Abstract
This report describes the presence and activity of 1,25-dihydroxyvitamin D3 (1,25-D3) in experimental bovine tuberculosis. Animals that went on to develop tuberculous lesions exhibited a rapid transient increase in serum 1,25-D3 within the first 2 weeks following infection with Mycobacterium bovis. 1,25-D3-positive mononuclear cells were later identified in all tuberculous granulomas by immunohistochemical staining of postmortem lymph node tissue. These results suggest a role for 1,25-D3 both at the onset of infection and in the development of the granuloma in these infected animals. Using a monoclonal antibody to the vitamin D receptor (VDR) as a VDR agonist, we confirmed that activation of the vitamin D pathway profoundly depresses antigen-specific, but not mitogenic, bovine peripheral blood T-cell responses (proliferation and gamma interferon production). Investigation of the mechanism of this suppression showed that the VDR antibody modified the expression of CD80 by accessory cells, such that a significant positive correlation between T-cell proliferation and accessory cell CD80 emerged.
Sunshine and cod liver oil both have a long association with the treatment of tuberculosis and represent the two sources of vitamin D available, via UV irradiation of the skin and diet. Reports from the mid-19th century suggest that vitamin D in the form of cod liver oil was beneficial in the treatment of tuberculosis, and calcium therapy with or without vitamin D was used before specific chemotherapy became available (18). More recent reports have associated low or disturbed serum vitamin D levels with increased susceptibility to tuberculosis (13, 18, 20, 51), and this is supported by genetic linkage studies of vitamin D receptor (VDR) gene polymorphism that indicate an association between the VDR and susceptibility or resistance to tuberculosis (5, 44, 45, 51).
The active component of vitamin D, 1,25-dihydroxyvitamin D3 (1,25-D3) acts exclusively via its specific nuclear receptor (49), and therefore the distribution of the VDR within the immune system provides information on the active sites of this steroid hormone. The VDR is constitutively expressed in human peripheral monocytes but can be induced in activated lymphocytes (6, 36). VDR expression in both monocytes and T cells can be further up-regulated by exposure to 1,25-D3. 1,25-D3 production by monocytes and macrophages has long been recognized (1, 26, 31), but it is also now apparent that T cells could equally be a major site of 1,25-D3 activity (7, 11, 47), and this may also be the case in tuberculosis (7, 11).
It is becoming increasingly accepted that 1,25-D3 has an important role in the development of immune responses. Reported activities of 1,25-D3 include the suppression of T-cell proliferation (40), inhibition of gamma interferon (IFN-γ) and interleukin-2 (IL-2) production (15, 25), differential regulation of CXC and CC chemokines (29), and enhanced generation of regulatory (23) and Th2 (8) T cells. Some of the effects of 1,25-D3 on T-cell responses are thought to be indirect via the antigen-presenting cell. Reports describe a reduced ability of monocytes to induce antigen-dependent T-cell proliferation, which is now known to be at least partly due to alterations in antigen-presenting and costimulatory molecules, and the retardation of dendritic cell development (12, 33, 34, 39).
In this report we describe the presence and activity of 1,25-D3 in cattle infected with Mycobacterium bovis. Bovine tuberculosis is an increasing problem in the United Kingdom herd, despite a government test-and-slaughter policy (35), and has led to an independent scientific review (28) that advocates the production of new and improved vaccines and diagnostic reagents. Underpinning this work is the investigation of the immunology of bovine tuberculosis, using an experimental regimen that generates pathology similar to that seen in naturally infected cattle (10). Using this experimental approach, we investigated the kinetics of serum 1,25-D3 following infection and the presence of 1,25-D3 within the tuberculous granuloma. The effects of VDR activation on antigen-specific lymphocyte responses were also assessed.
MATERIALS AND METHODS
Animals.
This study utilized eight Friesian cows from tuberculosis-free herds that were infected intratracheally with either 4 × 105 CFU (four animals), 6 × 104 CFU (two animals) or 6 × 103 CFU (two animals) of virulent M. bovis (AF2122/97). A naturally exposed M. bovis field reactor was also included in certain experiments as indicated. Sequential serum samples were taken from all experimentally infected animals. The experiments described were carried out with experimentally infected animals approximately 3 to 4 weeks prior to slaughter (ca. 17 weeks postinfection). The tuberculin skin test was carried out 2 weeks prior to slaughter of these experimentally infected cattle. Cattle exposed to the two higher doses of M. bovis (4 × 105 and 6 × 104 CFU) converted to skin test positivity. Cattle exposed to the lower dose of M. bovis (6 × 103 CFU) remained skin test negative. Euthanasia was carried out at approximately 20 weeks postinfection by intravenous injection of sodium pentobarbitone, and a detailed postmortem analysis was performed. Skin test-positive animals all presented with gross tuberculous lesions typical of natural bovine tuberculosis. Lymph nodes affected included the lateral and medial retropharyngeal, submandibular, bronchial, and mediastinal lymph nodes. Lung lesions were also identified. Lesions were confirmed as tuberculous by histopathological staining and M. bovis culture from affected tissues. No lesions were identified in either of the skin test-negative animals. Acid-fast bacilli could not be found in any tissues from these two low-dose-infected animals, which were also M. bovis culture negative. In addition to M. bovis-infected animals, we utilized a group of eight M. bovis BCG-vaccinated cattle in our final experiment. These animals were Fresian cows from tuberculosis-free herds that had been vaccinated subcutaneously with two doses (8 weeks apart) of 106 CFU of M. bovis BCG-Pasteur. The experiment described was carried out with these animals 2 weeks after their second dose of BCG-Pasteur, when strong specific immune responses (IFN-γ and proliferation) were still apparent.
