 0.05).
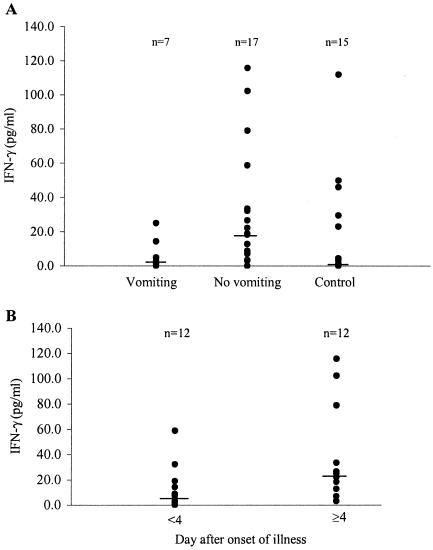
IFN-γ was detected in 22 (92%) of the 24 serum specimens from patients with rotavirus diarrhea, whereas it was detected in 8 (53%) of the 15 serum specimens from control children. Seven of the 24 patients experienced at least one vomiting episode on the day on which the first blood sample was collected and had significantly (P < 0.05) lower IFN-γ levels (median, 4.7 pg/ml; mean, 7.6 ± 3.4 pg/ml) than those of 17 patients without vomiting (median, 19.0 pg/ml; mean, 32.4 ± 8.6 pg/ml) (Fig. 4A). Control children had median and mean IFN-γ levels of 1.4 and 18.0 ± 8.1 pg/ml, respectively. There was no significant difference in the levels between control children and patients with or without vomiting. Of interest, 12 of the 24 serum samples from patients were collected 4 days after the onset of illness and had median and mean IFN-γ levels of 23.6 and 37.5 ± 11.3 pg/ml, respectively (Fig. 4B). Seven (58%) of these 12 patients had serum IFN-γ levels ≥20 pg/ml, and 2 (17%) experienced vomiting. In contrast, the other 12 serum samples collected in the first 3 days of illness had lower, although insignificant (P = 0.06), median and mean IFN-γ levels of 6.3 and 12.8 ± 5.0 pg/ml, respectively. Only 2 (17%) of these 12 patients had serum IFN-γ levels ≥20 pg/ml, and 5 (42%) experienced vomiting episodes.
FIG. 4.
Concentrations of IFN-γ in sera of patients with rotavirus infection. (A) Concentrations of IFN-γ in 7 serum specimens from patients with vomiting, 17 specimens from patients without vomiting, and 15 specimens from control children. Patients with vomiting had significantly lower levels of IFN-γ than those without vomiting. (B) Sera collected 4 days after the onset of illness had significantly higher levels of IFN-γ than those collected in the first 3 days of illness. The statistical significance of the differences was determined by the two-tailed t test (P < 0.05).
Age-dependent symptom expression.
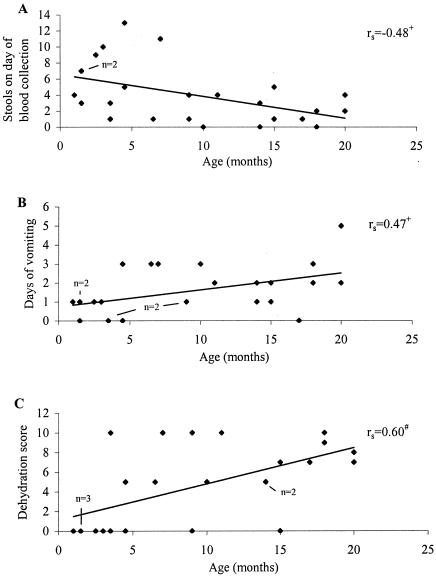
We conducted additional analyses to examine if cytokine responses and disease severity in children with rotavirus diarrhea were gender and age dependent. There were no significant differences in cytokine responses and disease severities between girls and boys. While cytokine responses were not age related, several symptom scores were. Older children with rotavirus infection tended to have fewer stools on the day of blood collection (P < 0.05) (Fig. 5A), but they were more likely than younger patients to vomit (P < 0.05) (Fig. 5B) and become dehydrated (P < 0.01) (Fig. 5C).
