Abstract
The objective of the present study was to investigate immunoglobulin G (IgG) and IgA antibody immune responses to Porphyromonas gingivalis, Prevotella intermedia, Bacteroides forsythus, and Candida albicans in the sera of patients with rheumatoid arthritis (RA), the synovial fluid (SF) of patients with RA (RA-SF samples), and the SF of patients without RA (non-RA-SF samples). An enzyme-linked immunosorbent assay was used to determine IgG and IgA antibody levels in 116 serum samples from patients with RA, 52 RA-SF samples, and 43 non-RA-SF samples; and these were compared with those in SF samples from 9 patients with osteoarthritis (OA-SF samples) and the blood from 100 donors (the control [CTR] group). Higher levels of IgG antibodies against B. forsythus (P < 0.0001) and P. intermedia (P < 0.0001) were found in non-RA-SF samples than in OA-SF samples, and higher levels of IgG antibodies against B. forsythus (P = 0.003) and P. intermedia (P = 0.024) were found in RA-SF samples than in OA-SF samples. Significantly higher levels of IgA antibodies against B. forsythus were demonstrated in both RA-SF and non-RA-SF samples than in OA-SF samples. When corrected for total Ig levels, levels of IgG antibody against B. forsythus were elevated in RA-SF and non-RA-SF samples compared to those in OA-SF samples. Lower levels of Ig antibodies against B. forsythus were found in the sera of patients with RA than in the plasma of the CTR group for both IgG (P = 0.003) and IgA (P < 0.0001). When corrected for total Ig levels, the levels of IgG and IgA antibodies against B. forsythus were still found to be lower in the sera from patients with RA than in the plasma of the CTR group (P < 0.0001). The levels of antibodies against P. gingivalis and C. albicans in the sera and SF of RA and non-RA patients were comparable to those found in the respective controls. The levels of IgG and IgA antibodies against B. forsythus were elevated in SF from patients with RA and non-RA-SF samples compared to those in OA-SF samples. Significantly lower levels of IgG and IgA antibodies against B. forsythus were found in the sera of patients with RA than in the plasma of the CTR group. This indicates the presence of an active antibody response in synovial tissue and illustrates a potential connection between periodontal and joint diseases.
Sufficient data are available to implicate Porphyromonas gingivalis, Bacteroides forsythus, and Prevotella intermedia as pathogens that initiate periodontal disease (2, 40, 43). These gram-negative anaerobic bacteria possess various antigens (32, 36) that provoke a host-mediated immune response to the offending species (2, 36). This is a complex immunopathogenic process which involves interactions between T and B lymphocytes, neutrophils, monocytes, and phagocytes and the subsequent production of cytokines and prostaglandins (15). The humoral immune response, in which immunoglobulin G (IgG) and IgA antibodies are produced, is considered to have a protective role in the pathogenesis of periodontal disease (2, 13, 22), but the mechanisms are not fully understood.
Rheumatoid arthritis (RA) is a systemic autoimmune disorder characterized by synovial hyperplasia and chronic inflammation (14). Peripheral joint disease is the most frequent feature of RA; but the eyes, skin, blood vessels, kidneys, and nervous system may also be involved. The prevalence of RA in the Western population is 0.5 to 1% and affects women about three times as often as it affects men (35). Experimental evidence has suggested that several microorganisms (bacterial proteins or viruses) may play an important role, in association with a genetic predisposition (3), in triggering the onset of the disease (25, 31, 44).
Clinical studies of RA and periodontal disease have provided evidence for a significant association between the two disorders (29). Patients with long-standing active RA have a substantially increased frequency of periodontal disease compared to that among healthy subjects (21). Patients with periodontal disease have a higher prevalence of RA than patients without periodontitis (28), and it may be hypothesized that periodontal disease plays a role as a triggering factor for RA.
Dry eyes and dry mouth have been shown to be major complaints among RA patients, but the prevalence is uncertain (4, 10, 11, 38). Decreased salivary secretion also affects the oral mucosa and may promote oral candidiasis (18). Interestingly, it has been shown that RA patients may have higher counts of oral Candida species than controls (19).
The last few decades of periodontal research have provided evidence for a correlation between elevated concentrations of antibodies against periodontal pathogens in serum and disease severity (2, 13, 22, 42). However, few studies that have attempted to search for a link between oral microorganisms and immune responses in the sera of patients with RA (RA sera) and synovial fluid (SF) samples from patients with RA (RA-SF samples) have been carried out. In a study of cell wall components from the periodontal pathogen Actinobacillus actinomycetemcomitans, it was shown that IgG antibody titers were significantly higher in RA sera than in the sera of healthy controls (41). Other findings suggest that while the levels of IgG antibodies against P. gingivalis were significantly elevated in sera from patients with periodontal disease, comparable levels were found in the sera of healthy controls and RA patients (42).
In order to further elucidate the role of periopathogens in RA, the purpose of this study was to screen for the levels of IgG and IgA antibodies against P. gingivalis, P. intermedia, B. forsythus, and Candida albicans in serum and RA-SF samples and samples from other arthritis patients compared to those in patients with osteoarthritis (OA) and healthy subjects.
MATERIALS AND METHODS
Patients and sampling.
All rheumatic patients were examined at the Rheumatology Clinic at Haukeland University Hospital, Bergen, Norway. A medical history was taken from each patient. Recorded serological data included rheumatoid factor (RF) titers and C-reactive protein (CRP) levels. CRP levels were determined by endpoint analysis (Tina Quant method) at a central laboratory, Haukeland University Hospital, during hospitalization. The cutoff level for a positive RF titer was set as a titer of 128 (11, 39), as determined by the hemagglutination method. The study was approved by the Ethical Committee, Faculty of Medicine, University of Bergen.
Group 1 consisted of 116 patients with RA. Eighty-two (70.7%) of these patients were women, and 34 (29.3%) were men. The mean ages were 58.6 years (range, 33 to 80 years) for women and 60.4 years (range, 33 to 80 years) for men. Ninety-two (79.3%) were treated with disease-modifying antirheumatic drugs (DMARDs), and the most frequent drug administered was methotrexate (MTX), which was administered to 63 patients. Steroids were given orally to 42 (36.2%) of the patients. The mean duration of DMARD treatment in this group was 7.0 years (range, 0 to 27 years).