Immunohistochemistry.
Lymph nodes (left bronchial and left medial retropharyngeal in this investigation) were removed at postmortem, cut into approximately 0.3- by 0.5- by 1.0-cm pieces, fixed in 10% buffered formalin, and processed and embedded in paraffin wax by standard histological procedures. Four-micrometer sections were cut and stored at 4°C until required. Sections were brought to room temperature and dewaxed in xylene (three times for 6 min) followed by absolute ethanol (two times for 6 min). Endogenous peroxidase was blocked by incubation in 1% H2O2 in methanol for 15 min. The sections were then rinsed in running water for 5 min before incubation in 1% Triton X-100 (Merck, Poole, Dorset, United Kingdom) in water for 90 s. Sections were rinsed again in running water for 5 min and finally washed twice in Tris-buffered saline (TBS). Epitope demasking was carried out by incubating sections in a 50:50 mix of trypsin-chymotrypsin (Sigma, Poole, Dorset, United Kingdom) at a total concentration of 1 mg/ml in 0.1% CaCl2 (pH 7.8) for 10 min at 37°C. The sections were then rinsed in running water for 10 min and washed twice in TBS. Sections were blocked in 2% normal goat serum (NGS) in TBS for 2 h at room temperature. Excess buffer was then blotted off, primary antibody (mouse anti-1,25-D3 [Biogenesis, Poole, Dorset] or normal mouse serum control [Sigma] diluted 1/100 in TBS-2% NGS) was added, and sections were incubated overnight at 4°C. The sections were washed three times in TBS, and biotinylated goat anti-mouse immunoglobulins (Vector Laboratories, Peterborough, United Kingdom) diluted 1/200 in TBS-2% NGS was added and left for 30 min at room temperature. Sections were washed three times in TBS, and 1,25-vitamin D staining was enhanced and visualized by using Vectastain Elite ABC reagent (Vector Laboratories) followed by diaminobenzidine substrate (Vector Laboratories). The sections were rinsed for 5 min in running water and then dehydrated through increasing concentrations of ethanol and cleared in xylene before mounting with Vectamount (Vector Laboratories).
Serum 1,25-vitamin D3.
1,25-D3 in serum samples was measured by using a radiometric assay kit specific for the active vitamin D molecule (IDS Ltd., Tyne and Wear, United Kingdom) according to the manufacturer's instructions. Results were determined with a Wallac gamma counter and expressed as picomolar concentrations.
Lymphocyte proliferation assay.
Peripheral blood mononuclear cells (PBMC) were separated from whole blood by centrifugation on Histopaque-1077 (Sigma). PBMC were washed in Hanks balanced salt solution (HBSS) (Life Technologies, Paisley, Scotland) twice, and viable cells were counted by trypan blue dye exclusion and set to 2 × 106/ml in culture medium (RPMI with Glutamax and 25 mM HEPES [Life Technologies] supplemented with 5% controlled protein supplement replacement 1 [Sigma], 100 U of penicillin per ml, 100 μg of streptomycin [Life Technologies] per ml, MEM nonessential amino acids [Sigma], and 5 × 10−5 M 2-mercaptoethanol [Life Technologies]). A total of 2 × 105 cells per well were incubated in 96-well flat-bottomed plates (Nunc, Life Technologies) in triplicate with antigen (bovine tuberculin purified protein derivative [PPD-B], 10 μg/ml; ESAT-6, 5 μg/ml), concanavalin A (ConA) (5 μg/ml), and medium only as a control. Rat anti-VDR antibody (rat immunoglobulin G2b [IgG2b] ascites; Biogenesis) was titrated into the cultures at final dilutions of 1/100, 1/200, 1/400, and 1/800. The concentration of specific antibody in the ascites was not known, and therefore we used two control rat antibodies diluted as described above: an isotype control rat IgG2b (Biogenesis), also of unknown concentration, and a total rat IgG (Sigma) with a stock concentration of 10 mg/ml (hence the final concentrations following dilution of this IgG control antibody were known to be 100 μg/ml [1/100], 50 μg/ml [1/200], 25 μg/ml [1/400], and 12.5 μg/ml [1/800]). Cultures were incubated for 5 days at 37°C with 5% CO2, pulsed with tritiated thymidine (Amersham, Little Chalfont, United Kingdom) at 1μCi/well, incubated for a further 24 h, and harvested, and results were determined by beta-scintillation counting.
IFN-γ enzyme-linked immunosorbent assay.
IFN-γ was measured in the culture supernatants of PBMC after 6 days of stimulation with PPD-B and in no-antigen controls by using the Bovigam enzyme-linked immunosorbent assay kit (Biocor, AH, Omaha, Nebr.) according to the manufacturer's instructions.
Flow cytometry.
