Abstract
We describe a simple test for the evaluation of phagocytosis and provide a chart of reference values to evaluate normal phagocytosis by age. We assessed the postnatal maturation of phagocytic function of neutrophils and monocytes. Phagocytosis was evaluated in newborn children delivered vaginally or by cesarean section, infants, preschool children, schoolchildren, and adult subjects. Two drops of blood were placed on a microscope slide and incubated with Saccharomyces cerevisiae yeasts, and phagocytosis was evaluated by microscopy. Our technique showed results comparable to or better than those obtained by other usual techniques. The neutrophils of newborn children delivered by cesarean section showed a phagocytic capacity 45% higher than those of neonates delivered vaginally, whereas neutrophils from children in the latter group showed the lowest phagocytic capacity of all age groups. Phagocytosis by neutrophils reached the levels seen in adults at about the first year of life, while there were no important variations in phagocytosis by monocytes in the different age groups. The technique described is reliable and fast, uses only a few drops of blood, and allows better preservation of cell function due to the minimal manipulation to which the cells are submitted. The delayed maturation of the phagocytic function by neutrophils may account for the high levels of susceptibility of newborn and infant children to bacterial infections. This practical method of assessment of phagocytosis may allow the diagnosis of primary or secondary phagocytic deficiencies to be made more easily and may allow better monitoring and treatment of those with dysfunctions of these cells.
Extracellular bacteria are the main agents of infections in neonates and infant children, and these pathogens depend on phagocytes for their elimination. Gram-negative enteric rods, Staphylococcus aureus, and Streptococcus spp. are the major causal agents of infections in newborn children, whereas Haemophilus influenzae and Streptococcus pneumoniae predominate in young infants (24, 27, 36). Antibodies and complement factors may act together with phagocytes in the defense against these infectious agents (4). Indeed, it has been shown that deficiencies in phagocytosis, humoral immunity, and complement factors during the first months of life increase susceptibility to infectious diseases and cause high rates of mortality in this period of life (4, 29).
The evaluation of phagocytic function in children has shown conflicting results (4, 16). Some investigators have found phagocytosis to be normal, whereas others have shown deficiencies. It has been observed that phagocytosis by neonatal leukocytes is abnormal when these cells are suspended in neonatal serum but not in adult serum, suggesting a role for a deficiency of opsonin components in the serum of newborn children. However, the phagocytic function in healthy children is not completely clarified (4, 16).
Although testing of phagocytic function has long been used for evaluation of the defense mechanisms of children, the available tests are not properly standardized. Moreover, they are laborious and time-consuming and often use large amounts of blood, which are difficult to obtain from children. Furthermore, they usually involve extensive manipulation of phagocytes, which reduces the reliability of the results (14, 26). Therefore, a chart of reference value that can be used to evaluate normal phagocytosis by age is still not available.
This work (i) describes the development of a novel simple, fast, reliable, and inexpensive microtechnique for the assessment of phagocytosis with a small amount of blood; (ii) aims to determine age-related reference values for phagocytosis by this novel microtechnique; and (iii) compares phagocytosis for different age groups, from healthy newborn children to adult individuals.
(The description of the technique for evaluation of phagocytosis was part of the M.Sc. thesis of Maria Cecília de Almeida Cardoso, supervised by Carlos Eduardo Tosta.)
MATERIALS AND METHODS
Ethical issues.
The ethical rules of the Declaration of Helsinki and those of the Brazilian National Council of Health, Ministry of Health, for experimentation with humans were strictly observed throughout this investigation. This work received approval from the Ethics Committee of the Faculty of Medicine, University of Brasilia. All volunteers or their parents gave formal consent to participate in this study.
Subjects and study groups.
For standardization of the test of phagocytic function, blood from 45 healthy individuals (28 males, 17 females; age range, 14 to 51 years) was assessed.
Different groups were studied to evaluate the influence of age on phagocytosis. Group a consisted of 31 healthy full-term newborn children (19 males, 12 females) who were delivered vaginally, whose weight and height were appropriate for gestational age (mean ± standard deviation [SD], 39.8 ± 1.3 weeks), and who were tested at 37.9 ± 27.3 h (mean ± SD) of life. Group b consisted of 32 healthy full-term newborn children (18 males, 14 females) who were delivered by cesarean section, whose weight and height were appropriate for gestational age (mean ± SD, 40.0 ± 1.3 weeks), and who were tested at 41.7 ± 28.5 h (mean ± SD) of life. Group c consisted of 30 healthy infants (14 males, 16 females; ages, 1 to 11 months) who were tested at 4.9 ± 3.1 months (mean ± SD) of life. Group d consisted of 30 healthy children (15 males, 15 females; age range, 1 to 5 years, mean ± SD age, 34.5 ± 13.1 months). Group e consisted of 29 healthy children (16 males, 13 females; age range, 6 to 12 years old; mean ± SD age, 92.3 ± 18.8 months). Group f consisted of 32 healthy adult individuals (18 males, 14 females; mean ± SD age, 27 ± 5 years).
All children presented with a normal weight for age, a normal height for age, and normal neurodevelopment; and all individuals evaluated were healthy at the time of examination. None of the newborn children selected for the investigation showed signs of fetal distress.
Separation of phagocytes.