Group 2 consisted of 104 patients who were different from those in group 1. Fifty-six (53.8%) of these patients were women, and 48 (46.2%) were men, and all had some type of inflammatory arthritis. Among these, 52 patients (34 women, 18 men) had RA, 9 (5 women, 4 men) had OA, and 43 (16 women, 27 men) had various arthritides (non-RA). Among the patients in the non-RA group, 14 had psoriatic arthritis, 10 had morbus Bechterew, 9 had reactive arthritis, 2 had morbus Reiter, and 8 were characterized as having arthritis in connection with ulcerative colitis and nonspecific mono- or polyarthritis. The mean ages for the RA patients were 53.0 years (range, 23 to 86 years) for women and 60.3 years (range, 20 to 76 years) for men. For the OA patients, the mean ages were 37.9 years (range, 13 to 68 years) for women and 43.1 years (range, 22 to 78 years) for men, and for the non-RA patients the mean ages were 57.2 years (range, 54 to 58 years) for women and 59.5 years (range, 52 to 68 years) for men. SF samples from OA patients (OA-SF samples) were used as controls (see Tables 2, 4, and 5).
TABLE 2.
Specific levels of IgG antibodies against P. gingivalis, P. intermedia, and B. forsythus in SF
| Sample | No. of samples | Mean (SD) IgG level (μg/ml)
|
Mean (SD) total IgG level (mg/ml) | ||
|---|---|---|---|---|---|
| P. gingivalis | P. intermedia | B. forsythus | |||
| RA-SF | 52 | 1.43 (1.08) | 0.82a (0.525) | 1.51b (1.309) | 8.80b (4.18) |
| Non-RA-SF | 43 | 1.56 (0.641) | 0.96c (0.401) | 1.68c (1.156) | 11.61c (7.44) |
| OA-SF | 9 | 1.36 (0.519) | 0.47 (0.056) | 0.45 (0.118) | 4.22 (1.13) |
P < 0.05 for differences in antibody levels between each patient group and controls (OA-SF samples) calculated by the Mann-Whitney U test.
P < 0.01 for differences in antibody levels between each patient group and controls (OA-SF samples) calculated by the Mann-Whitney U test.
P < 0.0001 for differences in antibody levels between each patient group and controls (OA-SF samples) calculated by the Mann-Whitney U test.
TABLE 4.
Levels of IgA antibodies against P. gingivalis, P. intermedia, and B. forsythus in SF
| Sample | No. of samples | Mean (SD) IgA level (μg/ml)
|
Mean (SD) total IgA level (mg/ml) | ||
|---|---|---|---|---|---|
| P. gingivalis | P. intermedia | B. forsythus | |||
| RA-SF | 52 | Not detectablea | Not detectable | 0.275b (0.246) | 1.32c (0.803) |
| Non-RA-SF | 43 | Not detectable | Not detectable | 0.501d (0.440) | 1.59d (0.949) |
| OA-SF | 9 | Not detectable | Not detectable | 0.100 (0.071) | 0.47 (0.575) |
Not detectable, values less than the lowest standard value used.
P < 0.05 for differences in antibody levels between each patient group and controls (OA-SF samples) calculated by the Mann-Whitney U test.
P < 0.01 for differences in antibody levels between each patient group and controls (OA-SF samples) calculated by the Mann-Whitney U test.
P < 0.0001 for differences in antibody levels between each patient group and controls (OA-SF samples) calculated by the Mann-Whitney U test.
TABLE 5.
Levels of antibodies against B. forsythus and P. intermedia corrected for total Ig levels
| Sample | No. of samples | Mean (SD) ratio (105) of IgG level/total IgG level
|
Mean (SD) ratio (105) of IgA level/total IgA level
|
||
|---|---|---|---|---|---|
| B. forsythus | P. intermedia | B. forsythus | P. intermedia | ||
| RA serum | 116 | 15.7a (12.4) | 8.90b (5.5) | 20.6a (23.0) | Not detectablec |
| Control plasma | 100 | 28.3 (23.2) | 12.4 (11.1) | 28.9 (22.0) | Not detectable |
| RA-SF | 52 | 18.3 (14.4) | 10.3 (6.7) | 22.2b (16.6) | Not detectable |
| Non-RA-SF | 43 | 19.7 (17.5) | 11.3 (7.9) | 40.1 (39.5) | Not detectable |
| OA-SF | 9 | 11.7 (5.0) | 11.8 (5.9) | 32.4 (12.8) | Not detectable |
P < 0.0001 for differences in antibody levels between the patient group (RA serum) and the CTR group (CTR plasma) and between RA-SF, non-RA-SF, and OA-SF samples calculated by the Mann-Whitney U test.
P < 0.05 for differences in antibody levels between the patient group (RA serum) and the CTR group (CTR plasma) and between RA-SF, non-RA-SF, and OA-SF samples calculated by the Mann-Whitney U test.
Not detectable, values less than the lowest standard value used.
Twenty-seven (53.0%) of the patients in group 2 were medically treated with DMARDs, and 14 of them were treated with MTX. Steroids were administered orally to 22 patients. One OA patient who provided an SF sample and three non-RF patients who provided an SF sample were treated with MTX. Steroids were given orally to four of the non-RA patients who provided an SF sample.
All RA patients fulfilled the revised criteria of the American College of Rheumatology (formerly the American Rheumatism Association) (7). Peripheral blood samples were collected from RA patients in group 1 at the time of medical examination and were immediately centrifuged, and the sera were stored at −20°C.
SF, mainly from larger joints, was obtained only from patients in group 2, centrifuged, and stored at −20°C.
Group 3 consisted of 100 healthy blood donors (71 women, 29 men) who were matched by gender and age with the patients in group 1 and who had no history of active rheumatic disease. The mean ages were 60.4 years (range, 41 to 70 years) for women and 62.3 years (range, 33 to 80 years) for men. The plasma was stored at −20°C and served as the control (CTR) group. In this study blood donor plasma was found to fulfill the criteria as controls for RA sera.