PBMC were incubated at 2 × 106/ml in 5-ml volumes in six-well plates (Costar; Corning Inc., High Wycombe, United Kingdom) in the presence of PPD-B (10 μg/ml) or PPD-B plus anti-VDR antibody at a 1/400 final dilution. On day 6 of the culture, adherent cells were harvested from each well; nonadherent cells were removed by gently resuspending with a pipette, followed by two washes with 5 ml of warm (37°C) culture medium. The medium was removed, and adherent cells were lifted by incubation for 10 to 20 min in 5 ml of nonenzymatic cell dissociation medium (Sigma). Cells were then washed once in HBSS (Gibco, Life Technologies) and divided into the wells of a 96-well V-bottom plate (Nunc, Life Technologies) for staining with monoclonal antibodies. The cells were washed once, and cell pellets were suspended in 30 μl of primary antibodies for CD80 (IL-A159) and CD86 (IL-A190) (both provided by N. MacHugh, Centre for Tropical Medicine, Roslin, United Kingdom.), CD40, major histocompatibility complex (MHC) class I (IL-A88), and MHC class II (CC-158). The isotype control antibodies used were TRT-1 (IgG1) and TRT-3 (IgG2a). All antibodies (except IL-A159 and IL-A190) were produced at the Institute for Animal Health. Antibodies were diluted in HBSS containing 1% bovine serum albumin (BSA) (Sigma) and 0.1% sodium azide (Sigma) where appropriate. Cells were incubated with the primary antibodies for 30 min on ice, washed once in HBSS-BSA-azide, and then resuspended in 30 μl of fluorescein isothiocyanate-conjugated sheep anti-mouse immunoglobulins (Vector Laboratories) diluted 1/50 in HBSS-BSA-azide for 30 min on ice. The cells were then washed once, resuspended in 4% paraformaldehyde-phosphate-buffered saline, and stored at 4°C until analysis with a FACScan and CellQuest software (Becton Dickinson, Oxford, United Kingdom). The percent positive staining for each surface marker in each animal for each culture condition (i.e., PPD-B only or PPD-B plus anti-VDR) was then generated by subtracting the percent background isotype control staining from the specific staining for each surface marker.
Statistics.
Paired t tests were used for the data in Fig. 4 to compare the inhibition of proliferation using VDR antibody with the antibody controls (IgG2b and IgG). Linear regression to measure correlation coefficients was carried out for the data in Fig. 5 and 6. The data were found to follow a Gaussian distribution and were therefore analyzed by using Pearson's product moment correlation. All statistical analyses were carried out with Prism software (GraphPad Software Inc., San Diego, Calif.) using 95% confidence intervals.
FIG. 4.
Inhibition of PBMC proliferation of seven animals (six experimentally infected animals and one naturally exposed field reactor) to antigen (PPD-B and ESAT-6) and mitogen (ConA) by the VDR antibody. Anti-VDR and control antibodies were used at a final dilution of 1/400 in PBMC cultures. The results are expressed as the mean (plus standard deviation) inhibition of proliferation compared to cultures of PBMC plus antigen or mitogen (no antibody). The VDR antibody (solid bars) significantly inhibited the proliferative response of PBMC to both PPD-B and ESAT-6 compared with either control antibody (IgG2b [shaded bars] or IgG [open bars]) (P < 0.0001 in all cases).
FIG. 5.
Correlation between inhibition of proliferation and inhibition of IFN-γ production in PPD-B-stimulated PBMC cultures in which anti-VDR (1/400 final dilution) was included. Each dot represents one experimentally infected animal. The data show a highly significant positive correlation (r2 = 0.9254; P = 0.0088) between the inhibition of proliferation and IFN-γ production caused by anti-VDR in these animals.
FIG. 6.
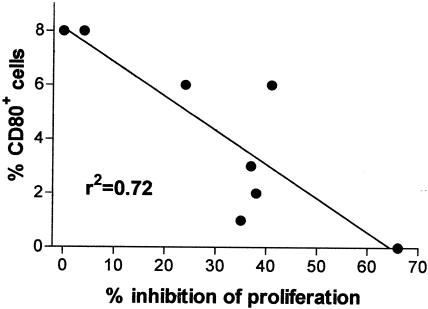
Correlation between percentage of CD80+ cells in the adherent population of PBMC and proliferation of the PBMC cultures after 6 days of incubation with PPD-B and VDR antibody (at a 1/400 final dilution). Each dot represents one BCG-vaccinated animal. The data show a significant negative correlation (r2 = 0.72; P = 0.0079) between the proportion of CD80+ accessory cells present and the level of proliferation.
RESULTS
Rapid mobilization of serum 1,25-D3 following infection.
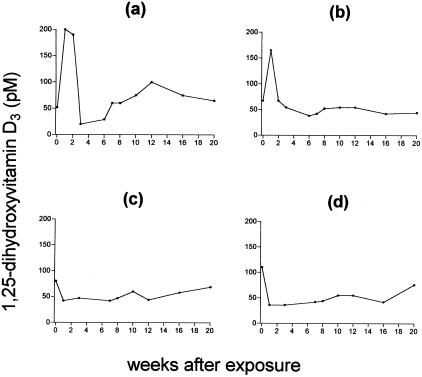
Figure 1 shows the serum 1,25-D3 concentrations in four animals prior to (week 0) and following exposure to virulent M. bovis. Each graph shows the results for one animal, monitored over a 20-week period. Figure 1a and b show the results for the two animals exposed to 6 × 104 CFU of M. bovis, which are representative of those for all six skin test-positive experimentally infected animals in this study. Figure 1c and d show the results for the two animals exposed to the lowest dose of 6 × 103 CFU of M. bovis, which remained skin test negative. The prechallenge serum 1,25-D3 levels ranged from 52 to 138 pM between individuals. Following infection, serum 1,25-D3 increased in all skin test-positive animals (peak levels of 132 to 200 pM within the group). This rapid increase in 1,25-D3 was transient, lasting 1 to 2 weeks, after which time the levels of 1,25-D3 dropped and gradually returned to preexposure levels. In contrast, the two animals exposed to the lowest dose (6 × 103 CFU) of M. bovis and that had remained skin test negative (Fig. 1c and d) did not exhibit such an increase in serum 1,25-D3, but instead the levels remained relatively stable (Fig. 1c) or even appeared to decrease (Fig. 1d) following exposure to M. bovis. Collectively, these results suggest a rapid mobilization of 1,25-D3 following exposure of cattle to M. bovis that can be detected in serum early (0 to 2 weeks) after infection, indicating a role for 1,25-D3 at the onset of host reactivity. However, the role of 1,25-D3 may be affected by the pathogen dose.