FIG. 5.
Age and disease severity by selected symptoms. Demographic and clinical data for 25 patients were analyzed as described in the text. Spearman's rank correlation coefficient (rs) is presented. +, P < 0.05; #, P < 0.01.
DISCUSSION
Cytokines comprise a diverse group of small proteins with both pro- and anti-inflammatory properties and are increasingly recognized to play critical roles in the pathogenesis and immunity of infectious diseases (4, 34). To examine cytokine profiles and their potential functions in children with natural rotavirus infections, we determined cytokine concentrations in serum using a newly developed bead-based immunoassay. This assay provides an easy, rapid, sensitive, and accurate means by which cytokine levels can be measured in the sera of children with rotavirus diarrhea. The ability to simultaneously measure the levels of multiple cytokines in a single reaction makes this assay even more appealing because a significantly lower volume of serum is required compared with the volume needed in traditional EIAs or bioassays, which measure the levels of only one cytokine at a time and also require 20 times more specimen (17, 44).
We demonstrated an overall increased cytokine response in children with acute rotavirus diarrhea compared with those in control children. The levels of three of the nine cytokines (IL-6, IL-10, and IFN-γ) in acute-phase sera were significantly elevated. These results indicate that both the Th1 and the Th2 types of cytokines are produced in young children with natural rotavirus infection, and Th1-like anti-inflammatory cytokines (IL-2 and IFN-γ) were predominately produced in the late phase of acute infection. Our results agree with those of a recent study performed in Bangladesh (2), in which the levels of three cytokines (IL-10, TNF-α, and IFN-γ) were found to be elevated in the sera of children with acute or persistent rotavirus diarrhea compared with the levels in uninfected, control children.
We present, for the first time, evidence for cytokine mediation of disease expression in children with rotavirus infection, as demonstrated by associations between elevated TNF-α and IL-6 levels and expression of fever in the acute phase of infection. Both TNF-α and IL-6 are multifunctional cytokines and are considered endogenous pyrogens. Once they are overproduced and present in the circulation, these cytokines can act as humoral signals and contribute to the acute febrile reaction by different mechanisms, including induction of the acute-phase protein response and inflammatory stimulation of the hypothalamus or afferent sensory nerves (11, 22, 36). Elevated levels of cytokines in serum or local secretions have also been documented to mediate disease expression or respiratory tract inflammation in children or adults infected with influenza A virus or respiratory syncytial virus (19, 27, 39, 40). In addition, we observed an association between the overproduction of TNF-α and more diarrheal episodes in patients with rotavirus infection, although the mechanism for this relationship is unknown. It could be due to the ability of TNF-α to induce increased levels of Cl− secretion in intestinal epithelial cells, as has been reported for patients with other infections or inflammatory processes (21, 31). We could not identify cytokines that might mediate the expression or severity of vomiting. A previous study, however, demonstrated that IFN-α levels were highly elevated and significantly correlated with the severity (the number of episodes) of vomiting in children with rotavirus diarrhea (7).
We demonstrated a delayed activation of IL-2 and IFN-γ in children with rotavirus infection, as evidenced by their predominant production 4 days after the onset of illness. Because IL-2 is secreted exclusively by antigen-activated T cells and IFN-γ is produced mainly by T cells, NK cells, and macrophages, these two cytokines constitute a second wave of the host response to infection in adaptive immunity (32, 41). The observation that IL-2 and IFN-γ were negatively associated with symptoms (diarrheal or vomiting episodes) on the day on which the first blood sample was collected indicates that these cytokines may be important components of host protective immunity and, consequently, mediate antiviral activities and help patients recover from severe rotavirus disease. Our results concur with those of previous studies that exogenously administered IFNs are protective against diarrhea in calves and pigs (23, 37), but they do not agree with results showing a lack of protection against disease in mice (1). IFN-γ was previously found to be secreted by rotavirus-stimulated peripheral blood mononuclear cells of healthy adults and to inhibit rotavirus entry into human intestinal epithelial cells (3, 47). Of note, IL-2 and IFN-γ have been demonstrated to confer resistance to other infectious diseases of humans and animals (12, 42).
