Abstract
To fulfill the need for a simple and rapid diagnostic test for human brucellosis, we used the immunochromatographic lateral flow assay format to develop two assays, one for the detection of Brucella-specific immunoglobulin M (IgM) antibodies and one for the detection of Brucella-specific IgG antibodies. The diagnostic values of these tests were examined. The tests are shown to detect acute, persistent, and relapsing disease and can be used to monitor treatment. The sensitivity of Brucella IgM and IgG flow assays calculated for the combined assay results is 96%, and specificity amounts to 99%. The flow assay requires neither specialized training nor equipment, the assay is very easy to perform and to read, and the components are stable without a requirement for refrigeration and well standardized. Together these characteristics indicate that the Brucella IgM and IgG flow assays are ideal for use in clinical settings in rural and suburban areas in which brucellosis is endemic.
Brucellosis is a multisystem disease showing great variability, and the clinical picture can be misleading (12, 27). A proper and prompt diagnosis is important, as the treatment of brucellosis is complicated, requiring specific antibiotics and, particularly in persistent disease, prolonged medication (24).
Culture provides definite proof of brucellosis but may not provide a positive result for all patients (5, 9, 17). Brucella bacteria are relatively slow growing, and the culture result may not become available for several days or weeks (25). In particular for patients with chronic disease, the sensitivity of culture can be low. Therefore, serological testing often is used for the confirmation of brucellosis (26). Almost all serological tests have been applied in the diagnosis of human brucellosis. The most commonly used test is the serum agglutination test (SAT) or Wright's test (23). The anti-human globulin test (Coombs) (19), the complement fixation test (14), and the enzyme-linked immunosorbent assay (ELISA) (4, 15, 18, 20) also are used. A laboratory that is capable of performing these slow and relatively complicated assays may not be available in many places. Under such conditions, the Rose Bengal test (RB) may be used not only as a simple screening test but also as a confirmatory test (10, 11).
In human brucellosis, specific immunoglobulin M (IgM) antibodies usually develop early in the infection and remain present for several weeks to months (4, 21). Specific IgG antibodies tend to develop somewhat later but may remain present, albeit at low levels, for months to years also after the patient has recovered. IgG but not IgM antibodies usually are present in recurrent infections. Specific agglutinating antibodies are detected by RB and SAT. Coombs, SAT in the presence of reducing agent, and more recently ELISA have been used for the detection of specific IgG antibodies. Coombs and IgG ELISA are particular useful when diagnosing patients with a chronic infection and to monitor relapse. Detection of specific IgM antibodies in ELISA is useful in diagnosing patients with acute illness, given the fact that the IgM antibody response tends to develop somewhat earlier than the IgG response. IgM detection also is used to discriminate between acute and chronic diseases, and for this SAT has been used as well (2, 16, 22). A significant reduction in titer in this assay when performed in the presence of reducing agent indicates the presence of specific IgM antibodies.
To address the need for a simple and rapid diagnostic test, we have applied the immunochromatographic lateral flow assay format to develop two assays, one for the detection of Brucella-specific IgM and one for Brucella-specific IgG antibodies.
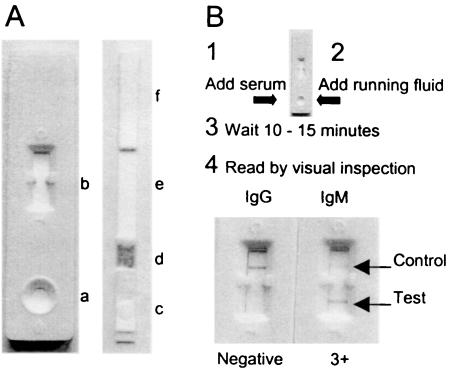
The lateral flow assay is a simplified version of ELISA (Fig. 1). The assay consists of a nitrocellulose detection strip flanked at one end by a reagent pad and at the other end by an absorption pad. A sample application pad flanks the reagent pad in turn. The composite strip is contained in a plastic assay device. The detection strip contains Brucella lipopolysaccharide (LPS) as a Brucella-specific capture probe as well as a reagent control applied in distinct lines. The reagent pad contains dried and stabilized detection reagent consisting of a colloidal gold immune conjugate. Colloidal gold-conjugated anti-human IgM was used as detection reagent in the IgM flow assay, and colloidal gold-conjugated anti-human IgG was used as detection reagent in the IgG flow assay. LPS was prepared from a solid 3-day-old culture of Brucella abortus strain 1119-3 grown on Brucella agar in 250-ml culture flasks. Cells were harvested in 50 ml of 0.9% NaCl per flask and inactivated by the addition of 250 μl of phenol and left for 2 days at 37°C and 4 days at 4°C (1). The inactivated cell suspension was centrifuged for 30 min at 4,750 × g, and the pellet was suspended in 25 ml of H2O and autoclaved for 30 min at 121°C. The mixture was cleared by centrifugation for 30 min at 4,750 × g, and LPS was precipitated by the addition of 75 ml of ice-cold 