FIG. 1.
Serum 1,25-D3 concentrations in four individual cattle prior to and following exposure to M. bovis. (a and b) Cattle exposed to 6 × 104 CFU; (c and d) cattle exposed to 6 × 103 CFU. Concentrations in serum were measured with a commercial radiometric assay.
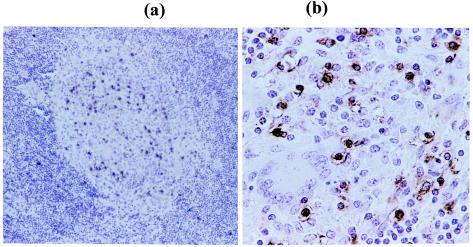
Lymphocytes within the granuloma produce 1,25-D3.
Figure 2 shows the immunohistochemical staining of an infected left bronchial lymph node section from one representative animal at magnifications of ×20 (Fig. 2a) and ×100 (Fig. 2b). Figure 2a shows that 1,25-D3-positive cells (stained brown) were concentrated almost exclusively within the granuloma, with very few 1,25-D3-positive cells present in the surrounding lymphoid tissue. Figure 2b shows these 1,25-D3-positive cells to be mononuclear cells. This concentration of 1,25-D3-positive cells within the granuloma was found in all animals investigated (six experimentally infected animals) and in all granulomas, large or small, suggesting that 1,25-D3 has an important role in granuloma development and/or maintenance in bovine tuberculosis.
FIG. 2.
Sections of lymph node tissue (left bronchial) from one representative animal stained with a monoclonal antibody to 1,25-D3. Sections show a granuloma at magnifications of ×20 (a) and ×100 (b). 1,25-D3-positive mononuclear cells are stained brown.
VDR ligation down-regulates T-cell responses.
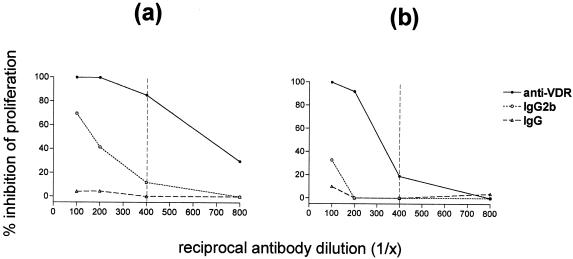
We next investigated the effects of 1,25-D3 on peripheral T-cell responses in both experimentally infected and naturally exposed animals. To exert its effect, 1,25-D3 must bind and activate the nuclear VDR. This allows the mobilization of the VDR into the nucleus, where it binds to specific response elements within the promoter regions of target genes (9). We found that a commercially available monoclonal antibody raised against the VDR acted as an agonist, in that this specific VDR antibody inhibited lymphocyte proliferation in a dose-dependent manner. Figure 3 shows the increased inhibition of PBMC proliferation of a naturally exposed field reactor animal with increased concentration (lower dilution) of VDR antibody. Two different rat antibody controls were incorporated in these experiments, an isotype-matched IgG2b and a total IgG (see Materials and Methods) used at the same dilutions as the anti-VDR antibody. Figure 3a and b show the effect of VDR antibody on proliferation to the dominant mycobacterial antigen ESAT-6 and the mitogen ConA, respectively. These results show that the inhibition by the VDR antibody is dependent upon antibody concentration within the culture, as the effect titrates out with increased dilution. It can be seen that at a dilution of 1/400, the inhibition of antigen-specific proliferation remains high (>85%) while the inhibition of mitogenic proliferation is low (<20%). The inhibitory effects of control antibodies were minimal in comparison at this antibody dilution. We therefore chose 1/400 for subsequent experiments using this antibody.
FIG. 3.
Inhibition of PBMC proliferation to ESAT-6 (a) and ConA (b) by a monoclonal antibody to the VDR. Antigen and mitogen concentrations were both 5 μg/ml. Antibodies were at final dilutions of 1/100, 1/200, 1/400, and 1/800. The anti-VDR antibody (IgG2b) was of unknown concentration, and therefore two control antibodies were used: an IgG2b isotype control (also of unknown concentration) and a total IgG control of known concentration (12.5 μg/ml at 1/800, 25 μg/ml at 1/400, 50 μg/ml at 1/200, and 100 μg/ml at 1/100). Results are expressed as the percent inhibition of proliferation compared to cultures of PBMC plus antigen or mitogen (no antibody). The vertical dashed lines indicate the chosen dilution of antibody (1/400) for subsequent experiments.
The inhibitory effect of the VDR antibody was then tested in a group of seven infected animals. Figure 4 shows the mean inhibition of proliferation induced by the VDR antibody in six experimentally infected animals plus the M. bovis field reactor (see above) to PPD-B, ESAT-6, and ConA. Antigen-specific responses were effectively blocked by the VDR antibody (>95% inhibition by antibody at a 1/400 final dilution), while mitogenic responses were barely affected (the mean represents one animal at 39% inhibition plus six animals with zero inhibition of ConA proliferation). Inhibition of antigen (PPD-B or ESAT-6)-specific proliferation by the VDR antibody was statistically significant compared with that by either the IgG2b or IgG control antibody (P < 0.0001 in all cases).
In a repeat experiment using five experimentally infected animals, we also confirmed the inhibitory effect of the VDR antibody on PPD-B-induced IFN-γ production. VDR antibody was again used at 1/400. Figure 5 shows the correlation between the inhibition of proliferation and the inhibition of IFN-γ production by PBMC in these animals. In this experiment the inhibition caused by the VDR antibody was more variable than that in the previous experiment with the same animals, with ranges of inhibition of 0 to 84% for proliferation and 24 to 96% for IFN-γ. However, a significant positive correlation (r2 = 0.9254; P = 0.0088) was observed between the inhibition of proliferation and inhibition of IFN-γ production mediated by the VDR antibody, demonstrating the potential of vitamin D to interfere with antigen-specific type 1 responses in these animals.