Phagocytes were separated by allowing them to adhere to microscope slides, which were prepared by marking 7-mm-diameter round fields with oil ink mixed with epoxy resin. Whole blood was collected without anticoagulant, and 40 μl (about 2 drops) was placed on each field. The slides were then incubated at 37°C for 45 min in a wet chamber to allow the phagocytes to adhere to the glass. The slides were then washed with 0.15 M phosphate-buffered saline (PBS; pH 7.2) at 37°C to eliminate nonadherent cells, fixed with absolute methanol, and stained with 10% Giemsa solution. The number of adherent phagocytes per marked area was assessed by microscopy, with 10% of the total area of the preparation evaluated in duplicate for each individual. Microscopic fields were randomly selected from throughout the preparation. To avoid examination of the same field twice, a previously established order of observation was followed.
Previous standardization showed no difference in the numbers and functions of adherent cells when heparin or no anticoagulant was used. In this study, the clot was removed with a hook-tipped needle after 45 min of incubation. Furthermore, there was no difference in the number of cells that adhered to the slide between the blood from children and the blood from adult individuals.
The efficiency of the method of phagocyte collection was compared with that of two standardized techniques, dextran sedimentation (5) and Percoll density gradient centrifugation (13), by using blood from healthy adult individuals. Blood was obtained by finger puncture (40 μl) and placed directly on the slide by the technique described above, whereas the two other techniques used heparinized venous blood. Leukocytes separated by the dextran sedimentation or Percoll density gradient centrifugation technique were individually suspended to the initial volume, and 40 μl of the suspension was placed on the marked area of the slide. The slide was then incubated for 45 min in a wet chamber at 37°C and washed with 0.15 M PBS (pH 7.2) at 37°C, and the number of phagocytes that adhered to the 7-mm-diameter fields was assessed by microscopy, as described above.
Test of phagocytosis.
Blood phagocytes were obtained by allowing the phagocytes to adhere to the microscope slide, as described above, and incubated in duplicate preparations with 2.5 × 105 S. cerevisiae yeasts in 20 μl of Hanks-Tris solution (pH 7.2; Sigma, St. Louis, Mo.) in a wet chamber at 37°C for 30 min. To evaluate the influence of opsonins (antibody and complement) on phagocytosis, the yeasts were incubated at 37°C for 30 min with 10% fresh serum from the donor in Hanks-Tris solution, and the level of phagocytosis of the yeasts was compared with that of yeasts treated with heat-inactivated fetal calf serum (Cultilab, Campinas, Brazil). After the preparations were rinsed with PBS at 37°C to eliminate nonphagocytosed yeasts, they were treated with 30% normal human serum in Hanks-Tris solution, dried with hot air, fixed with absolute methanol, and stained with 10% Giemsa solution.
The number of yeasts that attached to or that were ingested by 200 monocytes or 200 neutrophils in individual preparations was assessed by microscopy, and the source of the individual preparation was revealed only at the end of the evaluation. Microscopic fields were randomly selected from throughout the slide, and all monocytes and neutrophils in each particular microscopic field were examined. The phagocytic index was calculated as the average number of attached plus ingested yeasts per phagocytosing monocyte or neutrophil multiplied by the percentage of these cells engaged in phagocytosis (25). It was previously observed (12, 21) that 93.4% of the yeasts were ingested by phagocytes when the preparations were treated with 1% tannic acid, with only 6.6% of them appearing to be attached to these cells.
Baker's yeast (S. cerevisiae) was prepared by the technique of Lachmann and Hobart (18). In short, 50 g of fresh live yeast (Fleischmann, Jundiaí, SP, Brazil) were suspended in 220 ml of PBS (pH 7.2), autoclaved at 120°C for 30 min, and washed with PBS until a clear supernatant was obtained. The sediment was suspended in 28 ml of a 0.1 M 2-mercaptoethanol solution in PBS. After 2 h of incubation with stirring, the yeasts were washed again and suspended in 55 ml of 0.02 M iodoacetamide in PBS. After an additional 2 h of incubation at room temperature with stirring, the yeasts were washed three times and suspended in 220 ml of PBS (pH 7.2). The yeasts were again autoclaved, washed, suspended in 110 ml of Veronal-buffered saline (pH 7.2) containing sodium azide, and stored at 4°C until use. In each experiment, the yeast suspensions were washed in PBS, quantified, and suspended in Hanks-Tris solution.
The yeasts were sensitized with fresh human serum at 37°C for 30 min, and the phagocytes were tested for phagocytosis by using serum before or after inactivation of complement at 56°C for 30 min to evaluate the influence of complement molecules on phagocytosis. The presence of human immunoglobulins adsorbed to yeast cells was detected by immunofluorescence with fluorescein-conjugated anti-human immunoglobulin (Sigma).
Statistical analysis.
The results were analyzed by analysis of variance (ANOVA) followed by the Student-Newman-Keuls method to compare multiple normal unrelated samples. The Kruskal-Wallis test followed by Dunn's method was used to compare multiple unrelated nonnormal samples. The Student t test was used to compare two normal unrelated samples. A P value of <0.05 was considered significant. SigmaStat software (Jandel Scientific, San Rafael, Calif.) was used for the statistical tests.
RESULTS
Separation of phagocytes and phagocytosis test.