Microbial antigens used in enzyme-linked immunosorbent assay (ELISA).
P. gingivalis ATCC 33277 and P. intermedia ATCC 25611 were obtained from the American Type Culture Collection (ATCC), Manassas, Va. Both were cultured in Lab 090-A Fastidious Anaerobic Agar (LabM, IDG [United Kingdom] Limited, Bury, England), to which sheep blood was added.
B. forsythus FDC 2008 was obtained from the Forsythe Dental Center, Boston, Mass., and was cultured in Trypticase soy agar with N-acetylmuramic acid stock solution.
Mannan (M7504) was obtained from Sigma Chemical Company, St. Louis, Mo. The powder was diluted in distilled water so that the concentration of the solution was 27.2 mg/ml.
The organisms were cultivated in an anaerobic glove box for 10 days at 35°C and then harvested and recultivated for 10 additional days. The second-generation bacterial cultures were inactivated in 1% formalin for 24 h, washed twice in phosphate-buffered saline (PBS), and centrifuged at 10,000 × g for 10 min. The inactivated whole cells were again washed and diluted in PBS to approximately 1,500 × 106 cells/ml of PBS solution by comparison with McFarland standards (24).
ELISAs for IgG and IgA antibody detection.
The concentrations of IgG and IgA antibodies against whole-cell P. gingivalis, P. intermedia, and B. forsythus and the C. albicans polysaccharide mannan in RA sera, CTR plasma, and SF were determined by ELISA with 96-well microtiter flat-bottom high-binding plates (Costar, Cambridge, Mass.) (12, 16). All analyses were carried out in duplicate and were performed with 100 μl of solution per well.
The plates were coated overnight at 4°C with suspensions of inactivated whole bacterial cells (1,500 × 106 cells/ml of PBS).
For IgG antibody determinations, the suspensions were 1/100 for P. gingivalis and B. forsythus and 1/80 for P. intermedia. C. albicans mannan was diluted to 5 μg/ml in 0.05 M carbonate buffer. For IgA antibody determination, the suspensions were diluted 1/100 in PBS for P. gingivalis, P. intermedia, and B. forsythus. C. albicans mannan was diluted to 5 μg/ml in 0.05 M carbonate buffer.
All plates were washed four times with PBS-0.05% Tween 20 (PBST) and were then blocked with PBST-5% milk (L 31 skim milk; Oxoid, Basingstoke, England) for 1 h at room temperature.
For specific IgG antibody determinations, RA sera and CTR plasma were diluted 1/100 for P. gingivalis and P. intermedia, while SF was diluted 1/10 for P. gingivalis and P. intermedia. For B. forsythus and C. albicans mannan the RA sera and the CTR plasma were diluted 1/50 and SF was diluted 1/40. The RA sera, CTR plasma, and SF samples were diluted 1/10 for specific IgA antibody determinations. IgG and IgA antibody standards (3.125 to 200 ng/ml) were prepared by diluting purified human IgG (1.274 mg/ml; I4506; Sigma) and human IgA (1 mg/ml; I1010; Sigma) in PBST-5% milk.
After 1.5 h at room temperature, the plates were washed four times with PBST. Peroxidase-conjugated goat anti-human IgG (A0293; Sigma) was diluted 1/2,000 in PBST-5% milk for all plates except those for P. intermedia, which were diluted 1/1,500 for RA sera and CTR plasma, and was then added to the plates. Peroxidase-conjugated goat anti-human IgA (A7032; Sigma) was diluted 1/3,000 for all plates.
After 1 h at room temperature the plates were washed four times and substrate was added. 1,2-Phenylenediamine dihydrochloride tablets (Dako, Glostrup, Denmark) were dissolved in water by the addition of H2O2. The reaction was stopped with 1 M H2SO4 and read at 490 nm with an E-max precision microplate reader (Molecular Devices Corporation, Sunnyvale, Calif.). The software SOFTmax (Molecular Devices Corporation) was used to calculate the values. Prior to all assays, pilot studies were performed with a limited number of randomized selected samples to find the most appropriate dilutions.
Total IgG and IgA levels were determined by coating the plates with goat anti-human IgG (1 mg/ml; I3382; Sigma) and goat anti-human IgA (3 mg/ml; I1261; Sigma) diluted 1/2,000 and 1/4,000 in PBS, respectively. IgG and IgA antbody standards (3.125 to 200 ng/ml) were prepared by diluting purified human IgG (1.274 mg/ml; I4506; Sigma) and human IgA (1 mg/ml; I1010; Sigma) in PBST-5% milk. The RA serum, CTR plasma, and SF samples were diluted 1/106 in PBST-5% milk. Peroxidase-conjugated goat anti-human IgG (A0293; Sigma) and peroxidase-conjugated goat anti-human IgA (A7032; Sigma) were diluted 1/2,000 and 1/3,000 in PBST-5% milk, respectively. The ELISA was performed as described above for the bacterial suspensions.
Calculation of antibody standard.
The plates were coated with the whole bacterial suspensions. After some of the sera were screened, one sample with high levels of IgG antibodies (human serum) was used as the reference standard. This serum sample was assigned a value of arbitrary units by endpoint titration. In the subsequent analysis, twofold serial dilutions of this serum sample were added to each ELISA plate. In one plate some wells were coated with goat anti-human IgG (1 mg/ml; I3382; Sigma), and then a known amount (in nanograms per milliliter) of purified human IgG (1.274 mg/ml; I4506, Sigma) was added. Some other wells of the same plate were coated with bacteria, and then the reference serum sample was added. The same amount of peroxidase-conjugated goat anti-human IgG (A0293; Sigma) was added to the plate. The IgG titers for the human serum was read against the known IgG values. The units were translated to nanograms per milliliter.
Antibody specificity.
The human serum was mixed with P. gingivalis cells (1 part diluted serum [1/50] and two parts P. gingivalis [1.5 × 109 cells/ml]). The mixture was shaken on a Schüttler MTS 4 shaker (IKA) for 4 h at room temperature and was then left overnight at room temperature. After centrifugation the supernatant was tested by ELISA with P. gingivalis, P. intermedia, and B. forsythus as antigens. Anti-human IgG (I3382; Sigma) served as coat for standard IgG (I4506; Sigma). ELISA was performed as described above with peroxidase-conjugated goat anti-human IgG (A0293; Sigma).