VDR ligation modifies CD80 expression on antigen-presenting cells.
In the light of the results obtained above, i.e., inhibition of antigen-specific rather than mitogenic responses, we reasoned that the effects of the VDR antibody were indirect, via the antigen-presenting cell. We therefore investigated the effect(s) of the VDR antibody upon MHC and costimulatory molecule expression by accessory cells within antigen-stimulated PBMC. This last experiment was carried out with PBMC from a group of eight BCG-vaccinated cattle, as M. bovis-infected cattle were not available at this time. These animals were all responding well to PPD-B, and PPD-B-specific PBMC proliferation could be inhibited by the VDR antibody as shown for M. bovis-infected animals above. PBMC were cultured in bulk in six-well plates, with PPD-B in the presence or absence of a 1/400 dilution of VDR antibody. After 6 days, the adherent cells were harvested, washed, and labeled with monoclonal antibodies specific for the surface molecules MHC class I, MHC class II, CD80, CD86, and CD40. Percent positive staining was calculated by using isotype-matched control antibodies.
Figure 6 shows the correlation between PBMC proliferation to PPD-B and the percentage of CD80-positive cells within the adherent cell population of PPD-B-stimulated PBMC following culture with VDR antibody. Proliferation inhibition by VDR antibody varied in this group of animals from zero to 66%. The percentage of CD80-positive cells within the adherent cell population, after subtracting the background staining of the isotype-matched control antibody (background staining was variable between individual animals), was low, and ranged from zero to 8%. Nevertheless, these data clearly show a significant correlation (r2 = 0.72; P = 0.0079) between the percentage of CD80-positive adherent cells and the level of proliferation within PBMC cultures, with the greatest proliferation (lowest inhibition) in those cultures with the highest percentage of CD80-positive cells and the lowest proliferation (greatest inhibition) in those cultures with the lowest percentage of CD80-positive cells. This correlation was not apparent in PPD-B-stimulated cultures that did not contain the VDR antibody. No other correlation was found between PBMC proliferation and any of the other surface markers investigated (MHC class I, MHC class II, CD86, or CD40). This experiment suggests that CD80 expression by accessory cells is important for antigen-specific T-cell responses and that the expression of CD80 by accessory cells can be modified by the activation of the vitamin D pathway.
DISCUSSION
In this study we have shown that the bioactive component of vitamin D, 1,25-D3, is rapidly mobilized following infection of cattle with M. bovis. This mobilization was identified as a transient increase in serum 1,25-D3 within 2 weeks of infection in those animals that went on to develop tuberculous lesions. A similar transient increase in plasma 1,25-D3 (mean increase from 37 to 118 pM) has been reported for postparturient dairy cattle (2) coincident with an increased susceptibility to other infections, such as mastitis, metritis, and paratuberculosis (24). Interestingly, dietary supplements of vitamin D and vitamin A (which is known to enhance the effects of vitamin D) are recommended for postparturient dairy cattle to prevent hypocalcaemia (50). Whether such supplements, combined with the increased 1,25-D3 levels in these animals at this time, contribute to a lowered resistance to infection is an interesting question. Further to this, we found that two animals that were exposed to a lower dose of M. bovis and that remained disease free did not display any increase in serum 1,25-D3 following exposure to M. bovis. We know that these animals were infected, however, as specific IL-4 responses, but no consistent IFN-γ responses, were detected (38).
The observed changes in serum 1,25-D3 occurred at least 2 weeks before specific immune responses (IFN-γ, IL-2, and proliferation) generally begin to be detected in this infection model (37), indicating a role for 1,25-D3 at the initiation of host immune reactivity to M. bovis. This role could conceivably include effects on cellular recruitment via modulation of chemokine production (29) and/or effects on early T-cell polarization (46) or apoptosis (14).
In those animals that went on to develop tuberculous lesions, 1,25-D3-positive mononuclear cells were identified within every granuloma, clearly placing 1,25-D3 at the heart of pathogenesis. Although we did not identify the cell type, previous reports have described the production of 1,25-D3 by local and peripheral CD8+ T cells (11) and the expression of the VDR on both CD4+ and CD8+ T cells in human tuberculosis (7). In addition to the above-mentioned activities of 1,25-D3 in cellular recruitment and polarization, which could be important within the granuloma, 1,25-D3 is also known to control the monokines transforming growth factor β (27) and tumor necrosis factor alpha (16), both of which have been identified in human tuberculous granulomas (4, 21). Furthermore, 1,25-D3 is known to activate monocytes and macrophages to kill intracellular mycobacteria (17, 19, 41, 42), at least in part via the induction of nitric oxide. This suggests that the function of 1,25-D3 within the tuberculous lesion lies in the construction and maintenance of the granuloma and the dampening of potentially harmful inflammatory T-cell responses, while at the same time activating macrophages to kill intracellular bacilli.
We further investigated the ability of 1,25-D3 to affect specific T-cell responses. The suppression of antigen-specific and mitogenic bovine T-cell responses by 1,25-D3 has previously been described (3), and a more recent study indicated that this inhibitory activity of 1,25-D3 was also to be found in cattle infected with M. bovis (48). We attempted to clarify this effect of 1,25-D3 in our M. bovis-infected cattle by using a monoclonal antibody raised against the VDR as a receptor agonist. We found that addition of VDR antibody to antigen-stimulated PBMC cultures could profoundly depress antigen-specific proliferation and IFN-γ production but had a far lesser effect upon mitogenic proliferation. We therefore hypothesized that the observed effects of the VDR antibody on T-cell responses in these animals were indirect, acting via the antigen-presenting cell by affecting either antigen-presenting (MHC class I and MHC class II) or costimulatory (CD80, CD86, and CD40) molecules.