The cells that adhered to the slides were mainly neutrophils, monocytes, and eosinophils. They remained present on the slides at approximately the same proportions observed in whole blood. No statistically significant differences between the numbers of adherent cells were observed when phagocytes were recovered from whole blood by the technique described here (n = 19 adult individuals; median, 24,157 adherent cells), dextran sedimentation (n = 10 adult individuals; median, 32,881 adherent cells), Percoll 1.077 density gradient centrifugation for mononuclear cells (n = 8 adult individuals; median, 15,882 adherent cells), or Percoll 1.094 density gradient centrifugation for polymorphonuclear cells (n = 8 adult individuals; median, 17,253 adherent cells) (P > 0.05, Kruskal-Wallis test). Phagocytes isolated by adherence by our technique showed phagocytic indices comparable to or higher (154 ± 54) than those separated by dextran sedimentation (19.5 ± 15.4) or Percoll gradient centrifugation (for Percoll 1.077 density gradient, 105.5 ± 53; for Percoll 1.094 density gradient, 65 ± 64) (P < 0.05, ANOVA).
Factors influencing phagocytosis.
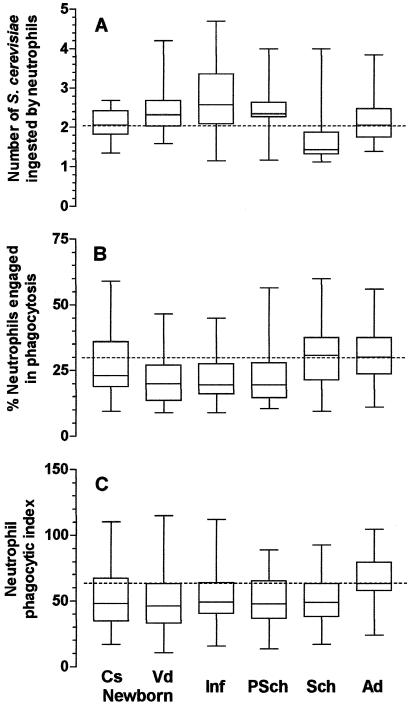
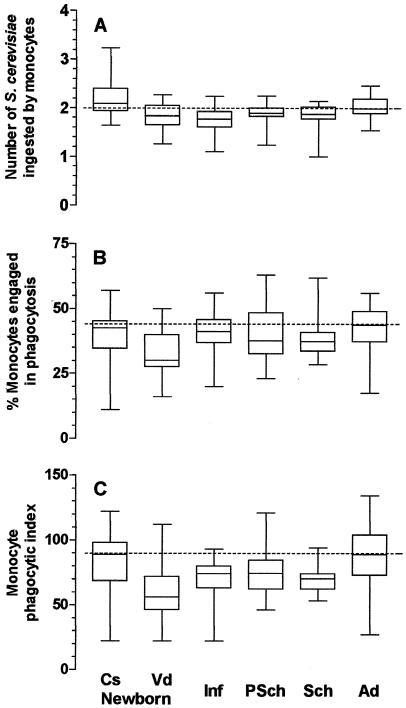
The levels of phagocytosis by neutrophils and monocytes varied by age group, the mode of delivery of the child, the type of phagocyte, and the presence or absence of phagocytosis-enhancing factors in serum. Using nonsensitized yeasts, the phagocytic indices of neutrophils and monocytes from children in all age groups, except for monocytes from children delivered by cesarian section, were lower than those of phagocytes from the adult population (Fig. 1C and 2C) due to the lower levels of engagement of those cells in phagocytosis (Fig. 1B and 2B) and/or to their intrinsic inability to ingest particles (Fig. 1A and 2A). The mode of delivery influenced phagocytosis: monocytes, but not neutrophils, from neonates delivered by cesarean section showed phagocytic indices comparable to those from adult individuals, while for neonates delivered vaginally, phagocytosis was significantly below the levels for adults (Fig. 1C and 2C).
FIG. 1.
Influence of age on phagocytosis of nonsensitized S. cerevisiae yeasts by neutrophils from peripheral blood. Cs newborn, 27 healthy newborn children delivered by cesarean section (group a); Vd newborn, 31 healthy newborn children delivered vaginally (group b); Inf, 30 healthy infants ages 1 to 11 months (group c); PSch, 30 healthy preschool children ages 1 to 5 years (group d); Sch, 30 healthy schoolchildren ages 5 to 12 years (group e); Ad, 31 healthy adult individuals (group f). Neutrophils were incubated with 2.5 × 105 nonsensitized S. cerevisiae yeasts in the presence of 10% fetal calf serum in Hanks-Tris solution. The data are expressed as medians (solid line in each box), quartiles (the tops and bottoms of each box), and minimum and maximum values (bars). The dotted lines indicate the medians for the adult population. (A) Average number of S. cerevisiae yeasts ingested by phagocytosing neutrophils; (B) proportion of neutrophils engaged in phagocytosis; (C) phagocytic index. In panel A, the values for group e were statistically significantly lower than those for groups b, c, d, and f (P < 0.001). In panel B, the values for group f were statistically significantly higher than those for groups b, c, and d; and the values for group e were statistically higher than those for groups b and c (P < 0.001). In panel C, the values for group f were statistically significantly higher than those for groups b and e (P = 0.018). The Kruskal-Wallis test followed by Dunn's method for multiple comparisons was used to determine the P values.
FIG. 2.