96% ethanol to the supernatant, incubated for 24 h at 4°C, and centrifuged at 2,325 × g for 15 min. Finally, the precipitate was dissolved in 5 ml of H2O. Before use, the concentration was adjusted by freeze-drying. Four-times-concentrated LPS was used as a capture probe in the IgM flow assay, and two-times-concentrated LPS was used as a capture probe in the IgG flow assay. The LPS capture probe was applied to the detection strip in a 2-mm-wide line by using a BioDot Quanti-2000 Biojet apparatus. Human IgM was applied in a second line to function as a reagent control in the IgM flow assay, and human IgG was applied in a second line to function as a reagent control in the IgG flow assay. The colloidal gold immunoconjugate detection reagents were prepared by Organon Teknika (Dublin, Ireland) by conjugation of affinity-purified polyclonal human IgM and IgG antibodies to 40-nm-diameter colloidal gold particles. The detection reagents were applied to the conjugate pad by using a BioDot Airjet Quanti 2000 apparatus. Strip tests were assembled by mounting the sample application pad, reagent pad, detection strip, and absorption pad onto a rigid support and placing the strips in a plastic assay device with a round sample well positioned above the sample pad and a square detection and control window above the detection strip. Finally, tests were sealed in a moisture-resistant protective foil containing a bag with desiccant. The antigen preparation method, the amount and concentration of LPS capture probe, and the amount of detection reagent were optimized in a step-by-step procedure using a panel of well-defined positive and negative control sera. The running fluid consisted of phosphate-buffered saline, pH 7.6, containing 1.67% bovine serum albumin and 3% Tween 20. Organon Teknika Ltd. manufactured the flow assays used for evaluation.
FIG. 1.
The Brucella IgM and IgG flow assays: physical presentation, components and method. The Brucella IgM and IgG flow assays (A) consist of a plastic assay device (left) containing a composite assay strip (right). The plastic assay device has a round sample application pad (a) and a square test and control window (b). The assay strip consists of a sample application pad (c), a conjugate pad (d) containing dried detection reagent, a detection strip (e) with distinct test and control lines, and an absorption pad (f). The assay procedure and test result are presented in panel B. The test result of a serum sample (SAT titer, 1:1,280; SAT-DTT, <1:40; Coombs, 1:1,280) giving a negative result in the IgG flow assay and a strong positive result in the IgM flow assay is presented.
The flow assay is simply performed by the addition of 5 μl of serum directly onto the sample application pad in the sample well of the plastic assay device. Following the addition of some test liquid, the result is read 10 to 15 min later by visual inspection for staining of the antigen and control lines in the test window of the device. The control line should stain in all cases. The assay is scored negative when no staining of the antigen line is observed and positive when a distinct staining of the antigen line is observed. The antigen line may stain at different intensities, and a positive result was subjectively rated 1+ when staining was weak, 2+ when staining was moderate, 3+ when staining was strong, and 4+ when staining was very strong. Undetermined staining represented by very weak (+/−) staining was considered negative. The results of exposed tests are stable after drying, but drying results in a slight loss of staining intensity. The assay is based on the binding of specific antibodies present in the clinical specimen to LPS antigen and staining of the bound antibodies by a colloidal gold-labeled antibody conjugate. By using two different conjugates, one for the detection of IgM antibodies and one for IgG antibodies, two complementary tests for the serodiagnosis of acute and persistent human brucellosis were developed.
The results of the Brucella IgM and IgG flow assays were compared with results by SAT (23), SAT performed in the presence of dithiothreitol (SAT-DTT) (3), and Coombs (7) for the initial serum samples collected from 205 patients who had been referred for laboratory testing to different laboratories in Spain because of clinical suspicion of brucellosis (Table 1). Patients had been referred to the Infectious Diseases Unit of the General Hospital of Albacete in Albacete (n = 54 sera), the Laboratory for Microbiology of the Hospital Virgen de la Victoria in Malaga (n = 81), and the Department of Microbiology of the University of Navarra in Pamplona (n = 70). The IgM flow assay tested positive in 51% of the sera, and the IgG flow assay tested positive in 67% of the sera. A total of 173 (84%) sera tested positive in at least one of the two flow assays. This number was significantly higher (P = 0.004) than the number of sera that tested positive by SAT (79%) at a cutoff value of 1:160 but was lower than the number testing positive by Coombs (97%). SAT-DTT indicated the presence of specific IgM antibodies in 38% of the sera.