Statistical analysis.
Statistical analysis of the differences between the patient groups and the controls was carried out by the Mann-Whitney U test. The level of significance was set at a P value of <0.05. The associations between specific antibody levels and total IgG and IgA levels were analyzed by use of Spearman's rho correlation coefficient. The statistical analyses were performed with SPSS software (release 10.0.0; SPSS Inc., Chicago, Ill.).
RESULTS
Total Ig levels.
The total IgG antibody levels were significantly higher in RA sera than in CTR plasma (P < 0.0001) (Table 1). Total IgG levels were also higher in RA-SF samples (P = 0.001) and SF samples from non-RA patients (non-RA-SF samples) (P < 0.0001) than in SF samples from patients with OA (OA-SF samples) (Table 2). No differences in total IgA antibody levels in sera were seen (Table 3), but significantly higher IgA antibody levels were found in RA-SF (P = 0.001) and non-RA-SF (P < 0.0001) samples than in OA-SF samples (Table 4).
TABLE 1.
Levels of IgG antibodies against P. gingivalis, P. intermedia, and B. forsythus in RA and CTR group sera
| Sample | No. of samples | Mean (SD) IgG level (μg/ml)
|
Mean (SD) total IgG level (mg/ml) | ||
|---|---|---|---|---|---|
| P. gingivalis | P. intermedia | B. forsythus | |||
| RA serum | 116 | 9.85 (12.08) | 2.21 (1.72) | 3.92a (3.34) | 26.50b (10.04) |
| CTR | 100 | 7.70 (9.99) | 1.91 (1.10) | 4.65 (2.96) | 19.68 (8.29) |
P < 0.01 for differences in antibody levels between the patient group (RA serum) and the CTR group (CTR plasma) calculated by the Mann-Whitney U test.
P < 0.0001 for differences in antibody levels between the patient group and the CTR group calculated by the Mann-Whitney U test.
TABLE 3.
Levels of specific IgA antibodies against P. gingivalis, P. intermedia, and B. forsythus in RA and CTR sera
| Sample | No. of samples | Mean (SD) IgA level (μg/ml)
|
Mean (SD) total IgA level (mg/ml) | ||
|---|---|---|---|---|---|
| P. gingivalis | P. intermedia | B. forsythus | |||
| RA-serum | 116 | 0.169 (0.176) | Not detectablea | 0.349b (0.275) | 2.33 (1.50) |
| CTR | 100 | 0.129 (0.121) | Not detectable | 0.532 (0.390) | 2.28 (2.06) |
Not detectable, values less than the lowest standard value used.
P < 0.0001 for differences in antibody levels between the patient group (RA serum) and the CTR group (CTR plasma) calculated by the Mann-Whitney U test.
Specific IgG and IgA antibody levels.
RA sera contained significantly lower levels of antibodies against B. forsythus than CTR plasma (Tables 1 and 3). The P values for differences in IgG antibody levels were 0.003 and <0.0001 for IgA, respectively. In contrast, the levels of IgG antibodies against B. forsythus were significantly higher in both RA-SF (P = 0.003) and non-RA-SF (P < 0.0001) samples than in OA-SF samples (Table 3). Significantly higher levels of IgA antibodies against B. forsythus were also seen in RA-SF (P = 0.027) and non-RA-SF (P < 0.0001) samples than in OA-SF samples (Table 4).
The levels of IgG antibodies against P. intermedia were significantly higher in both RA-SF (P = 0.024) and non-RA-SF (P < 0.0001) samples than in OA-SF samples (Table 2). Similarly, non-RA-SF samples had stronger antibody responses against B. forsythus and P. intermedia than RA-SF samples.
The levels of IgA antibodies against P. intermedia in RA sera and SF samples and against P. gingivalis in SF samples were below the lowest standard value (<3.125 ng/ml) and were excluded as a result of the findings from the pilot studies with five randomly selected samples (Tables 3 and 4).
Although the levels of IgG and IgA antibodies against P. gingivalis tended to be higher in RA serum and SF samples than in the plasma from the CTR group, the differences were not statistically significant (Tables 1 to 3).
Higher levels of IgG antibodies against C. albicans mannan were seen in RA sera than in CTR plasma, although these differences were not significant (P = 0.072). The differences in IgG antibody levels in RA-SF and non-RA-SF samples compared to those in OA-SF samples were not statistically significant, and the detectable IgA levels were below the lowest standard value.
Total Ig and specific antibody levels.
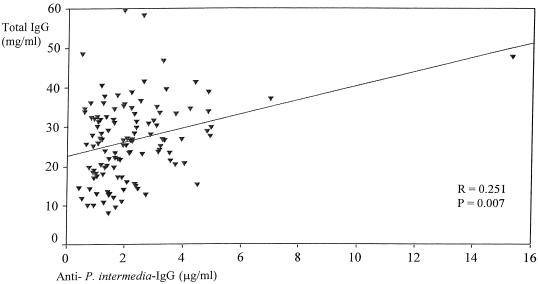
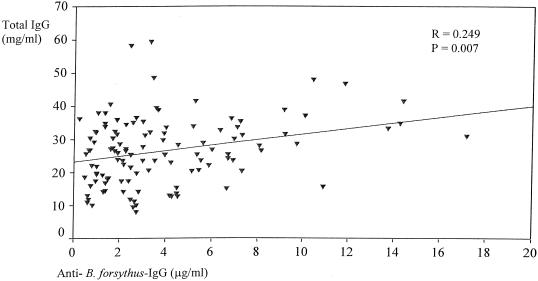
Significant associations between specific antibodies and the total Ig levels in serum and SF samples were detected. In RA serum (Fig. 1 and 2), total IgG antibody levels correlated with the levels of IgG antibodies against P. intermedia (r = 0.251; P = 0.007) and B. forsythus (r = 0.249; P = 0.007). No significant correlation was found between the levels of IgG antibodies against P. gingivalis and the total IgG level in RA sera.