Influence of age on phagocytosis of nonsensitized S. cerevisiae yeasts by monocytes from peripheral blood. Cs newborn, 27 healthy newborn children delivered by caesarean section (group a); Vd newborn, 29 healthy newborn children delivered vaginally (group b); Inf, 30 healthy infants ages 1 to 11 months (group c); PSch, 30 healthy preschool children ages 1 to 5 years (group d); Sch, 29 healthy schoolchildren ages 5 to 12 years (group e); Ad, 28 healthy adult individuals (group f). Monocytes were incubated with 2.5 × 105 nonsensitized S. cerevisiae yeasts in the presence of 10% fetal calf serum in Hanks-Tris solution. The data are expressed as medians (solid line in each box), quartiles (the tops and bottoms of each box), and minimum and maximum values (bars). The dotted lines indicate the medians for the adult population. (A) Average number of S. cerevisiae yeasts ingested by phagocytosing monocytes; (B) proportion of monocytes engaged in phagocytosis; (C) phagocytic index. For panel A, the values for group a were statistically significantly higher than those for groups b, c, d, and e; and the values for group f were statistically significantly higher than those for groups b and c (P < 0.001 by ANOVA, followed by the Student-Newman-Keuls method for multiple comparisons). In panel B, the values for group b were statistically significantly lower than those for groups c, d, and f (P = 0.012 by ANOVA, followed by the Student-Newman-Keuls method for multiple comparisons). In panel C, the values for group b were statistically significantly lower than those for groups a and f, and the values for group e were lower than those for group f (P < 0.001 by the Kruskal-Wallis test, followed by Dunn's method for multiple comparisons).
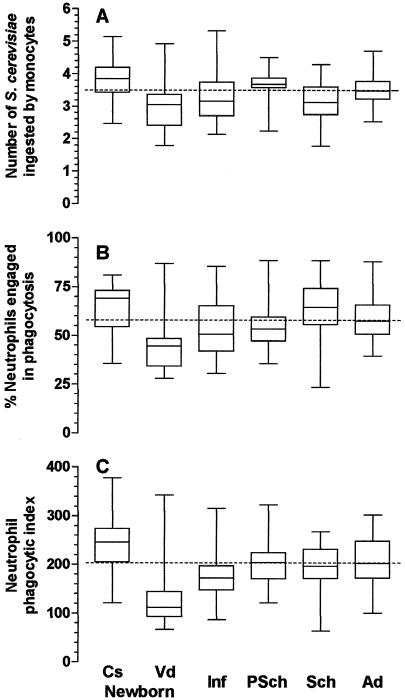
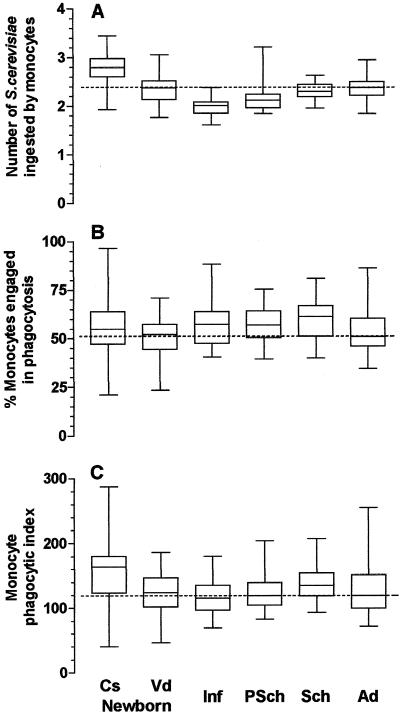
The influence of phagocytosis-enhancing factors (immunoglobulin and complement) in serum was assessed by using S. cerevisiae yeasts sensitized with fresh serum from the tested individual in the in vitro system. This procedure significantly increased the levels of phagocytosis by neutrophils and monocytes and allowed a better characterization of the influence of the mode of delivery on phagocytosis. Cesarean delivery significantly enhanced the phagocytic indices of both neutrophils and monocytes, whereas phagocytosis by neutrophils, but not by monocytes, was significantly lower in children delivered vaginally (Fig. 3C and 4C) (P < 0.001; Kruskal-Wallis test). The enhancing effect of delivery by cesarean section on phagocytosis by neutrophils affected both the proportion of cells engaged in phagocytosis and the ability of these cells to phagocytose (Fig. 3A and B). The latter feature was critical for the increased phagocytic indices of monocytes from children delivered by cesarean section (Fig. 4A to C). This enhancing effect of cesarean delivery on phagocytosis by monocytes disappeared during the first month of life, since the mean ± SD phagocytic index for children ages 1 to 11 months who had been delivered by cesarean section was 129 ± 30, comparable to the mean ± SD phagocytic index of 112 ± 26 for children ages 1 to 11 months who had been delivered vaginally (P > 0.05, the Student t test). The same was observed for neutrophils: the mean ± SD phagocytic index for children ages 1 to 11 months who had been delivered by cesarean section (164 ± 46) was comparable to that for children ages 1 to 11 months who had been delivered vaginally (180 ± 55) (P > 0.05, the Student t test). Different from the low degree of phagocytosis observed with phagocytes from children ages 1 month to 12 years incubated with nonsensitized yeasts (Fig. 1 and 2), the phagocytic indices of neutrophils and monocytes from children incubated with sensitized S. cerevisiae yeasts were generally comparable to those of neutrophils and monocytes from adults. The exception was for neutrophils from children ages 1 to 11 months, for which the phagocytic indices were significantly lower (Fig. 3 and 4).
FIG. 3.