TABLE 1.
Results of Brucella IgM and IgG flow assays for different groups of sera and comparison with SAT, DTT-SAT, and Coombs
| Serum group (no. of samples) | No. (%) of sera testing positive by indicated assay
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| SAT, ≥1:320 | SAT, ≥1:160 | SAT-DTT, ≥1:160 | SAT-DTT, ≥1:80 | SAT-DTT, ≥4-fold reduction in titer | Coombs, ≥1:320 | IgM flow, ≥1+ | IgG flow, ≥1+ | IgM + IgG flow, ≥1+f | |
| Initial samples from patients with clinical suspicion of brucellosis | |||||||||
| Albacete (54) | 31 (57) | 39 (72) | 24 (44) | 31 (57) | 25 (46) | 53 (98) | 28 (52) | 33 (61) | 41 (76) |
| Malaga (81) | 43a (57) | 57a (76) | 45b (62) | 53b (73) | 20 (27)b | 68 (94)d | 29 (36) | 54 (67) | 67 (87) |
| Pamplona (70) | 52c (78) | 58c (87) | 53c (79) | 49c (79) | 30 (45)c | 66 (99)c | 47 (67) | 50 (71) | 65 (93) |
| Total (205) | 127 (65) | 154 (79) | 122 (63) | 133 (69) | 75 (38) | 187 (97) | 104 (51) | 137 (67) | 173 (84) |
| Rev-1 contacts (10) | 0 (0) | 0 (0) | NTe | NT | 0 (0) | 6 (60) | 0 (0) | 4 (40) | 4 (40) |
| Patients with fever from an area of nonendemicity for brucellosis (79) | NT | NT | NT | NT | NT | NT | 1 (1) | 1 (1) | 1 (1) |
| Cases with illnesses other than brucellosis (197) | NT | NT | NT | NT | NT | NT | 2 (1) | 1 (1) | 2 (1) |
Six sera were not tested.
Eight sera were not tested.
Three sera were not tested.
Nine sera were not tested.
NT, not tested.
Positive (≥1+) test result in at least one of the two flow assays.
The IgM and IgG flow assays tested positive for just 1 of 79 patients from a control group of hospitalized patients with fever from an area where the disease is not endemic. A positive result also was obtained for 1 of 31 rheuma factor-positive sera, and for the serum of one of 34 patients with malaria. The rheuma factor-positive serum that tested positive was from a patient from an area where brucellosis is endemic. The flow assays scored negative for samples from patients with various diseases other than brucellosis, including patients with tuberculosis (n = 24), human immunodeficiency virus-positive patients (n = 19), and patients with typhoid fever (n = 17), leptospirosis (n = 16), hepatitis B (n = 16), hepatitis C (n = 10), and arthritis (n = 30). Reactivity of the IgG flow assay was noted to be present in the sera from four veterinarians who had been involved in the vaccination of animals with the Rev-1 vaccine. Six other sera from Rev-1 contacts tested negative. The IgM flow assay was negative for all 10 sera. The Coombs test gave positive resutls for the sera from six Rev-1 contacts, two of which also tested positive in the IgG flow assay. The titers observed in SAT for the control sera were all below the cutoff used for diagnosis.
Sensitivity and specificity are two important characteristics of a diagnostic test. The clinical symptoms of brucellosis may closely resemble those of other illnesses, and in areas of endemicity SAT and Coombs may give positive results in nonbrucellosis patients due to residual antibodies from previous exposure. The sensitivity of the flow assays thus cannot be calculated based on the results obtained for the samples from the patients with clinical suspicion of brucellosis and confirmed by serological testing in SAT and Coombs. The sensitivity of the IgM and IgG flow assays therefore was calculated based on the results for 89 patients who were confirmed by culture (n = 73 cases) and or radial immunodiffusion test (RID) (n = 49) (Table 2). Culture provides definite proof of brucellosis, and RID using native hapten, a homopolymer of N-formylperoxamine presenting together with LPS on the surface of the Brucella organisms, is highly specific (13). The sensitivities of the IgM flow assay and the IgG flow assay were calculated to be 67 and 71%, respectively. The combined results gave a sensitivity of 96%, which is significantly higher (P = 0.002) than the sensitivity of 78% calculated for SAT for the same group of sera at the diagnostic threshold titer of 1:320 (21). The sensitivity of SAT at a cutoff value of ≥1:160, however, was similar to that of the flow assays (P > 0.05). The sensitivity of Coombs at a cutoff value of ≥1:320 was 100% for this group of confirmed patients. The specificity of the flow assay was calculated to be 99% (95% confidence interval [CI]; range, 97 to 100), based on the results of the control groups of hospitalized patients with fever and patients with illnesses other than brucellosis. In this group of 276 sera, all but three tested negative. The weak (1+) reactivity of the IgG flow assay with some of the sera from Rev-I contacts, however, indicates that the specificity could be somewhat lower in specific risk groups.