FIG. 1.
Relation between levels of IgG antibodies against P. intermedia and total IgG levels in RA serum.
FIG. 2.
Relation between levels of IgG antibodies against B. forsythus and total IgG levels in RA serum.
A significant correlation between the total IgA antibody level and the B. forsythus-specific IgA level was evident in RA serum (r = 0.198; P = 0.033).
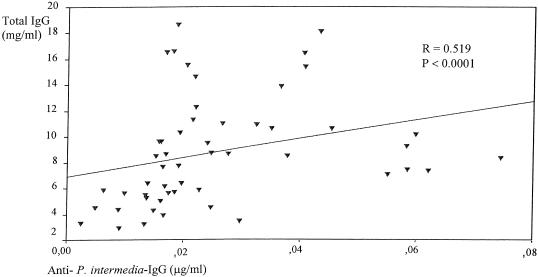
A significant correlation between the levels of IgG antibodies against P. intermedia and total IgG levels in RA-SF samples was present (r = 0.519; P < 0.0001) (Fig. 3). Increased levels of IgG (r = 0.372; P = 0.007) and IgA (r = 0.588; P < 0.0001) antibodies against B. forsythus correlated with increased total IgG and IgA antibody levels in RA-SF samples. No significant relation between the total antibody levels and specific Ig antibody levels in non-RA-SF samples was shown. Significant relations between total antibody levels and C. albicans mannan specific antibodies were absent for RA serum, CTR plasma, and SF samples.
FIG. 3.
Relation between levels of IgG antibodies against P. intermedia and total IgG levels in RA-SF samples.
When corrected for the total Ig levels (Ig level/total Ig level), lower levels of IgG and IgA antibodies against B. forsythus in RA sera than in CTR plasma (P < 0.0001) were demonstrated (Table 5). Although the differences were not significant (P = 0.120), a tendency toward higher levels of IgG antibodies against B. forsythus in the non-RA-SF and RA-SF samples than in OA-SF samples was seen. For IgA this tendency was also evident in non-RA-SF samples (Table 5). Minor differences were detected for P. intermedia.
Serological data and antibody levels.
The sera of 63 (54.3%) of the RA patients were RF positive, and significant relations between RF titers and total IgA antibody levels (r = 0.698; P < 0.0001) and between RF titers and the levels of IgG antibodies against P. gingivalis (r = 0.189; P = 0.043) were found. No significant associations between RF titers and total IgG levels or between RF levels and the levels of specific Ig against the other bacteria and C. albicans were seen.
The SF of 16 (32%) of the 50 RA patients was RF positive, and a significant correlation between RF levels and total IgA antibody levels was found (r = 0.449; P = 0.001). The SF of all of the non-RA patients was RF negative, and the SF of one of the OA patients was RF positive. No significant correlation was seen between RF titers and total IgG antibody levels or between RF levels and the levels of Ig against the other bacteria and C. albicans in SF.
The median CRP level among the serum samples from RA patients was 20.8 mg/liter (range, 0 to 139 mg/liter). A significant association between CRP and total IgG levels (r = 0.291; P = 0.002) was found. Positive associations between CRP levels and both the levels of IgG antibodies against B. forsythus (r = 0.187; P = 0.047) and the levels of IgG antibodies against P. intermedia (r = 0.227; P = 0.016) were also demonstrated.
The mean CRP level for the RA-SF group was 52.0 mg/liter (range, 2.0 to 122.0 mg/liter), that for the OA-SF group was 5.8 mg/liter (range, 4.0 to 9.0 mg/liter), and that for the non-RA-SF group was 43.4 mg/liter (range, 0 to 240 mg/liter). For the RA-SF group, there was a significant correlation between CRP levels and the levels of IgA antibodies against B. forsythus (r = 0.302; P = 0.037) and between CRP levels and total IgA levels (r = 0.489; P < 0.0001).
Antigen specificity.
Testing of the antigen specificity between P. gingivalis and the other two bacteria showed that 70% of the anti-P. gingivalis IgG antibodies did not react when they were mixed with the P. intermedia suspension and that 77% of the anti-P. gingivalis IgG antibodies did not react when they were mixed with the B. forsythus suspension.
DISCUSSION
In this study we have shown that there is a different immune response against some selected periodontal pathogens in serum and SF between arthritis patients and controls. Significantly higher levels of IgG and IgA antibodies against B. forsythus and P. intermedia were found in SF from RA- and non-RA-patients than in SF from OA patients. However, the levels of antibodies against P. gingivalis in RA sera were comparable to those found in the plasma of the CTR group, which has also been demonstrated by others (42). These findings could indicate that a stronger activation of specific B cells with the subsequent formation of antibodies directed especially against B. forsythus and P. intermedia is present in the joints of RA patients and patients with other arthritides.
The lower total antibody levels in SF compared to those in serum, findings which are in agreement with those of other investigators (27), imply that this immune response predominantly takes place in the surrounding tissue and to a lesser degree in the joint cavity. A possible mechanism for this is the capture of bacterial DNA or bacterial products in the synovial tissue, where they promote proinflammatory responses. Plasma cells may also be recruited nonspecifically to the joint. Higher mean levels of Ig antibodies (Tables 1 to 5) against B. forsythus compared to the levels of antibodies against P. intermedia were found. This and the fact that only IgA antibodies against B. forsythus could be detected in SF support the assumption of a particularly strong synovial immune response to B. forsythus.
We also demonstrated significantly lower levels of IgG and IgA antibodies against B. forsythus in RA serum than in the plasma of the CTR group. Given the higher SF antibody concentrations, it could be assumed that the B-cell homing mechanism toward synovial tissue is more pronounced in RA patients and patients with other arthritides and that this homing mechanism is particularly strengthened for B. forsythus. It has also been demonstrated that mucosal lymphocytes in particular are able to home to the synovium by expression of relevant adhesion molecules (8). The explanation why the levels of antibodies against this pathogen were particularly lower in RA serum is disputable. However, studies have shown reduced systemic levels of IgG against peptidoglycans from intestinal bacteria, indicating insufficient protection from spreading peptidoglycans, which was hypothesized to contribute to inflammatory processes (30).