Influence of age on phagocytosis of sensitized S. cerevisiae yeasts by neutrophils from peripheral blood. Cs newborn, 31 healthy newborn children delivered by caesarean section (group a); Vd newborn, 29 healthy newborn children delivered vaginally (group b); Inf, 30 healthy infants ages 1 to 11 months (group c); PSch, 29 healthy preschool children ages 1 to 5 years (group d); Sch, 30 healthy schoolchildren ages 5 to 12 years (group e); Ad, 30 healthy adult individuals (group f). Neutrophils were incubated with 2.5 × 105 S. cerevisiae yeasts sensitized for 30 min at 37°C with 10% fresh serum from the individual donor in Hanks-Tris solution. The data are expressed as medians (solid line in each box), quartiles (the tops and bottoms of each box), and minimum and maximum values (bars). The dotted lines indicate the medians for the adult population. (A) Average number of S. cerevisiae yeasts ingested by phagocytosing neutrophils; (B) proportion of neutrophils engaged in phagocytosis; (C) phagocytic index. In panel A, the values for group a were statistically significantly higher than those for groups b, c, and e; and the values for group d were statistically significantly higher than those for groups b and e (P < 0.001 by the Kruskal-Wallis test, followed by the Dunn's method for multiple comparisons). In panel B, the values for group b were statistically significantly lower than those for groups a, c, d, e, and f (P < 0.001 by ANOVA, followed by the Student-Newman-Keuls method for multiple comparisons). In panel C, the values for group a were statistically significantly higher than those for groups b, c, d, e, and f; and the values for group b were statistically significantly lower than those for groups a, c, d, e, and f (P < 0.001 by ANOVA, followed by the Student-Newman-Keuls method for multiple comparisons).
FIG. 4.
Influence of age on phagocytosis of sensitized S. cerevisiae yeasts by monocytes from peripheral blood. Cs newborn, 29 healthy newborn children delivered by cesarean section (group a); Vd newborn, 30 healthy newborn children delivered vaginally (group b); Inf, 30 healthy infants ages 1 to 11 months (group c); PSch, 30 healthy preschool children ages 1 to 5 years (group d); Sch, 29 healthy schoolchildren ages 5 to 12 years (group e); Ad, 31 healthy adult individuals (group f). Monocytes were incubated with 2.5 × 105 S. cerevisiae yeasts sensitized for 30 min at 37°C with 10% fresh serum from the individual donor in Hanks-Tris solution. The data are expressed as medians (solid line in each box), quartiles (the tops and bottoms of each box), and minimum and maximum values (bars). The dotted lines indicate the medians for the adult population. (A) Average number of S. cerevisiae yeasts ingested by phagocytosing neutrophils; (B) proportion of neutrophils engaged in phagocytosis; (C) phagocytic index. In panel A, the values for group a were statistically significantly higher than those for groups b, c, d, e, and f; the values for group f were statistically significantly higher than those for groups c and d; and the values for group c were statistically significantly lower than those for groups a, b, e, and f (P < 0.001 by the Kruskal-Wallis test, followed by Dunn's method for multiple comparisons). In panel B, the values for group b were statistically significantly lower than those for group e (P = 0.032 by ANOVA, followed by the Student-Newman-Keuls method for multiple comparisons). In panel C, the values for group a were statistically significantly higher than those for groups c and d (P = 0.002 by the Kruskal-Wallis test, followed by Dunn's method for multiple comparisons).
On the basis of the evaluation of phagocytosis by the technique described above with sera from 184 individuals, from neonates to adults, charts of the percentiles of the phagocytic indices of neutrophils (Table 1) and monocytes (Table 2) were constructed to help interpret the test results.
TABLE 1.
Percentiles of phagocytic indices for neutrophils, by age group, obtained by the described techniquea
| Group | No. of individuals | Phagocytic index at the following percentiles:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 10 | 25 | 50 | 75 | 90 | 95 | 97 | 99 | ||
| Neonates delivered by cesarean section | 31 | 121 | 129 | 140 | 157 | 204 | 246 | 274 | 320 | 361 | 372 | 378 |
| Neonates delivered vaginally | 29 | 66 | 70 | 75 | 78 | 91 | 112 | 151 | 243 | 252 | 307 | 343 |
| Infants | 30 | 87 | 89 | 91 | 101 | 145 | 172 | 202 | 244 | 256 | 292 | 316 |
| Preschool children | 29 | 121 | 121 | 121 | 137 | 168 | 204 | 228 | 247 | 305 | 316 | 323 |
| Schoolchildren | 30 | 64 | 79 | 101 | 140 | 170 | 197 | 231 | 259 | 263 | 265 | 267 |
| Adults | 30 | 100 | 107 | 118 | 147 | 164 | 203 | 248 | 283 | 297 | 300 | 302 |
Phagocytosis was evaluated with fresh serum from each subject tested. The phagocytic index was calculated as the average number of attached plus ingested S. cerevisiae yeasts per phagocytosing neutrophil multiplied by the percentage of these cells engaged in phagocytosis.
TABLE 2.
Percentiles of phagocytic indices for monocytes, by age group, obtained by the described techniquea
| Group | No. of individuals | Phagocytic index at the following percentiles:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 10 | 25 | 50 | 75 | 90 | 95 | 97 | 99 | ||
| Neonates delivered by cesarean section | 29 | 41 | 60 | 90 | 96 | 121 | 164 | 186 | 217 | 226 | 264 | 288 |
| Neonates delivered vaginally | 30 | 46 | 54 | 67 | 78 | 101 | 124 | 148 | 167 | 179 | 183 | 186 |
| Infants | 30 | 69 | 73 | 80 | 85 | 95 | 116 | 136 | 155 | 167 | 175 | 180 |
| Preschool children | 30 | 83 | 83 | 84 | 94 | 103 | 119 | 140 | 158 | 165 | 188 | 204 |
| Schoolchildren | 31 | 93 | 96 | 101 | 102 | 116 | 135 | 159 | 176 | 193 | 202 | 207 |
| Adults | 31 | 71 | 76 | 83 | 94 | 98 | 119 | 152 | 172 | 184 | 224 | 255 |
Phagocytosis was evaluated with fresh serum from each subject tested. The phagocytic index was calculated as the average number of attached plus ingested S. cerevisiae yeasts per phagocytosing monocyte multiplied by the percentage of these cells engaged in phagocytosis.