TABLE 2.
Sensitivity of Brucella IgM and IgG flow assays for initial serum samples from patients with confirmed brucellosis and comparison with other serological tests
| Assay | No. of positive samples (n = 89) | % Sensitivity (95% CI) |
|---|---|---|
| SAT, ≥1:320 | 69 | 78 (67-85) |
| SAT, ≥1:160 | 77 | 88 (78-93) |
| SAT-DTT, ≥1:160 | 55 | 62 (51-72) |
| SAT-DTT, ≥1:80 | 64 | 70 (61-81) |
| Coombs, ≥1:320 | 89 | 100 (95-100) |
| IgM flow | 60 | 67 (57-77) |
| IgG flow | 70 | 71 (61-79) |
| IgM + IgG flow | 85 | 96 (88-99) |
Results of the flow assay are easy to read (Table 3). A moderate (2+) to very strong (4+) staining in either or both of the flow assays was observed for 88% (75 of 85) of the initial sera from the confirmed brucellosis patients with a positive result. For the other 10 positive patients a 1+ staining was recorded as the strongest test result. In the control groups a clear negative result was obtained for 270 (97.8%) sera. The sera of three patients tested positive, and the sera of three other patients gave an undetermined (+/−) result in the IgM flow assay. The correlation of the SAT titer with the reactivity in the flow assays for the sera from the confirmed patients shows that the results of the flow assays closely parallel those of the agglutination test (Table 4). The five sera that had a SAT titer of ≤1:40 and a moderate to very strong positive result in the IgG flow assay all had high titers, ranging from 1:2,560 to 1:81,920 with Coombs.
TABLE 3.
Staining intensity of Brucella IgM and IgG lateral flow assays for serum samples collected at the time of first diagnosis
| Assay and patient group | No. of serum samples with the indicated staining intensity
|
|||||
|---|---|---|---|---|---|---|
| Negative | +/− | 1+ | 2+ | 3+ | 4+ | |
| Brucella IgM flow assay | ||||||
| Confirmed case patients | 29 | 14 | 17 | 20 | 9 | |
| Unconfirmed patients with clinical suspicion of brucellosis | 55 | 18 | 17 | 12 | 14 | 1 |
| Controlsa | 271 | 3 | 1 | 1 | ||
| Brucella IgG flow assay | ||||||
| Confirmed case patients | 16 | 3 | 24 | 27 | 13 | 6 |
| Unconfirmed patients with clinical suspicion of brucellosis | 34 | 15 | 37 | 19 | 5 | 6 |
| Controls | 273 | 1 | 2 | |||
Patients with fever and patients with illnesses other than brucellosis.
TABLE 4.
Correlation of staining intensity of Brucella IgM and IgG flow assays with SAT titer for patients with confirmed brucellosis
| Staining intensity in flow assaya | No. of sera with indicated SAT titer
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤1:20 | 1:40 | 1:80 | 1:160 | 1:320 | 1:640 | 1:1,280 | 1:2,560 | 1:5,120 | 1:10,240 | |
| 4+ | 1 | 1 | 1 | 3 | 3 | 4 | 2 | |||
| 3+ | 1 | 3 | 2 | 3 | 4 | 9 | 6 | 2 | ||
| 2+ | 1 | 2 | 8 | 10 | 9 | 2 | 3 | 1 | ||
| 1+ | 2 | 1 | 2 | 2 | 2 | |||||
| Negative | 2 | 1 | 1 | |||||||
The staining intensity for either the IgM or IgG flow assay is given, whichever was highest.