To the best of our knowledge, our study is the first to investigate the role of periodontal pathogens in SF from RA patients and patients with other arthritides. Previous studies have focused on the role of antibody levels in the sera of patients with periodontal disease (2, 13, 22, 42). Researchers have also been interested in the role of the concentrations of antibodies against periodontal pathogens in the serum of RA patients and have investigated the relationship between periodontal disease and RA (21, 28, 29, 33, 41, 42). However, very few studies have been able to provide clinical periodontal data as a supplement to the immune status. Periodontal status has been shown to vary between RA patients and controls, and it is assumed that patients with long-standing active RA have an increased frequency of periodontal disease (21). Our findings regarding the mean antibody levels (Tables 1 to 5) could support this assumption that the incidence of periodontal disease could be higher in RA patients and patients with other arthritides than in healthy blood donors and OA patients, respectively.
Many similarities between the immunopathologies of RA and periodontal disease have been detected (29, 41, 42). Both are destructive, chronic, and inflammatory conditions and may have an acute or chronic presentation. Periodontitis affects single or multiple sites within the oral cavity, leading to the loss of the supporting periodontal tissue (23). In parallel with the pathogenesis seen in RA, the degenerative capability of periodontitis may be initiated and maintained by the presence of endotoxins and exotoxins introduced by microbial organisms (13, 32, 44). It is suggested that while T lymphocytes and polymorphonuclear cells dominate in the early stages of plaque accumulation and gingivitis, B cells dominate in more advanced lesions, with a higher degree of soft tissue destruction and bone loss (9, 36). As is seen in periodontal disease (5, 15, 20, 36), B cells are activated in the synovial tissue in patients with arthritis, leading to a spontaneous secretion of Igs (IgG, IgA, and IgM) (1). This lymphocytic response may lead to the subsequent formation of immune complexes, which are capable of inducing a spectrum of inflammatory mediators (17, 34). In patients with periodontal disease, inflammatory mediators are connected to tissue destruction and bone loss (15).
The cell wall polysaccharide mannan is considered a major antigen of the yeast C. albicans and has the ability to stimulate specific cell-mediated and humoral IgG and IgA immune responses (26). It has been shown that mannan antibodies more frequently appear in sera from patients with Crohn's disease and ulcerative colitis than in controls (6). In our study, we did not find any significant differences in seroreactivity to C. albi-cans between RA sera and the plasma of the CTR group or between RA-SF, non-RA-SF, and OA-SF samples. This could indicate that the activation of B cells against C. albicans in serum or synovial tissue is a rare event.
In this study we found that high levels of antigen-specific antibodies against periodontal pathogens weakly correlated with the total IgG and IgA antibody levels (Fig. 1 and 2). However, when the levels were corrected for total IgG levels (Table 5), lower IgG antibody levels in RA serum compared to those in CTR plasma and higher levels in RA-SF and non-RA-SF samples compared to those in OA-SF samples still predominated. Corrected values for the non-RA-SF group supported the initial findings of the stronger generation of IgA against antibodies B. forsythus in the SF of non-RA patients than in the SF of the OA patients (Table 5). These results are further strengthened by the fact that CRP levels correlated significantly with the levels of IgA antibodies against B. forsythus in the RA-SF group.
Considering the mean age differences in the non-RA-SF group compared to the RA-SF group and the OA-SF group, it might also be suggested that the immune response is stronger in younger patients.
The RF titers did not seem to correlate with the Ig levels against the different bacteria. These findings demonstrate that elevated levels of Ig antibodies against periodontal bacteria in arthritis patients are independent of an up-regulated immune response in general and underline the initial assumption of elevated levels of antibodies against P. intermedia and B. forsythus in the RA-SF and non-RA-SF samples compared to those in the OA-SF samples.
Research regarding the role of intestinal bacteria and the connection between arthritis and bacterial products or bacterial DNA has been presented (30, 37). In light of this, one might assume that our results elucidate an association between oral microbial flora and arthritis. The higher levels of antibodies against these bacteria in arthritis patients demonstrate that they have a higher affinity for the antigens possessed by the cell membranes of periodontal pathogens than those of antibodies in other patient groups. This could be due to earlier exposure to bacterial antigens or products in certain regions such as the synovium or connective tissue as a result of shared antigens between related species in the oral and the intestinal floras. More research should be carried out in this field to elucidate the similarities between oral and intestinal pathogens and their possible arthritogenic capacities.
In conclusion, we have demonstrated evidence for raised levels of antibodies against some selected periodontal pathogens in the serum and intra-articular space of patients with different forms of arthritis, with a special focus on RA. Although the levels of antibodies were significantly higher against P. intermedia and B. forsythus in serum and SF samples from RA patients than in SF samples from OA patients, we demonstrated that the levels of antibodies against P. gingivalis were only slightly elevated. This could indicate a potential connection between periodontal and joint diseases, and our data should lead to more studies in order to evaluate the effect of oral microorganisms in relation to the pathogenesis of RA and related arthritis disorders.
Acknowledgments
This work was supported by grants from the Faculty of Dentistry, University of Bergen, Bergen, Norway, and the Broegelmann Foundation.
We thank Nils Skaug, Elling Ulvestad, Stein Atle Lie, Einar Kristoffersen, Peter Garred, Brita Lofthus, Øyunn Nielsen, and Atle Håland for valuable advice, help, and discussions.