We demonstrated no significant associations of elevated cytokine levels in the acute phase with total severity scores, which are a combined assessment of symptoms (fever, vomiting, diarrhea, and dehydration) and length of hospital stay. Furthermore, we found no significant associations between elevated cytokine levels in the convalescent phase and symptom scores. These data suggest that the associations between elevated levels of several cytokines in patients with acute diarrhea and individual symptom scores are specific.
Another important finding of this study is the significant elevation of IL-10 levels in the sera of children with rotavirus diarrhea. Like most other cytokines, IL-10 exhibits numerous effects on the modulation of immune responses and ion transport in the small intestine, and it presents both potential benefits (anti-inflammatory effect) and severe risks (association with fatality) to patients with infectious diseases (24, 26, 30). However, in our study, IL-10 was not associated with either protection against rotavirus disease or the expression or severity of fever, vomiting, or diarrhea. Whether IL-10 is associated with other systemic symptoms or even fatal outcomes, as reported in humans and animals with meningococcal or pneumococcal disease (24, 43), remains to be determined.
We demonstrated no distinct age-related differences in cytokine responses to rotavirus infection but found a positive association between age and disease severity, as evidenced by less severe vomiting and dehydration among infants in the first several months of life. Our finding that older children have fewer stools than younger infants suggests that age itself is a deterrent of diarrhea severity, an observation reported previously (10, 33). Further studies are needed to examine and compare the cytokine and other host responses between younger and older children.
Our present study has several limitations. First, the standard antigens used in our bead-based Luminex assay were recombinant cytokines expressed in Escherichia coli or insect cells, and antibodies were raised against these recombinant cytokines. Despite their specificity, affinity, and ability to recognize most isoforms of recombinant cytokines, it is not clear if these antibodies bind to all isoforms of the native cytokines. Consequently, the use of these recombinant cytokines, together with the inclusion of nonlabeled detector antibodies (Table 1), in the assay may result in underestimation of the levels of some cytokines. Second, the relatively small numbers of children and the large variations in cytokine concentrations may have affected the accuracy of our results. Future studies will need to examine larger numbers of age- and sex-matched control and patient populations to substantiate associations between cytokine levels and disease severity, examine possible differential cytokine profiles in children infected with various rotavirus serotypes, and subsequently identify potential associations between serotypes and disease severity. In addition, because the half-lives of most cytokines are believed to be short, a series of serum specimens collected at various time points would be needed to examine the kinetics of the cytokine profiles. However, we were able to collect only one serum specimen during each of the acute and convalescent phases of infection from young children with rotavirus diarrhea. Finally, the origins of the serum cytokines detected in our study are not clear. They could be secreted by NK cells, lymphocytes, monocytes, or macrophages in the circulation or in intestinal tissues. Since most cytokines exhibit their function locally in a paracrine or even autocrine fashion, further studies are needed to examine the cytokine profiles present in intestinal fluids or expressed in lymphoid or epithelial cells of the small intestine from children with rotavirus diarrhea.
In summary, we have examined the cytokine responses to rotavirus in children with diarrhea and identified several cytokines that are potential mediators for or effectors against the expression of clinical symptoms or severity of disease. These new findings should help us redefine our research direction to include the contributions of host factors to disease initiation and progression, a proposition that has not yet received much attention. We may also potentially use early cytokine profiles to predict disease outcome or vaccine efficacy in hosts infected with or vaccinated against rotavirus, as recently reported for other virus infections (48). Finally, these findings may allow us to design new therapeutic strategies and vaccines against rotavirus disease in children.
Acknowledgments
We thank Kathi Kellar and Ralph Tripp for help with the Luminex assays and Claudia Chesley for editorial assistance.