DISCUSSION
Phagocytic tests may have a wide range of uses, from the investigation of individuals with repeated infections to the evaluation of the effects of drugs or infectious agents on defense mechanisms. However, the tests available at present have several important limitations: they are laborious and time-consuming, and principally, they submit phagocytes to extensive manipulation, which can affect their function (34). We describe a novel method for the evaluation of the phagocytic capacities of neutrophils and monocytes based on the adherence of these cells to microscopy glass slides, followed by phagocytosis of the yeast S. cerevisiae, sensitized or not with fresh serum from the individual tested. The method is very easy, fast, and inexpensive and uses only a few drops of blood. Other advantages are the fact that it does not require a sterile environment and the fact that it permits the concomitant evaluation of phagocytosis by neutrophils and monocytes. However, the major advantage of the method is the minimal manipulation to which phagocytes are submitted, since cells are not exposed to centrifugation, separation media, or anticoagulants, as usually happens; and therefore, their functions are better preserved and the results of the evaluation are more reliable. In fact, we showed that the phagocytic capacities of phagocytes obtained directly by adherence to glass slides were higher than those obtained after the dextran sedimentation and Percoll gradient centrifugation separation techniques.
Some aspects of the technique deserve special attention. The reduced amount of blood used (40 μl) makes it critical to keep a high humidity in the environment to avoid evaporation of the preparation, which is capable of influencing cell function. In addition, the glass slides used to collect phagocytes need to be absolutely clean to allow proper adherence of the cells. The wide range of individual variability of the results found with the present technique was not different from that observed with other functional tests of the immune system (8).
S. cerevisiae yeasts were chosen as the particle to be phagocytosed because of the ease of preparation, storage, and quantification by microscopy. Moreover, by using sensitized and nonsensitized yeasts, it was possible to assess phagocytosis via different receptors, such as those for immunoglobulins and complement components (33), and some pattern recognition receptors (30). We tested phagocytosis via opsonins by incubating phagocytes with yeasts previously sensitized with fresh human serum. It was shown by immunofluorescence and functional tests that both immunoglobulin and complement were present on the surfaces of the sensitized yeasts. These opsonins caused average increases in the phagocytic indices of monocytes and neutrophils of 1.8- and 3.8-fold, respectively. Phagocytosis via pattern recognition receptors was assessed with nonsensitized yeast particles. In this case, mannose residues on the surface of the yeast were the major ligand (19).
We found that phagocytosis was influenced by the age of the individual, the type of phagocyte evaluated, the presence of opsonins, and the way in which the child was delivered. Both neutrophils and monocytes from those delivered by cesarean section showed increased levels of phagocytosis, while phagocytes from those delivered vaginally had phagocytic indices below those for phagocytes from adults. Our results are in accordance with those of Szymanska-Toczek and colleagues (32), who observed an increased phagocytic capacity of neutrophils from newborn children delivered by cesarean section.
A lack of consideration of the way in which the neonate is delivered may explain some contrasting results observed when the phagocytic function of newborn children was evaluated (4, 6, 11, 17, 22). It is not yet clear why the phagocytic function of neonates delivered by cesarean section is increased, but some possibilities merit consideration. Parturition modifies the production of several molecules that influence the function of the immune system, such as prostaglandin E, sexual hormones, hypothalamic-pituitary-adrenal axis hormones, and cytokines (7, 15, 23). The way in which the neonate is delivered may interfere with the levels of these molecules. Thus, progesterone (20), cortisol (31), and catecholamines (35) are found at higher concentrations in serum from children delivered vaginally than in those delivered by cesarean section. On the other hand, the concentrations of the inflammatory cytokines interleukin 1 (IL-1), IL-6, tumor necrosis factor alpha, IL-8, and transforming growth factor β are decreased in children delivered by cesarean section (10). Therefore, it is possible that the finding of alterations of phagocytosis associated with the type of delivery was due to the influence of the different molecules acting in each situation. In fact, it was previously shown that phagocytosis by monocytes is influenced by the local cytokine concentration (26).
The reasons for the reduced levels of phagocytosis found in neonates delivered vaginally have not yet been established. A possible factor is the low concentrations of opsonic factors capable of enhancing phagocytosis, such as immunoglobulins (2) and complement components (9), in children from the time they are born to age 11 months. However, our data indicate that the functions of both neutrophils and monocytes from this age group were intrinsically affected, since the level of phagocytosis was low even in the absence of the child's serum, as observed in Fig. 1 and 2. Therefore, it is possible that a degree of immaturity of phagocytes and the reduced levels of expression of opsonin receptors (1) also play a part in the deficiency of phagocytosis in neonates and infants.
Many unfavorable consequences, such as a predisposition to infections, may result from an inadequate function of phagocytes in newborns delivered vaginally (24, 36). However, it should be stressed that the consequences of the enhanced phagocytic capacities of neutrophils and monocytes observed in neonates delivered by cesarean section have still not been clarified; and possible benefits seem to be unlikely, since these phagocytes appear to be working close to their highest capacity and neonates do not have a good pool of phagocytes stored in their bone marrow (17). It is possible that in these children a superimposed infection may not be accompanied by an equivalent increase in the function of phagocytes. In fact, it has been suggested that during infections in these children even adequate numbers of leukocytes may be insufficient since their function may be altered (17), and cesarean delivery following an uncomplicated pregnancy is a risk factor for an adverse neonatal outcome (3).