Of the 89 patients with confirmed brucellosis, 15 tested positive in the IgM flow assay and were negative in the IgG flow assay, 44 tested positive in both flow assays, 26 tested positive in the IgG flow assay only, and 4 tested negative. A ≥4-fold reduction in SAT titer in the presence of DTT was observed in the serum samples from 12 of the confirmed patients with a positive result in the IgM flow assay and a negative result in the IgG flow assay. The Coombs titers (median titer, 1:1,280) were relatively low for these patients. Patients with a positive result in both flow assays had high Coombs titers (median titer, 1:10,240). Agglutination in SAT was DTT insensitive for 22 (50%) of these patients. The median Coombs titer for the sera from these 22 patients was 1:20,480, compared with 1:5,120 for the patients with a DTT-sensitive agglutination in SAT, suggesting that the high levels of specific IgG antibody in these patients masked the detection of specific IgM antibodies in SAT-DTT. The detection of specific IgM antibodies in the IgM flow assay apparently was not affected by the presence of these high specific IgG antibody levels. Most of the 26 patients with a positive result in the IgG flow and a negative result in the IgM flow assay had relatively high Coombs titers (median titer, 1:10,240), and only 7 showed a reduction in SAT titer in the presence of DTT. These results suggest that the flow assays provide a more accurate tool for the discrimination between specific IgM and IgG antibodies than the agglutination test. The initial sample from four patients with confirmed brucellosis scored negative in both flow assays. SAT showed low, insignificant titers (1:80) for the sera of two of these four patients and tested positive for the two other patients. Coombs was positive in all four patients. One of these four patients was diagnosed with a small hepatic abscess years after he had been treated for brucellosis. Brucella melitensis was isolated from the abscess. Coombs gave a titer of 1:640, but the IgG in the sera did not agglutinate in SAT. IgA purified from the serum showed agglutinating activity that was sensitive for DTT. The second patient presented an acute case with a blood culture positive for B. melitensis. Only Coombs tested positive (1:640) for this patient. The third patient also was culture positive, and the serum of this patient reacted in SAT (1:320) and Coombs (1:2,560). The fourth patient was culture negative with a serum that agglutinated in SAT (1:1,280), was DTT sensitive, and reacted weakly in Coombs (1:1,280).
The duration of illness at the time of diagnosis was recorded for 36 patients with confirmed brucellosis (Table 5). Of these patients, 23 had acute illness and 13 suffered from persistent disease. Of the serum samples from acute-phase patients, 2 samples tested negative in both tests, 4 tested positive in the IgM assay and negative in the IgG flow assay, 10 tested positive in both tests, and 7 tested positive only in the IgG flow assay. Of the sera from the patients with persistent disease, one tested negative in both tests, two reacted only in the IgM flow assay, two tested positive in both tests, and eight reacted only in the IgG flow assay. The sensitivity of the flow assays was 91% for the patients with acute illness and 92% for the patients with persistent disease. The sensitivity of the flow assays was higher than that of SAT but lower than that of Coombs for each of the two groups.
TABLE 5.
Presence of specific IgM and IgG antibodies in patients with acute and persistent brucellosis
| Stage of illnessa | No. of patients with positive result (% positive, 95% CI) in the indicated assay
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| SAT, ≥1:320 | SAT, ≥1:160 | SAT-DTT, ≥1:160 | SAT-DTT, ≥1:80 | SAT-DTT, ≥4-fold titer reduction | Coombs, ≥1:320 | IgM flow, ≥1+ | IgG flow, ≥1+ | IgM + IgG flow, ≥1+b | |
| Acute (n = 23) | 16 (70, 47-86) | 20 (87, 65-97) | 11 (48, 27-69) | 13 (57, 35-76) | 15 (65, 43-83) | 23 (100, 82-100) | 14 (61, 39-80) | 17 (74, 51-89) | 21 (91, 71-99) |
| Persistent (n = 13) | 7 (54, 26-80) | 9 (69, 39-90) | 3 (23, 6-54) | 5 (39, 15-68) | 5 (39, 15-68) | 13 (100, 72-100) | 4 (31, 10-61) | 10 (77, 46-94) | 12 (92, 62-100) |
Acute, <3 months of illness at diagnosis; persistent, >3 months of illness at diagnosis.
Positive (≥1+) test result in at least one of the two flow assays.
Culture and RID were negative (n = 46) or not performed (n = 70) for 116 patients with an unconfirmed clinical diagnosis of brucellosis. SAT with a titer of ≥1:320 (referred to hereafter as SAT, ≥1:320), SAT, ≥1:160, SAT-DTT, ≥1:160, SAT-DTT, ≥1:80, Coombs, and the IgM and IgG flow assays tested positive in 54, 72, 52, 66, 93, 38, and 58%, respectively, of the initial sera of this group of patients. SAT-DTT indicated the presence of specific IgM antibodies in 29% of the sera. The IgM and IgG flow assays combined gave a positive result for a total of 88 (76%) patients, and of these, 57 (64%) showed a moderate to very strong staining (Table 3). The other 31 positive sera gave a 1+ staining. For this heterogeneous group of patients also the results of the flow assays closely paralleled those of SAT, and given the demonstrated high sensitivity and specificity, the results of the flow assays confirmed the diagnosis in a high percentage of the cases. A number of patients that were considered negative gave an indeterminate reactivity in the flow assay. Such cases would require further investigation by testing follow-up sera or laboratory testing in other tests.