REFERENCES
- 1.Angel, J., M. A. Franco, H. B. Greenberg, and D. Bass. 1999. Lack of a role for type I and type II interferons in the resolution of rotavirus-induced diarrhea and infection in mice. J. Interferon Cytokine Res. 19:655-659. [DOI] [PubMed] [Google Scholar]
- 2.Azim, T., S. M. Ahmad, S. Khuda, M. S. Sarker, L. E. Unicomb, S. De, J. D. Hamadani, M. A. Salam, M. A. Wahed, and M. J. Albert. 1999. Immune response of children who develop persistent diarrhea following rotavirus infection. Clin. Diagn. Lab. Immunol. 6:690-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bass, D. M. 1997. Interferon gamma and interleukin 1, but not interferon alfa, inhibit rotavirus entry into human intestinal cell lines. Gastroenterology 113:81-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belardelli, F. 1995. Role of inteferons and other cytokines in the regulation of the immune response. APMIS 103:161-179. [DOI] [PubMed] [Google Scholar]
- 5.Bhan, M. K., J. F. Lew, S. Sazawal, B. K. Das, J. R. Gentsch, and R. I. Glass. 1993. Protection conferred by neonatal rotavirus infection against subsequent diarrhea. J. Infect. Dis. 168:282-287. [DOI] [PubMed] [Google Scholar]
- 6.Bishop, R. F., G. L. Barnes, E. Cipriani, and J. S. Lund. 1983. Clinical immunity after neonatal rotavirus infection: a prospective longitudinal study in young children. N. Engl. J. Med. 309:72-76. [DOI] [PubMed] [Google Scholar]
- 7.Boissieu, D. D., P. Lebon, J. Badoual, Y. Bompard, and C. Dupont. 1993. Rotavirus induces alpha-interferon release in children with gastroenteritis. J. Pediatr. Gastroenterol. Nutr. 16:29-32. [DOI] [PubMed] [Google Scholar]
- 8.Carson, R. T., and D. A. A. Vignali. 1999. Simultaneous quantitation of 15 cytokines using a multiplexed flow cytometric assay. J. Immunol. Methods 227:41-52. [DOI] [PubMed] [Google Scholar]
- 9.Das, B. K., J. R. Gentsch, H. G. Cicirello, P. A. Woods, A. Gupta, M. Ramachandran, R. Kumar, M. K. Bhan, and R. I. Glass. 1994. Characterization of rotavirus strains from newborns in New Delhi, India. J. Clin. Microbiol. 32:1820-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dennehy, P. H., G. C. Rodgers, R. L. Ward, A. J. Markwick, M. Mack, and E. T. Zito. 1996. Comparative evaluation of reactogenicity and immunogenicity of two dosages of oral tetravalent rhesus rotavirus vaccine. Pediatr. Infect. Dis. J. 15:1012-1018. [DOI] [PubMed] [Google Scholar]
- 11.Dinarello, C. A., J. G. Cannon, and S. M. Wolff. 1988. New concepts on the pathogenesis of fever. Rev. Infect. Dis. 10:168-189. [DOI] [PubMed] [Google Scholar]
- 12.Finke, D., U. G. Brinckmann, V. T. Meulen, and U. G. Liebert. 1995. Gamma interferon is a major mediator of antiviral defense in experimental measles virus-induced encephalitis. J. Virol. 69:5469-5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flores, J., I. Perez-Schael, M. Gonzalez, D. Garcia, M. Perez, N. Daoud, W. Cunto, R. M. Chanock, and A. Z. Kapikian. 1987. Protection against severe rotavirus diarrhea by rhesus rotavirus vaccine in Venezuelan infants. Lancet i:882-884. [DOI] [PubMed]
- 14.Gentsch, J. R., R. I. Glass, P. Woods, V. Gouvea, M. Gorziglia, J. Flores, B. K. Das, and M. K. Bhan. 1992. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 30:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guidotti, L. G., P. Borrow, A. Brown, H. McClary, R. Koch, and F. V. Chisari. 1999. Noncytopathic clearance of lymphocytic choriomeningitis virus from the hepatocyte. J. Exp. Med. 189:1555-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guidotti, L. G., T. Ishikawa, M. V. Hobbs, B. Matzke, R. Schreiber, and F. V. Chisari. 1996. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 4:25-36. [DOI] [PubMed] [Google Scholar]