REFERENCES
- 1.Al-Balaghi, S., H. Strøm, and E. Møller. 1982. High incidence of spontaneous Ig-producing lymphocytes in peripheral blood and synovial fluid of patients with active seropositive rheumatoid arthritis. Scand. J. Immunol. 16:69-76. [DOI] [PubMed] [Google Scholar]
- 2.Albandar, J. M., A. M. DeNardin, M. R. Adesanya, S. R. Diehl, and D. M. Winn. 2001. Associations between serum antibody levels to periodontal pathogens and early-onset periodontitis. J. Periodontol. 72:1463-1469. [DOI] [PubMed] [Google Scholar]
- 3.Albani, S., D. A. Carson, and J. Roudier. 1992. Genetic and environmental factors in the immune pathogenesis of rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 18:729-740. [PubMed] [Google Scholar]
- 4.Andonopoulos, A. P., A. A. Drosos, F. N. Skopouli, N. C. Acritid, and H. M. Moutsopoulos. 1987. Secondary Sjögren's syndrome in rheumatoid arthritis. J. Rheumatol. 14:1098-1103. [PubMed] [Google Scholar]
- 5.Anil, S., P. Remani, R. Ankathil, and T. Vijayakumar. 1990. Circulating immune complexes in diabetic patients with periodontitis. Ann. Dent. 49:3-5. [PubMed] [Google Scholar]
- 6.Annese, V., A. Andreoli, A. Andriulli, R. D'Inca, P. Gionchetti, A. Latiano, A., G. Lombardi, A. Piepoli, D. Poulain, B. Sendid, and J. F. Colombel. 2001. Familial expression of anti-Saccaromyces cerevistae mannan antibodies in Crohn's disease and ulcerative colitis: a GISC study. Am. J. Gastroenterol. 96:2407-2412. [DOI] [PubMed] [Google Scholar]
- 7.Arnett, F. C., S. M. Edworthy, D. A. Bloch, D. J. McShane, J. F. Fries, N. S. Copper, L. A Healey, S. R Kaplan, M. H. Liang, H. S. Luthra, T. A. Medsker, Jr., D. M. Mitchell, D. H. Neustadt, R. S. Pinals, J. G. Schaller, J. T. Sharp, R. L. Wilder, and G. G. Hunder. 1988. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 31:315-324. [DOI] [PubMed] [Google Scholar]
- 8.Brandtzaeg, P. 1997. Homing of mucosal immune cells—a possible connection between intestinal and articular inflammation. Aliment. Pharmacol. Ther. 11:24-37. [DOI] [PubMed] [Google Scholar]
- 9.Brecx, M. C., B. Lehman, C. M. Siegwart, P. Gehr, and N. P. Lang. 1988. Observation on the initial stages of healing following human experimental gingivitis. A clinical and morphometric study. J. Clin. Periodontol. 15:123-129. [DOI] [PubMed] [Google Scholar]
- 10.Brun, J. G., H. Jacobsen, R. Kloster, M. Cuida, A. C. Johannesen, H. M. Hoyeraal, and R. Jonsson. 1994. Use of a sicca symptoms questionnaire for the identification of patients with Sjögren's syndrome in a heterogeneous hospital population with various rheumatic diseases. Clin. Exp. Rheumatol. 12:649-652. [PubMed] [Google Scholar]
- 11.Brun, J. G., T. M. Madland, and R. Jonsson. 2003. A prospective study of sicca symptoms in patients with rheumatoid arthritis. Arthritis Rheum. 49:187-192. [DOI] [PubMed] [Google Scholar]
- 12.Cuida, M., A.-K. Halse, A. C. Johannessen, T. Tynning, and R. Jonsson. 1997. Indicators of salivary gland inflammation in primary Sjögren's syndrome. Eur. J. Oral Sci. 105:228-233. [DOI] [PubMed] [Google Scholar]
- 13.Ebersole, J. L., D. Capelli, and S. C. Holt. 2001. Periodontal diseases: to protect or not to protect is the question. Acta Odontol. Scand. 59:161-166. [DOI] [PubMed] [Google Scholar]
- 14.Fox, D. A. 2001. Etiology and pathogenesis of rheumatoid arthritis, p. 1085-1102. In W. J Koopman (ed.), Arthritis and allied conditions. A textbook of rheumatology, 14th ed. Lippincott, Williams & Wilkins, Baltimore, Md.
- 15.Gemell, E., K. Yamazaki, and G. J. Seymour. 2002. Destructive periodontitis lesions are determined by the nature of the lymphocyte response. Crit. Rev. Oral Biol. Med. 13:17-34. [DOI] [PubMed] [Google Scholar]
- 16.Hordnes, K., T. Tynning, A. I. Kvam, R. Jonsson, and B. Haneberg. 1996. Colonization in the rectum and uterine cervix with group B streptococci may induce specific antibody responses in cervical secretions of pregnant women. Infect. Immun. 64:1643-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarvis, J. N., W. Wang, H. T. Moore, L. Zhao, and C. Xu. 1997. In vitro induction of proinflammatory cytokine secretion by juvenile rheumatoid arthritis synovial fluid immune complexes. Arthritis Rheum. 40:2039-2046. [DOI] [PubMed] [Google Scholar]
- 18.Jensen, J. L., and P. Barkvoll. 1998. Clinical implications of the dry mouth. Ann. N. Y. Acad. Sci. 842:156-162. [DOI] [PubMed] [Google Scholar]
- 19.Jensen, J. L., T. Uhlig, T. K. Kvien, and T. Axell. 1997. Characteristics of rheumatoid arthritis patients with self-reported sicca symptoms: evaluation of medical, salivary and oral parameters. Oral Dis. 3:254-261. [DOI] [PubMed] [Google Scholar]
- 20.Jonsson, R., A. Pitts, C. Lue, S. Gay, and J. Mestecky. 1991. Immunoglobulin isotype distribution of locally produced autoantibodies to collagen type I in adult periodontitis. Relationship to periodontal treatment. J. Clin. Periodontol. 18:703-707. [DOI] [PubMed] [Google Scholar]
- 21.Kasser, U. R., C. Gleissner, F. Dehne, A. Michel, B. Willerhausen-Zönnchen, and W. W. Bolten. 1997. Risk for periodontal disease in patients with long standing rheumatoid arthritis. Arthritis Rheum. 40:2248-2251. [DOI] [PubMed] [Google Scholar]
- 22.Kinane, D. F., and D. F. Lappin. 2001. Clinical, pathological and immunological aspects of periodontal disease. Acta Odontol. Scand. 59:154-160. [DOI] [PubMed] [Google Scholar]
- 23.Leknes, K. 1997. The influence of anatomic and iatrogenic root surface characteristics on bacterial colonization and periodontal destruction. J. Periodontol. 68:507-516. [DOI] [PubMed] [Google Scholar]
- 24.Lennette, E. H., A. Balows, W. J. Hausler, Jr., and H. J. Shadomy (ed.). 1985. Manual of clinical microbiology, 4th ed. American Society for Microbiology, Washington, D.C.