The number of positive results in the IgM and IgG flow assays decreased during the follow-up of treated patients (Table 6). The number of positive results in the IgM flow assay decreased from 62 to 31% in the first 3 months after treatment and decreased further, to around 20%, during the following 3 months. In the IgG flow assay the number of patients with a positive result slightly increased during the first 2 months of treatment and gradually decreased to 38% for samples collected after more than 6 months of follow-up. The number of SAT-positive patients also rapidly declined during follow-up. Coombs titers remained high during follow-up, with some reduction in the number of positive patients observed after 6 months. The decrease in the number of patients with a positive result in the flow assays coincided with a reduction in the staining intensity of the antigen band (Table 7). The tests thus may be used to monitor the effects of treatment.
TABLE 6.
Brucella IgM and IgG flow assay results and comparison with standard serological assays during follow-up of treated patients
| Period (mo) (n)a | % Positive (95% CI) in the indicated assay
|
|||||
|---|---|---|---|---|---|---|
| IgM flow, ≥1+ | IgG flow, ≥1+ | SAT, ≥1:320 | SAT, ≥1:160 | SAT-DTT, ≥4-fold titer reduction | Coombs, ≥1:320 | |
| Initial sample (26) | 62 (41-79) | 69 (48-85) | 54 (34-73) | 73 (52-88) | 54 (34-73) | 100 (84-100) |
| 0-1 (16) | 75 (44-87) | 81 (50-92) | 44 (21-69) | 81 (50-92) | 68 (39-85) | 88 (62-98) |
| 1-2 (23) | 61 (39-80) | 87 (61-94) | 26 (11-49) | 48 (27-69) | 35 (17-57) | 91 (71-99) |
| 2-3 (16) | 31 (12-59) | 69 (42-88) | 19 (5-46) | 31 (12-89) | 31 (12-59) | 88 (58-96) |
| 3-6 (18) | 22 (7-48) | 61 (36-82) | 6 (0-29) | 22 (7-48) | 18 (4-42) | 83 (58-96) |
| >6 (21) | 19 (6-43) | 38 (19-61) | 0 (0-20) | 10 (2-32) | 19 (6-43) | 57 (34-77) |
n, number of samples.
TABLE 7.
Decreased staining intensity of Brucella IgM and IgG lateral flow assays during follow-up of treated patients
| Period (mo) (n)a | No. of serum samples with the indicated staining intensity in:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Brucella IgM flow assay
|
Brucella IgG flow assay
|
|||||||||||
| Negative | +/− | 1+ | 2+ | 3+ | 4+ | Negative | +/− | 1+ | 2+ | 3+ | 4+ | |
| Initial sample (26) | 9 | 1 | 1 | 4 | 9 | 2 | 5 | 3 | 7 | 6 | 2 | 3 |
| 0-1 (16) | 4 | 1 | 3 | 3 | 5 | 1 | 2 | 2 | 5 | 4 | 4 | |
| 1-2 (23) | 9 | 1 | 3 | 9 | 1 | 3 | 1 | 9 | 7 | 2 | 1 | |
| 2-3 (16) | 6 | 5 | 3 | 2 | 2 | 3 | 5 | 4 | 2 | |||
| 3-6 (18) | 9 | 5 | 4 | 5 | 2 | 4 | 7 | |||||
| >6 (21) | 15 | 3 | 3 | 7 | 6 | 7 | 1 | |||||
n, number of samples.
For two relapsing patients, the reactivity observed in the IgG flow assay was strongly increased for the samples collected during and after relapse over that observed for the sample collected at first diagnosis. For one of these two patients who became blood culture positive 1 year after treatment, the initial serum sample collected at first diagnosis tested 2+ in the IgM flow assay and 1+ in the IgG flow assay and the serum collected after relapse tested indeterminate in the IgM flow assay and 3+ in the IgG flow assay. These results were in agreement with those of SAT, SAT-DTT, and Coombs.