Although phagocytosis by neutrophils and monocytes represents the first line of defense against the major pathogens that affect children (17), its evaluation has been greatly neglected. The main reason for this appears to be the limitations of the presently available tests, which involve laborious and time-consuming techniques, with extensive manipulation of the phagocytes, which is capable of affecting their functionality. The simple, reliable, inexpensive, and fast test of phagocytic function described here allows the more frequent evaluation of the functions of monocytes and neutrophils in patients with several diseases with inadequate phagocyte function, such as protein energy malnutrition, diabetes mellitus, nephrotic syndrome, and Down's syndrome, among others (28), in children and adult individuals, with just a few drops of capillary or venous blood and virtually no cell manipulation. Our data broaden the understanding of the function of phagocytes and their maturation among different age groups. The delayed maturation of phagocytosis by neutrophils may explain the high degrees of susceptibility of newborn and infant children to bacterial infections. This practical method of assessment of phagocytosis with only a minute amount of blood may ease the diagnosis of primary or secondary phagocytic deficiencies and allows better monitoring and treatment of those with dysfunctions of these cells.
Acknowledgments
L.M.F.P. and V.L.D.S.-F. were the recipients of fellowships from PIBIC/CNPq-UnB at the Cellular Immunology Laboratory.
We thank Luiz Alberto Lima and Antonio José Duarte Jácomo for allowing access to neonates; Sebastião Peçanha for access to healthy children; Renê Pires, Josélia de Souza, and José Siqueira for technical assistance; and Luiz Fernando Junqueira Jr., and Fernanda Muniz Junqueira Ottoni for preparing and editing the illustrations.
REFERENCES
- 1.Abughali, N., M. Berger, and M. F. Tosi. 1994. Deficient total cell content of CR3 (CD11b) in neonatal neutrophils. Blood 83:1086-1092. [PubMed] [Google Scholar]
- 2.Alford, C. A. 1971. Immunoglobulin determinations in the diagnosis of fetal infection. Pediatr. Clin. N. Am. 18:99-113. [DOI] [PubMed] [Google Scholar]
- 3.Annibale, D. J., T. C. Hulsey, C. L. Wagner, and W. M. Southgate. 1995. Comparative neonatal morbidity of abdominal and vaginal deliveries after uncomplicated pregnancies. Arch. Pediatr. Adolesc. Med. 149:862-867. [DOI] [PubMed] [Google Scholar]
- 4.Bellanti, J. A., Y.-A. Pung, and B. J. Zeligs. 1994. Immunology, p. 1000-1052. In G. B. Avery, M. A. Fletcher, and M. G. MacDonald (ed.), Neonatology: pathophysiology and management of the newborn. J. B. Lippincott, Philadelphia, Pa.
- 5.Boyum, A. 1984. Separation of lymphocytes, granulocytes and monocytes from human blood using iodinated density gradient media. Methods Enzymol. 108:88-102. [DOI] [PubMed] [Google Scholar]
- 6.Carr, R. 2000. Neutrophil production and function in newborn infants. Br. J. Haematol. 110:18-28. [DOI] [PubMed] [Google Scholar]
- 7.Challis, J. R. G., S. G. Matthews, W. Gibb, and S. J. Lye. 2000. Endocrine and paracrine regulation of birth at term and preterm. Endocrine Rev. 21:514-550. [DOI] [PubMed] [Google Scholar]
- 8.Cohen, J. J. 1999. Individual variability and immunity. Brain Behav. Immun. 13:76-79. [DOI] [PubMed] [Google Scholar]
- 9.Colten, H. R., and G. Goldberger. 1979. Ontogeny of serum complement proteins. Pediatrics 64(Suppl.):775-780. [PubMed] [Google Scholar]
- 10.Dudley, D. J., D. Collmer, M. D. Mitchell, and M. S. Trautman. 1996. Inflammatory cytokine mRNA in human gestational tissues: implications for term and preterm labor. J. Soc. Gynecol. Investig. 3:328-335. [PubMed] [Google Scholar]
- 11.Forman, M. L., and E. R. Stiehm. 1969. Impaired opsonic activity but normal phagocytosis in low-birth-weight infants. N. Engl. J. Med. 281:926-931. [DOI] [PubMed] [Google Scholar]
- 12.Giaimis, J., Y. Lombard, M. Makaya-Kumba, P. Fonteneau, and P. Poindron. 1992. A new and simple method for studying the binding and ingestion steps in the phagocytosis of yeasts. J. Immunol. Methods 154:185-193. [DOI] [PubMed] [Google Scholar]
- 13.Giudicelli, J., P. J. M. Philip, P. Delque, and P. Sudaka. 1982. A single-step centrifugation method for separation of granulocytes and mononuclear cells from blood using discontinuous density gradient of Percoll. J. Immunol. Methods 54:43-46. [DOI] [PubMed] [Google Scholar]
- 14.Hallwirth, U., G. Pomberger, D. Zaknun, Z. Szepfalusi, E. Horcher, A. Pollak, E. Roth, and A. Spittler. 2002. Monocyte phagocytosis as a reliable parameter for predicting early-onset sepsis in very low birthweight infants. Early Hum. Dev. 67:1-9. [DOI] [PubMed] [Google Scholar]
- 15.Harris, S. G., J. Padilla, L. Koumas, D. Ray, and R. P. Phipps. 2002. Prostaglandins as modulators of immunity. Trends Immunol. 23:144-150. [DOI] [PubMed] [Google Scholar]
- 16.Johnston, R. B., Jr. 1998. Function and cell biology of neutrophils and mononuclear phagocytes in the newborn infant. Vaccine 16:1363-1368. [DOI] [PubMed] [Google Scholar]
- 17.Kanwar, V. S., and M. S. Cairo. 1993. Neonatal neutrophil maturation, kinetics, and function, p. 1-21. In J. S. Abramson and J. G. Wheeler (ed.), The neutrophil. IRL Press, Oxford, United Kingdom.