- 17.Helle, M., L. Boeije, E. d. Groot, A. D. Vos, and L. Aarden. 1991. Sensitive ELISA for interleukin-6. Detection of IL-6 in biological fluids: synovial fluids and sera. J. Immunol. Methods 138:47-56. [DOI] [PubMed] [Google Scholar]
- 18.Jiang, B., J. R. Gentsch, and R. I. Glass. 2002. The role of serum antibodies in the protection against rotavirus disease: an overview. Clin. Infect. Dis. 34:1351-1361. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser, L., R. S. Frit, S. E. Straus, L. Gubareva, and F. G. Hayden. 2001. Symptom pathogenesis during acute influenza: interleukin-6 and other cytokine responses. J. Med. Virol. 64:262-268. [DOI] [PubMed] [Google Scholar]
- 20.Kapikian, A. Z., Y. Hoshino, and R. M. Chanock. 2002. Rotavirus, p. 1787-1833. In D. M. Knipe and P. M. Howley (ed.), Fields virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 21.Kendil, H. M., H. M. Berschneider, and R. A. Argenzio. 1994. Tumor necrosis factor alpha changes porcine intestinal ion transport through a paracrine mechanism involving prostaglandins. Gut 35:934-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le, J., and J. Vilcek. 1989. Interleukon-6: a multifunctional cytokine regulating immune reactions and the acute phase protein response. Lab. Investig. 61:588-602. [PubMed] [Google Scholar]
- 23.Lecce, J. G., J. M. Cummins, and A. B. Richards. 1990. Treatment of rotavirus infection in neonate and weanling pigs using natural human interferon alpha. Mol. Biother. 2:211-216. [PubMed] [Google Scholar]
- 24.Lehmann, A. K., A. Halstensen, S. Sornes, O. Rokke, and A. Waage. 1995. High levels of interleukin 10 in serum are associated with fatality in meningococcal disease. Infect. Immun. 63:2109-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehmann, E. L. 1975. Nonparametrics: statistical methods based on ranks. Holden-Day, Inc., San Francisco, Calif.
- 26.Madsen, K. L., M. M. Tavernini, T. R. Mosmann, and R. N. Fedorak. 1996. Interleukin 10 modulates ion transport in rat small intestine. Gastroenterology 111:936-944. [DOI] [PubMed] [Google Scholar]
- 27.Noah, T. L., F. W. Henderson, I. A. Wortman, R. B. Devlin, J. Handy, H. S. Koren, and S. Becker. 1995. Nasal cytokine production in viral acute upper respiratory infection of childhood. J. Infect. Dis. 171:582-592. [DOI] [PubMed] [Google Scholar]
- 28.Offit, P. A. 1996. Host factors associated with protection against rotavirus disease: the skies are clearing. J. Infect. Dis. 174(Suppl. 1):S59-S64. [DOI] [PubMed] [Google Scholar]
- 29.Offit, P. A., and K. I. Dudzik. 1988. Rotavirus-specific cytotoxic T lymphocytes cross-react with target cells infected with different rotavirus serotypes. J. Virol. 62:127-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Opal, S. M., J. C. Wherry, and P. Grint. 1998. Interleukin-10: potential benefits and possible risks in clinical infectious diseases. Clin. Infect. Dis. 27:1497-1507. [DOI] [PubMed] [Google Scholar]
- 31.Oprins, J. C. J., H. P. Meijer, and J. A. Groot. 2000. TNF-alpha potentiates the ion secretion induced by muscarinic receptor activation in HT29cl. 19A cells. Am. J. Physiol. Cell Physiol. 278:C463-C472. [DOI] [PubMed] [Google Scholar]
- 32.Page, C. L., P. Genin, M. G. Baines, and J. Hiscott. 2000. Interferon activation and innate immunity. Rev. Immunogen 2:374-386. [PubMed] [Google Scholar]
- 33.Perez-Schael, I., M. J. Guntinas, M. Perez, V. Pagone, A. M. Rojas, R. Gonzalez, W. Cunto, Y. Hoshino, and A. Z. Kapikian. 1997. Efficacy of the rhesus rotavirus-based quadrivalent vaccine in infants and young children in Venezuela. N. Engl. J. Med. 337:1181-1209. [DOI] [PubMed] [Google Scholar]
- 34.Ramshaw, I. A., A. J. Ramsay, G. Karupiah, M. S. Rolph, S. Mahalingam, and J. C. Ruby. 1997. Cytokines and immunity to viral infections. Immunol. Rev. 159:119-135. [DOI] [PubMed] [Google Scholar]
- 35.Roitt, I. M. 1997. Essential immunology. Blackwell Science Ltd., Oxford, United Kingdom.