We investigated the presence of these molecules by flow cytometry on peripheral blood monocytes (plastic-adherent cells) isolated from PBMC following stimulation with antigen, with or without the addition of the VDR antibody. We found that the addition of the VDR antibody modified the expression of CD80 on accessory cells, such that a significant correlation emerged between the percentage of CD80-positive cells present and the level of PBMC proliferation observed in the individual cultures. These results were surprising given that these accessory cells would have been a heterogeneous population of cells and that the percentages of CD80-positive cells measured within the adherent cell populations were low (although they were similar to levels measured in 3-day plastic-adherent PBMC in other, unrelated experiments [J. Hope, unpublished observations]). Our results therefore clearly suggest that the expression of CD80 by accessory cells is an important factor for bovine antigen-specific T-cell responses and that the modification of the expression of this molecule on accessory cells represents one mechanism by which vitamin D may affect downstream T-cell responses in cattle.
This conclusion is supported by murine studies that have described the inhibition of Th1 cells by CD80 suppression on Langerhans cells (32), the prevention of graft-versus-host disease by the blockade of Th1 responses with monoclonal antibodies to CD80 and CD86 (43), and the correlation between CD80-positive accessory cells and elevated Th1 responses in experimental autoimmune encephalomyelitis (22). In addition, CD80 expression by human and mouse accessory cells has been shown to be crucial for the induction of CD4+ cytotoxic T cells (30). Whether such cytotoxic T cells are active in bovine tuberculosis is yet to be discovered.
In summary, our work on bovine tuberculosis supports a role for 1,25-D3 at the very onset of infection with M. bovis and in the development of pathogenesis. Our data support an anti-inflammatory role for 1,25-D3 in the reduction of Th1 responses, for which the expression of accessory cell CD80 may be important.
Acknowledgments
This work was funded by the Department of Environment, Food and Rural Affairs (DEFRA), United Kingdom.
REFERENCES
- 1.Adams, J. S., O. P. Sharma, M. A. Gacad, and F. R. Singer. 1983. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J. Clin. Investig. 72:1856-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ametaj, B. N., D. C. Beitz, T. A. Reinhardt, and B. J. Nonnecke. 1996. 1,25-Dihydroxyvitamin D3 inhibits secretion of interferon-γ by mitogen- and antigen-stimulated bovine mononuclear leukocytes. Vet. Immunol. Immunopathol. 52:77-90. [DOI] [PubMed] [Google Scholar]
- 3.Ametaj, B. N., B. J. Nonnecke, R. L. Horst, and D. C. Beitz. 2000. Effects of retinoic acid and 1,25-dihydroxyvitamin D3 on IFN-γ secretion by mononuclear leukocytes from nulliparus and postparturient dairy cattle. Int. J. Nutr. Res. 70:92-101. [DOI] [PubMed] [Google Scholar]
- 4.Aung, H., Z. Toossi, S. M. McKenna, P. Gogate, J. Sierra, E. Sada, and E. A. Rich. 2000. Expression of transforming growth factor-β but not tumor necrosis factor-α, interferon-γ and interleukin-4 in granulomatous lung lesions in tuberculosis. Tuberc. Lung Dis. 80:61-67. [DOI] [PubMed] [Google Scholar]
- 5.Bellamy, R. 2000. Identifying genetic susceptibility factors for tuberculosis in Africans: a combined approach using a candidate gene study and a genome-wide screen. Clin. Sci. (Colch). 98:245-250. [PubMed] [Google Scholar]
- 6.Bhalla, A. K., E. P. Amento, T. L. Clemens, M. F. Holick, and S. M. Krane. 1983. Specific high affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J. Clin. Endocrinol. 57:1308-1310. [DOI] [PubMed] [Google Scholar]
- 7.Biyoudi-Vouenze, R., J. Cadranel, D. Valeyre, B. Milleron, A. J. Hance, and P. Soler. 1991. Expression of 1,25(OH)2D3 receptors on alveolar lymphocytes from patients with pulmonary granulomatous diseases. Am. Rev. Respir. Dis. 143:1376-1380. [DOI] [PubMed] [Google Scholar]
- 8.Boonstra, A., F. J. Barrat, C. Crain, V. L. Heath, H. F. Savelkoul, and A. O'Garra. 2001. 1α,25-Dihydroxyvitamin D3 has a direct effect on naïve CD4+ T cells to enhance the development of Th2 cells. J. Immunol. 167:4974-4980. [DOI] [PubMed] [Google Scholar]
- 9.Bouillon, R., W. H. Okamura, and A. W. Norman. 1995. Structure-function relationships in the vitamin endocrine system. Endocrinol. Rev. 16:200-257. [DOI] [PubMed] [Google Scholar]