In areas of endemicity the diagnosis of brucellosis may be hampered by lack of expertise and laboratory facilities to perform the currently available diagnostic tests. The Brucella IgM and IgG flow assays fulfill this need. The sensitivity and specificity of this assay system for samples collected at the time of first diagnosis are high, 96 and 99%, respectively, allowing an accurate and early diagnosis. The sensitivity is high for acute as well as chronic cases. The assay is also user-friendly; it is very simple to perform without the need for specific equipment, training, or electricity. Importantly, the assay gives a very clear result and is very easy to read by visual inspection for staining of a line in the test zone of the assay device. Moreover, the assay components are highly stable and devices can be stored for a prolonged time without the need for refrigeration.
The high sensitivity and specificity of the flow assays allow their use as a confirmatory test in all cases in which symptoms and signs lead to suspect the presence of brucellosis. In clinical practice the flow assays may be used as a confirmatory test in combination with RB as a screening assay. A positive result by RB together with a positive test in either of the two flow assays should be considered consistent with brucellosis. It may be noted, though, that the results of RB may vary depending on the quality of the particular antigen batch used (8) and that culture remains the preferred method because it provides definite proof.
When using the flow assays, the Brucella IgM and the Brucella IgG flow assays should be used as complementary tests. Only some of the cases with suspected acute brucellosis gave a positive result in the IgM flow assay. Others gave a positive result in the IgG flow assay instead. Among the cases with suspected persistent brucellosis, some reacted in the IgG flow assay and some reacted in the IgM flow assay. The use of the flow assays will thus require the simultaneous or sequential use of the two tests, and this will allow the confirmation of a high percentage of acute cases with brucellosis as well as those with a longer duration of illness.
The lateral flow assay may also be used in the follow-up of patients undergoing treatment and to study relapse. A rapid decline in response in the lateral flow assay was observed for patients undergoing treatment. For patients showing relapse, a strong response in the IgG flow assay was noticed in the sera collected at the time of relapse. However, this observation requires investigation of a larger number of patients in order to be confirmed but is consistent with the notion that during relapse specific IgG but not IgM antibodies usually are formed (6).
In conclusion, the availability of a rapid and simple assay system for human brucellosis such as the Brucella IgM and IgG flow assays is important, as it takes testing out of the hands of well-equipped laboratories and puts it in the hands of physicians who can use it in health centers and district hospitals and also in private practice. The test is especially suited for use in suburban and rural areas in countries where the disease is endemic.
Acknowledgments
We thank Sophie Gill for dedicated technical advice and manufacturing of development and evaluation lots of flow assays.
REFERENCES
- 1.Alton, G. G., L. M. Jones, and D. E. Pietz. 1975. Laboratory techniques in brucellosis. Monogr. Ser. World Health Organ. 55:1-163. [PubMed] [Google Scholar]
- 2.Anderson, R. K., R. Jenness, H. P. Brumfield, and P. Gough. 1964. Brucella-agglutinating antibodies: relation of mercaptoethanol stability to complement fixation. Science 143:1334-1335. [PubMed] [Google Scholar]
- 3.Aragon, V., R. Diaz, E. Moreno, and I. Moriyon. 1996. Characterization of Brucella abortus and Brucella melitensis native haptens as outer membrane O-type polysaccharides independent from the smooth lipopolysaccharide. J. Bacteriol. 178:1070-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ariza, J., T. Pellicer, R. Pallares, A. Foz, and F. Gudiol. 1992. Specific antibody profile in human brucellosis. Clin. Infect. Dis. 14:131-140. [DOI] [PubMed] [Google Scholar]
- 5.Ariza, J., J. Corredoira, R. Pallares, P. Fernandez-Viladrich, G. Rufi, M. Pujol, and F. Gudiol. 1995. Characteristics of and risk factors for relapse of brucellosis in humans. Clin. Infect. Dis. 20:1241-1249. [DOI] [PubMed] [Google Scholar]
- 6.Avhonen, P., and K. Sievers. 1969. Yersinia enterocolitica infection associated with Brucella agglutinins. Clinical features of 24 patients. Acta Med. Scand. 185:121-125. [DOI] [PubMed] [Google Scholar]