- 36.Roth, J., and G. E. P. de Souza. 2000. Fever induction pathways: evidence from responses to systemic or local cytokine formation. Braz. J. Med. Bio. Res. 34:301-314. [DOI] [PubMed] [Google Scholar]
- 37.Schwers, A., B. C. Vanden, M. Maenhoudt, J. M. Beduin, J. Werenne, and P. P. Pastoret. 1985. Experimental rotavirus diarrhea in colostrum-deprived newborn calves: assay of treatment by administration of bacterially produced human interferon (Hu-IFN alpha 2). Ann. Rech. Vet. 16:213-218. [PubMed] [Google Scholar]
- 38.Seo, S. H., E. Hoffmann, and R. G. Webster. 2002. Lethal H5N1 influenza viruses escape host anti-viral cytokine responses. Nat. Med. 8:950-954. [DOI] [PubMed] [Google Scholar]
- 39.Sheeran, P., H. Jafri, C. Carubelli, J. Saavedra, C. Johnson, K. Krisher, P. Sanchez, and O. Ramilo. 1999. Elevated cytokine concentrations in the nasopharyngeal and tracheal secretions of children with respiratory syncytial virus disease. Pediatr. Infect. Dis. J. 18:115-122. [DOI] [PubMed] [Google Scholar]
- 40.Skoner, D. P., D. A. Gentile, A. Patel, and W. J. Doyle. 1999. Evidence for cytokine mediation of disease expression in adults experimentally infected with influenza A virus. J. Infect. Dis. 180:10-14. [DOI] [PubMed] [Google Scholar]
- 41.Smith, K. A. 1988. Interleukin 2: inception, impact, and implication. Science 240:1169-1176. [DOI] [PubMed] [Google Scholar]
- 42.Spruance, S. L., T. G. Evans, M. B. McKeough, L. Thai, B. A. Araneo, R. A. Daynes, E. M. Mishkin, and A. S. Abramovitz. 1995. Th1/Th2-like immunity and resistance to herpes simplex labialis. Antivir. Res. 28:39-55. [DOI] [PubMed] [Google Scholar]
- 43.Van der Poll, T., A. Marchant, C. V. Keogh, M. Goldman, and S. F. Lowry. 1996. Interleukin-10 impairs host defense in murine pneumococcal pneumonia. J. Infect. Dis. 174:994-1000. [DOI] [PubMed] [Google Scholar]
- 44.Vignali, D. A. A. 2000. Multiplexed particle-based flow cytometric assays. J. Immunol. Methods 243:243-255. [DOI] [PubMed] [Google Scholar]
- 45.Whiteside, T. L., and R. B. Herberman. 1989. The role of natural killer cells in human disease. Clin. Immunol. Immunopathol. 53:1-23. [DOI] [PubMed] [Google Scholar]
- 46.Yamamura, M., K. Uyemura, R. J. Deans, K. Weinberg, T. H. Rea, B. R. Bloom, and R. L. Modlin. 1991. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science 254:277-279. [DOI] [PubMed] [Google Scholar]
- 47.Yasukawa, M., O. Nakagomi, and Y. Kobayashi. 1990. Rotavirus induces proliferative response and augments non-specific cytotoxic activity of lymphocytes in humans. Clin. Exp. Immunol. 80:49-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zou, W., A. A. Lackner, M. Simon, I. Durand-Gasselin, P. Galanaud, R. C. Desrosiers, and D. Emilie. 1997. Early cytokine and chemokine gene expression in lymph nodes of macaques infected with simian immunodeficiency virus is predictive of disease outcome and vaccine efficacy. J. Virol. 71:1227-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]