- 10.Buddle, B. M., D. Keen, A. Thomson, G. Jowett, J. Heslop, G. W. deLisle, and J. L. Stanford. 1995. Protection of cattle from bovine tuberculosis by vaccination with BCG by the respiratory or subcutaneous route, but not by vaccination with killed Mycobacterium vaccae. Res. Vet. Sci. 59:10-16. [DOI] [PubMed] [Google Scholar]
- 11.Cadranel, J., M. Garabedian, B. Milleron, H. Guilozo, G. Akoun, and A. J. Hance. 1990. 1,25(OH)2D3 production by T lymphocytes and alveolar macrophages recovered by lavage from normocalcemic patients with tuberculosis. J. Clin. Investig. 85:1588-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canning, M. O., K. Grotenhuis, H. de Wit, C. Ruwhof, and H. A. Drexhage. 2001. 1-α,25-Dihydroxyvitamin D3 (1,25(OH)2D3) hampers the maturation of fully active immature dendritic cells from monocytes. Eur. J. Endocrinol. 145:351-357. [DOI] [PubMed] [Google Scholar]
- 13.Chan, T. Y. K. 1999. Seasonal variation in vitamin-D status and the incidence of tuberculosis in different countries. Respiration 66:196. [DOI] [PubMed] [Google Scholar]
- 14.Cippitelli, M., C. Fionda, D. Di Bona, F. Di Rosa, A. Lupo, M. Piccoli, L. Frati, and A. Santoni. 2002. Negative regulation of CD95 ligand gene expression by vitamin D3 in T lymphocytes. J. Immunol. 168:1154-1166. [DOI] [PubMed] [Google Scholar]
- 15.Cippitelli, M., and A. Santoni. 1998. Vitamin D3: a transcriptional modulator of the interferon-gamma gene. Eur. J. Immunol. 28:3017-3030. [DOI] [PubMed] [Google Scholar]
- 16.Cohen, M. L., A. Douvdevani, C. Chaimovitz, and S. Shany. 2001. Regulation of TNF-alpha by 1 alpha, 25-dihydroxyvitamin D3 in human macrophages from CAPD patients. Kidney Int. 59:69-75. [DOI] [PubMed] [Google Scholar]
- 17.Crowle, A. J., E. J. Ross, and M. H. May. 1987. Inhibition by 1,25(OH)2-vitamin D3 of the multiplication of virulent tubercle bacilli in cultured human macrophages. Infect. Immun. 55:2945-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies, P. D. O. 1985. A possible link between vitamin D deficiency and impaired host defence to Mycobacterium tuberculosis. Tubercle 66:301-306. [DOI] [PubMed] [Google Scholar]
- 19.Dhama, K., M. P. Bansal, and G. C. Ram. 1998. In vitro evaluation of nitrite production and intracellular killing activities of bovine peripheral blood monocytes pulsed with Mycobacterium bovis BCG. Indian Vet. J. 75:1075-1078. [Google Scholar]
- 20.Douglas, A. S., D. P. Strachan, and J. D. Maxwell. 1996. Seasonality of tuberculosis: the reverse of other respiratory diseases in the UK. Thorax 51:944-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fenhalls, G., A. Wong, J. Bezuidenhout, P. van Helden, P. Bardin, and P. T. Lukey. 2000. In situ production of gamma interferon, interleukin-4, and tumor necrosis factor alpha mRNAs in human lung tuberculous granulomas. Infect. Immun. 68:2827-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genc, K., D. L. Dona, and A. T. Reder. 1997. Increased CD80(+) B cells in active multiple sclerosis and reversal by interferon beta-1b therapy. J. Clin. Investig. 99:2664-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregori, S., M. Casorati, S. Amuchastegui, S. Smiroldo, A. M. Davalli, and L. Adorini. 2001. Regulatory T cells induced by 1α,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplant tolerance. J. Immunol. 167:1945-1953. [DOI] [PubMed] [Google Scholar]
- 24.Jakobsen, M. B., L. Alban, and S. S. Nielsen. 2000. A cross-sectional study of paratuberculosis in 1155 Danish dairy cows. Prev. Vet. Med. 46:15-27. [DOI] [PubMed] [Google Scholar]
- 25.Jordan, S. C., M. Toyoda, J. Prehn, J. M. Lemire, R. Sakai, and J. S. Adams. 1989. Interleukin-2 and interleukin-2 receptor levels and gene expression in human T cells. Mol. Immunol. 26:979-984. [DOI] [PubMed] [Google Scholar]
- 26.Koeffler, H. P., H. Reichel, J. E. Bishop, and A. W. Norman. 1985. Gamma-interferon stimulates production of 1,25-dihydroxyvitamin D3 by normal human macrophages. Biochem. Biophys. Res. Commun. 127:596-603. [DOI] [PubMed] [Google Scholar]
- 27.Koli, K., J. Saharinen, M. Hyytiainen, C. Penttinen, and J. Keski-Oja. 2001. Latency, activation and binding proteins of TGF-beta. Microsc. Res. Technol. 52:354-362. [DOI] [PubMed] [Google Scholar]
- 28.Krebs, J. R., R. M. Anderson, T. Clutton-Brock, W. I. Morrison, D. Young, and C. Donnely. 1997. Bovine tuberculosis in cattle and badgers. Report to the Rt. Hon. Dr. Jack Cunningham M. P. MAFF Publications, London, United Kingdom.