- 25.Mäki-Ikola, O., M. Penttinen, R. von Essen, C. Gripenberg-Lerche, H. Isomäki, and K. Granfors. 1997. IgM, IgG and IgA class enterobacterial antibodies in serum and synovial fluid in patients with ankylosing spondylitis and rheumatoid arthritis. Br. J. Rheumatol. 36:1051-1053. [DOI] [PubMed] [Google Scholar]
- 26.Martìnez, J. P., M. Luisa Gil, J. L. Lòpez-Ribot, and W. L. Chaffin. 1998. Serologic response to cell wall mannoproteins and proteins of Candida albicans. Clin. Microbiol. Rev. 11:121-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masson-Bessiere, C., M., Sebbag, J. J. Durieux, L. Nogueira, C. Vincent, E. Girbal-Neuhauser, R. Durroux, A. Cantagrel, and G. Serre. 2000. In the rheumatoid pannus, anti-filaggrin autoantibodies are produced by plasma cells and constitute a higher proportion of IgG than in synovial fluid and serum. Clin. Exp. Immunol. 119:544-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mercado, F., R. I. Marshall, A. C. Klestov, and P. M. Bartold. 2000. Is there a relationship between rheumatoid arthritis and periodontal disease? J. Clin. Periodontol. 27:267-272. [DOI] [PubMed] [Google Scholar]
- 29.Mercado, F. B., R. I. Marshall, A. C. Klestov, and P. M. Bartold. 2001. Relationship between rheumatoid arthritis and periodontitis. J. Periodontol. 72:779-787. [DOI] [PubMed] [Google Scholar]
- 30.Schrijver, I. A., A. De Man, M.-J. Melief, J. M. Van Laar, H. M. Markusse, I. S. Klasen, M. P. Hazenberg, and J. D. Laman. 2000. Reduced systemic IgG levels against peptidoglycan in rheumatoid arthritis (RA) patients. Clin. Exp. Immunol. 123:140-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simelyte, E., E. Rimpiläinen, L. Leehtonen, X. Zhang, and P. Toivanen. 2000. Bacterial cell wall induced arthritis: chemical composition and tissue distribution of four Lactobacillus strains. Infect. Immun. 68:3535-3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sims, T. J., R. W. Ali, E. S. Brockman, N. Skaug, and R. C. Page. 1998. Antigenic variation in Porphyromonas gingivalis ribotypes recognized by serum immunoglobulin G of adult periodontitis patients. Oral Microbiol. Immunol. 13:1-13. [DOI] [PubMed] [Google Scholar]
- 33.Snyderman, R., and G. A. McCarty. 1982. Analogous mechanism of tissue destruction in rheumatoid arthritis and periodontal disease, p. 354-362. In R. J. Genco and S. E. Mergenhagen (ed.), Host-parasite interactions in periodontal diseases. American Society for Microbiology, Washington, D.C.
- 34.Stanilova, S. A., and E. S. Slavov. 2001. Comparative study of circulating immune complexes quantity detection by three assays—CIF-ELISA, C1q-ELISA and anti-C3 ELISA. J. Immunol. Methods 253:13-21. [DOI] [PubMed] [Google Scholar]
- 35.Symmons, D. P. M., E. M. Barrett, C. R. Bankhead, D. G. I. Scott, and A. J. Silman. 1994. The incidence of rheumatoid arthritis in the United Kingdom: results from the Norfolk Arthritis Register. Br. J. Rheumatol. 33:735-739. [DOI] [PubMed] [Google Scholar]
- 36.Tew, J., D. Engel, and D. Mangan. 1989. Polyclonal B-cell activation in periodontitis. J. Periodontal Res. 24:225-241. [DOI] [PubMed] [Google Scholar]
- 37.Toivanen, P. 2000. From reactive arthritis to rheumatoid arthritis. J. Autoimmun. 16:369-371. [DOI] [PubMed] [Google Scholar]
- 38.Uhlig, T., T. K. Kvien, J. L. Jensen, and T. Axell. 1999. Sicca symptoms, saliva and tear production, and disease variables in 636 patients with rheumatoid arthritis. Ann. Rheum. Dis. 58:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ulvestad, E., A. Kanestrøm, T. M. Madland, E. Thomassen, and H.-J. Haga. 2001. Clinical utility of diagnostic tests for rheumatoid factor. Scand. J. Rheumatol. 30:87-91. [DOI] [PubMed] [Google Scholar]
- 40.Vasel, D. T., J. Sims, B. Bainbridge, L. Houston, R. Darveau, and R. C. Page. 1996. Shared antigens of Porphyromonas gingivalis and Bacteroides forsythus. Oral Microbiol. Immunol. 11:226-235. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida, A., Y. Nakano, Y. Yamashita, T. Oho, H. Ito, M. Kondo, M. Ohishi, and T. Koga. 2001. Immunodominant region of Actinobacillus actinomycetemcomitans 40-kilodalton heat shock protein in patients with rheumatoid arthritis. J. Dent. Res. 80:346-350. [DOI] [PubMed] [Google Scholar]
- 42.Yousof, Z., S. R. Porter, J. Greenman, and C. Scully. 1995. Levels of serum IgG against Porphyromonas gingivalis in patients with rapidly progressive periodontitis, rheumatoid arthritis and adult periodontitis. J. Nihon Univ. Sch. Dent. 37:197-200. [DOI] [PubMed] [Google Scholar]
- 43.Zambon, J. J. 1996. Periodontal diseases: microbial factors. Ann. Periodontol. 1:879-925. [DOI] [PubMed] [Google Scholar]
- 44.Zhang, X., E. Rimpiläinen, E. Simelyte, and P. Toivanen. 2000. What determines arthritogenicity of bacterial cell wall? A study on Eubacterium cell wall-induced arthritis. Rheumatology 39:274-282. [DOI] [PubMed] [Google Scholar]