- 18.Lachmann, P. J., and M. J. Hobart. 1978. Complement technology, p. 1A-5A. In D. M. Weir (ed.), Handbook of experimental immunology, vol. I. Immunochemistry. Blackwell, Oxford, United Kingdom.
- 19.Linehan, S. A., L. Martínez-Pomares, and S. Gordon. 2000. Macrophage lectins in host defence. Microbes Infect. 2:279-288. [DOI] [PubMed] [Google Scholar]
- 20.Löfgren, M., and T. Bäckström. 1997. High progesterone is related to effective human labor: study of serum progesterone and 5α-pregnane-3,20-dione in normal and abnormal deliveries. Acta Obstet. Gynecol. Scand. 76:423-430. [DOI] [PubMed] [Google Scholar]
- 21.Lombard, Y., J. Giaimis, M. Makaya-Kumba, P. Fonteneau, and P. Poindron. 1994. A new method for studying the binding and ingestion of zymosan particles by macrophages. J. Immunol. Methods 174:155-165. [DOI] [PubMed] [Google Scholar]
- 22.Maródi, L., P. C. J. Leijh, and R. van Furth. 1984. Characteristics and functional capacities of human cord blood granulocytes and monocytes. Pediatr. Res. 18:1127-1131. [DOI] [PubMed] [Google Scholar]
- 23.McEwen, B. S., C. A. Biron, K. W. Brunson, K. Bulloch, W. H. Chambers, F. S. Dhabhar, R. H. Goldfarb, R. P. Kitson, A. H. Miller, R. L. Spencer, and J. M. Weiss. 1997. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Res. Rev. 23:79-133. [DOI] [PubMed] [Google Scholar]
- 24.Mulholland, K. 1998. Serious infections in young infants in developing countries. Vaccine 16:1360-1362. [DOI] [PubMed] [Google Scholar]
- 25.Muniz-Junqueira, M. I., A. Prata, and C. E. Tosta. 1992. Phagocytic and bactericidal function of mouse macrophages to Salmonella typhimurium in schistosomiasis mansoni. Am. J. Trop. Med. Hyg. 46:132-136. [DOI] [PubMed] [Google Scholar]
- 26.Muniz-Junqueira, M. I., L. L. Santos-Neto, and C. E. Tosta. 2001. Influence of tumor necrosis factor-α on the ability of monocytes and lymphocytes to destroy intraerythrocytic Plasmodium falciparum in vitro. Cell. Immunol. 208:73-79. [DOI] [PubMed] [Google Scholar]
- 27.Pabst, H. F., and H. W. Kreth. 1980. Ontogeny of the immune response as a basis of childhood disease. J. Pediatr. 97:519-534. [DOI] [PubMed] [Google Scholar]
- 28.Shearer, W. T., and D. C. Anderson. 1989. The secondary immunodeficiencies, p. 400-438. In E. R. Stiehm (ed.), Immunologic disorders in infants and children, 3rd ed. The W. B. Saunders Co., Philadelphia, Pa.
- 29.Siegrist, C.-A. 2001. Neonatal and early life vaccinology. Vaccine 19:3331-3346. [DOI] [PubMed] [Google Scholar]
- 30.Stahl, P. D., and R. A. B. Ezekowitz. 1998. The mannose receptor is a pattern recognition receptor involved in host defense. Curr. Opin. Immunol. 10:50-55. [DOI] [PubMed] [Google Scholar]
- 31.Sybulski, S., and G. B. Maughan. 1976. Cortisol levels in umbilical cord plasma in relation to labor and delivery. Am. J. Obstet. Gynecol. 125:236-238. [DOI] [PubMed] [Google Scholar]
- 32.Szymanska-Toczek, Z., J. Dabek, A. Hrycek, R. Osuch-Jaczewska, and M. Baumert. 2001. Comparative study of neutrophil activities in adults and full-term neonates in relation to the method of delivery. Folia Biol. (Praha) 47:71-74. [PubMed] [Google Scholar]
- 33.Underhill, D. M., and A. Ozinsky. 2002. Phagocytosis of microbes. Complexity in action. Annu. Rev. Immunol. 20:825-852. [DOI] [PubMed] [Google Scholar]
- 34.van Eeden, S. F., M. E. Klut, B. A. M. Walker, and J. C. Hogg. 1999. The use of flow cytometry to measure neutrophil function. J. Immunol. Methods 232:23-43. [DOI] [PubMed] [Google Scholar]
- 35.Wang, L., W. Zhang, and Y. Zhao. 1999. The study of maternal and fetal plasma catecholamine levels during pregnancy and delivery. J. Perinat. Med. 27:195-198. [DOI] [PubMed] [Google Scholar]
- 36.Wright, P. F., and P. F. Wright. 1998. Infectious diseases in early life in industrialized countries. Vaccine 16:1355-1359. [DOI] [PubMed] [Google Scholar]