- 7.Beaton, C. P., and J. R. Forsyth. 1984. A micromethod for agglutination and antiglobulin tests for antibody to Brucella abortus. J. Biol. Stand. 12:271-275. [DOI] [PubMed] [Google Scholar]
- 8.Blasco, J. M., B. Garin-Bastuji, C. M. Marin, G. Gerbier, and J. Fanlo, M. P. Jimenez de Bagues, and C. Cau. 1994. Efficacy of different Rose Bengal and complement fixation antigens for the diagnosis of Brucella melitensis infection in sheep and goats. Vet. Rec. 134:415-420. [DOI] [PubMed] [Google Scholar]
- 9.Buchanan, T. M., J. C. Faber, and R. A. Feldman. 1974. Brucellosis in the United States 1960-1972. An abattoir associated disease. Part I. Clinical features and therapy. Medicine (Baltimore) 53:403-413. [DOI] [PubMed] [Google Scholar]
- 10.Daddod, W. A., and Z. A. Abdulia. 2000. A panel of eight tests in the serodiagnosis and immunological evaluation of acute brucellosis. East. Med. Health J. 6:304-312. [PubMed] [Google Scholar]
- 11.Diaz, R., E. Maravi-Poma, and A. Rivero. 1976. Comparison of counter-immunoelectrophoresis with other serological tests in the diagnosis of human brucellosis. Bull. W. H. O. 53:417-424. [PMC free article] [PubMed] [Google Scholar]
- 12.Colmenero, J. D., L. M. Reguera, F. Martos, D. Sanchez-Mora, M. Delgado, M. Causse, A. Martin-Farfan, and C. Juarez. 1996. Complications associated with Brucella melitensis infection: a study of 530 cases. Medicine (Baltimore) 75:195-211. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Lago, L., and R. Diaz. 1986. Demonstration of antibodies against Brucella melitensis 16M lipopolysaccharide and native hapten in human sera by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 24:76-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foz, A., and L. Arcalis. 1953. Die Komplementbindungs-Reaktion in der Diagnosis der menschlichen Brucellosis. Z. Hyg. 136:55-66. [Google Scholar]
- 15.Gazapo, E., J. Gonzales Lahoz, J. L. Subiza, M. Baquera, J. Gil, E. G. de la Concha. 1989. Changes in IgM and IgG antibody concentrations in brucellosis over time: importance for diagnosis and follow-up. J. Inf. Disease. 159:219-225. [DOI] [PubMed] [Google Scholar]
- 16.Kerr, W. R., W. J. McCaughey, J. D. Coghlan, D. J. Payne, R. A. Quaife, L. Robertson, and I. D. Farrell. 1968. Techniques and interpretations in the serological diagnosis of brucellosis in man. J. Med. Microbiol. 1:181-193. [DOI] [PubMed] [Google Scholar]
- 17.Kiel, F. W., and M. Y. Khan. 1987. Analysis of 506 consecutive positive serological tests. J. Clin. Microbiol. 25:1384-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lulu, A. R., M. Y. Mustafa, and M. I. Khateeb. 1986. Evaluation of ELISA in the diagnosis of acute and chronic brucellosis in human beings. J. Hyg. 97:457-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otero, J. R., A. Fuertes, E. Palenque, and A. R. Noriega. 1982. Microtitre-adapted method that facilitates the Coombs test for brucellosis. J. Clin. Microbiol. 16:737-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Memish, Z. A., M. Almuneef, M. W Mah, L. A. Qassem, and A. O. Osoba. 2002. Comparison of the Brucella Standard Agglutination Test with the ELISA IgG and IgM in patients with Brucella bacteremia. Diagn. Microbiol. Infect. Dis. 44:129-132. [DOI] [PubMed] [Google Scholar]
- 21.Pellicer, T., J. Ariza, A. Foz, R. Pallares, and F. Gudiol. 1988. Specific antibodies detected during relapse of human brucellosis. J. Infect. Dis. 157:918-924. [DOI] [PubMed] [Google Scholar]
- 22.Reddin, L., R. K. Anderson, R. Jenness, and W. W. Spink. 1965. Significance of 7S and macroglobulin brucella agglutinins in human brucellosis. N. Engl. J. Med. 272:1263-1268. [DOI] [PubMed] [Google Scholar]
- 23.Renoux, G., M. Plommet, and A. Philippon. 1971. Microréactions d'agglutination et de fixation de complément pour le diagnostic des brucelloses. Ann. Rech. Vet. 2:263-269. [Google Scholar]
- 24.Solera, J., E. Martinez-Alfaro, and A. Espinosa. 1997. Recognition and optimum treatment of brucellosis. Drugs 53:245-256. [DOI] [PubMed] [Google Scholar]
- 25.Yagupsky, P. 1994. Detection of Brucella melitensis by BACTEC NR660 blood culture system. J. Clin. Microbiol. 32:1899-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young, E. J. 1991. Serological diagnosis of human brucellosis: analysis of 214 cases by agglutination tests and review of the literature. Rev. Infect. Dis. 13:359-372. [DOI] [PubMed] [Google Scholar]
- 27.Young, E. J. 1994. An overview of human brucellosis. Clin. Inf. Dis. 21:283-290. [DOI] [PubMed] [Google Scholar]