- 29.Kruger, S., and B. Kreft. 2001. 1,25-Dihydroxyvitamin D3 differentially regulates IL-1α-stimulated IL-8 and MCP-1 mRNA expression by human proximal tubular epithelial cells. Exp. Nephrol. 9:223-228. [DOI] [PubMed] [Google Scholar]
- 30.Mauri, D., and W. J. Pichler. 1996. Involvement of CD80 in the generation of CD4+ cytotoxic T cells. Immunol. Res. 15:126-140. [DOI] [PubMed] [Google Scholar]
- 31.Monkawa, T., T. Yoshida, M. Hayashi, and T. Saruto. 2000. Identification of 25-hydroxyvitamin D3 1-alpha-hydroxylase gene expression in macrophages. Kidney Int. 58:559-568. [DOI] [PubMed] [Google Scholar]
- 32.Ozawa, H., S. Aiba, S. Nakagawa, and H. Tagami. 1996. Interferon-γ and interleukin-10 inhibit antigen presentation by Langerhans cells for T helper type 1 cells by suppressing their CD80 (B7-1) expression. Eur. J. Immunol. 26:648-652. [DOI] [PubMed] [Google Scholar]
- 33.Penna, G., and L. Adorini. 2000. 1α,25-Dihydroxyvitamin D3 inhibits differentiation, maturation, activation and survival of dendritic cells leading to impaired alloreactive T cell activation. J. Immunol. 164:2405-2411. [DOI] [PubMed] [Google Scholar]
- 34.Piemonti, L., P. Monti, M. Sironi, P. Fraticelli, B. E. Leone, E. D. Cin, P. Allavena, and V. DiCarlo. 2000. Vitamin D3 affects differentiation, maturation and function of human monocyte-derived dendritic cells. J. Immunol. 164:4443-4451. [DOI] [PubMed] [Google Scholar]
- 35.Pritchard, D. G. 1988. A century of bovine tuberculosis 1888-1988: conquest and controversy. J. Comp. Pathol. 15:651-663. [DOI] [PubMed] [Google Scholar]
- 36.Provvedini, D. M., C. D. Tsoukas, L. J. Deftos, and S. C. Manolagas. 1983. 1,25-Dihydroxyvitamin D3 receptors in human leukocytes. Science 221:1181-1183. [DOI] [PubMed] [Google Scholar]
- 37.Rhodes, S. G., D. Gavier-Widen, B. M. Buddle, A. O. Whelan, M. Singh, R. G. Hewinson, and H. M. Vordermeier. 2000. Antigen specificity in experimental bovine tuberculosis. Infect. Immun. 68:2573-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rhodes, S. G., N. Palmer, S. P. Graham, A. E. Bianco, R. G. Hewinson, and H. M. Vordermeier. 2000. Distinct response kinetics of gamma interferon and interleukin-4 in bovine tuberculosis. Infect. Immun. 68:5393-5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rigby, W. F. C., M. Waugh, and R. F. Graziano. 1990. Regulation of human monocyte HLA-DR and CD4 antigen expression and antigen presentation by 1,25-dihydroxyvitamin D3. Blood 1:189-197. [PubMed] [Google Scholar]
- 40.Rigby, W. F. C., B. Yirinec, R. L. Oldershaw, and M. W. Fanger. 1987. Comparison of the effects of 1,25-dihydroxyvitamin D3 on T lymphocyte subpopulations. Eur. J. Immunol. 17:563-566. [DOI] [PubMed] [Google Scholar]
- 41.Rockett, K. A., R. Brookes, I. Udalova, V. Vidal, A. V. S. Hill, and D. Kwiatkowski. 1998. 1,25-Dihydroxyvitamin D3 induces nitric oxide synthase and suppresses the growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect. Immun. 66:5314-5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rook, G. A. W., J. Steele, L. Fraher, S. Barker, P. Karmali, J. O'Riordan, and J. Stanford. 1986. Vitamin D3, gamma-interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology 57:159-163. [PMC free article] [PubMed] [Google Scholar]
- 43.Saito, K., H. Yagita, H. Hashimoto, K. Okumura, and M. Azuma. 1996. Effect of CD80 and CD86 blockade and anti-interleukin-12 treatment on mouse acute graft-versus-host disease. Eur. J. Immunol. 26:3098-3106. [DOI] [PubMed] [Google Scholar]
- 44.Selvaraj, P., S. M. Kurian, H. Uma, and P. R. Narayanan. 2000. Influence of non-MHC genes on lymphocyte response to Mycobacterium tuberculosis antigens and tuberculin reactive status in pulmonary tuberculosis. Indian J. Med. 112:86-92. [PubMed] [Google Scholar]
- 45.Selvaraj, P., P. R. Narayanan, and A. M. Reetha. 2000. Association of vitamin D receptor genotypes with the susceptibility to pulmonary tuberculosis in female patients and resistance in female contacts. Indian J. Med. Res. 111:172-179. [PubMed] [Google Scholar]
- 46.Staeva-Vieira, T., and L. P. Freedman. 2002. 1,25-Dihydroxyvitamin D3 inhibits IFN-γ and IL-4 levels during in vitro polarization of primary murine CD4+ T cells. J. Immunol. 168:1181-1189. [DOI] [PubMed] [Google Scholar]
- 47.Veldman, C. M., M. T. Cantorna, and H. F. DeLuca. 2000. Expression of 1,25-dihydroxyvitamin D3 receptor in the immune system. Arch. Biochem. Biophys. 374:334-338. [DOI] [PubMed] [Google Scholar]
- 48.Waters, W. R., B. J. Nonnecke, T. E. Rahner, M. V. Palmer, D. L. Whipple, and R. L. Horst. 2001. Modulation of Mycobacterium bovis-specific responses of bovine peripheral blood mononuclear cells by 1,25-dihydroxyvitamin D3. Clin. Diagn. Lab. Immunol. 8:1204-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wecksler, W. R., and A. W. Norman. 1980. Biochemical properties of 1,25 dihydroxyvitamin D3 receptors. J. Steroid Biochem. 13:977-989. [DOI] [PubMed] [Google Scholar]
- 50.Weiss, W. P. 1998. Requirements of fat-soluble vitamins for dairy cows: a review. J. Dairy Sci. 81:2493-2501. [DOI] [PubMed] [Google Scholar]
- 51.Wilkinson, R. J., M. Llewelyn, Z. Toossi, P. Patel, G. Pasvol, A. Lalvani, D. Wright, M. Latif, and R. N. Davidson. 2000. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet 355:618-621. [DOI] [PubMed] [Google Scholar]