Abstract
To elucidate the pathological roles of staphylococcal enterotoxin C (SEC) in bovine staphylococcal mastitis, a histopathological analysis of SEC-inoculated mammary glands was performed. SEC-inoculated mammary glands exhibited interstitial inflammation, and the leukocytes that migrated into the gland were predominantly polymorphonuclear neutrophils (PMN). In the gland cistern tissues dissected from SEC-inoculated mammary glands, epithelial cellular degeneration was observed. We also investigated the physiological effects of SEC on PMN in vitro. PMN migration was induced by culture supernatant of SEC-stimulated peripheral blood mononuclear cells (S-PBMC sup) but not by that of nonstimulated PBMC (N-PBMC sup). The concentration of interleukin-8 was significantly (P < 0.05) higher in S-PBMC sup than N-PBMC sup, and a significantly (P < 0.05) higher mRNA expression of growth-regulated oncogenes was detected in SEC-stimulated PBMC than in nonstimulated PBMC. Milk PMN collected from SEC-inoculated mammary glands produced more than 2 times the amount of superoxide at 1 day postinoculation (dpi) than at 0 dpi in the presence of phorbol 12-myristate 13-acetate (PMA). PMN cultured with S-PBMC sup for 24 h also produced significantly (P < 0.05) larger amounts of superoxide than those cultured with N-PBMC sup in the presence of PMA. Moreover, S-PBMC sup induced the long-time survival of PMN. These results indicate that SEC induces the activation of PMN via the stimulation of mononuclear cells.
In the dairy industry, staphylococcal mastitis causes enormous economic losses through discards of contaminated milk and reductions of milk yields (6). In many cases, staphylococci cause subclinical and/or chronic mastitis because of persistent intramammary infection (33). Staphylococcal enterotoxins (SEs), which are well-known causative agents of staphylococcal food poisoning, show superantigenic activity for various mammalian T lymphocytes and major histocompatibility complex (MHC) class II-positive cells (11, 15, 37). Superantigens exhibit mitogenic activity for T lymphocytes, which express specific Vβ molecules of the T-cell receptor in the presence of MHC class II molecules and induce the production of various inflammatory cytokines (11, 15, 37). Because of their strong immunomodulating activity, SEs have been examined for pathological roles in various diseases, such as toxic shock syndrome (15). Although geographical variation exists, many staphylococci isolated from bovine mastitis produce SEs, especially SEC (16, 18). Therefore, SEC is considered to be one of the most important pathological agents in staphylococcal mastitis. We previously reported that the concentration of SEC in milk significantly correlates with the severity of mastitis, and acute and significant increases of somatic cell counts (SCC) in milk were induced by the intramammary inoculation of SEC (16).
The increase of SCC that accompanies mastitis is caused mainly by the migration of polymorphonuclear neutrophils (PMN) into mammary glands (26). Many chemotactic factors, such as interleukin-8 (IL-8), leukotriene B4 (LTB4), and complement fragment C5a, are detected in milk collected from animals with naturally occurring and experimentally induced mastitis (26, 27, 29, 31). These chemotactic factors are produced in response to bacterial toxins, such as SEs and lipopolysaccharide, of gram-negative bacteria (17, 20, 32).
The PMN that migrate to inflammatory sites produce several bactericidal and cytocidal agents (3). Oxygen metabolites, such as superoxide, hydrogen peroxide, and hydroxyl radical, are produced by activated PMN and play an important role in inflammation (36). These mechanisms are very important for the host defense; however, excessive activation of PMN induces environmental tissue damage (36). Therefore, the appropriate exclusion of PMN from inflammatory sites is essential for the termination of inflammation.
Several reports on the effects of SEC on bovine T lymphocytes are available (11, 37). On the other hand, investigations of the effects on PMN, major leukocytes that increase in number with mastitis, are important to elucidate the pathological roles of SEC in mastitis. Because of their strong immunomodulatory activity, physiological effects of SEs on PMN are expected, and some reports on indirect effects via the stimulation of mononuclear cells (MNC) are available (21, 30). However, conflicting results on the survival of PMN cocultured with SEs have been reported (21, 30). Therefore, the correlation between SEs and PMN in inflammation has not been clarified.
In this study, we evaluated the histopathological findings for mammary glands inoculated with SEC. Moreover, to elucidate the correlation between SEC and PMN, we investigated the inflammatory responses of PMN induced by SEC in vitro. We discuss the mechanism by which mastitis is caused by SEC.
MATERIALS AND METHODS
Intramammary inoculation of SEC and histopathological analysis.
Intramammary inoculation of SEC was carried out as previously described (16). Either 100 or 5 μg of SEC (Toxin Technology Inc., Sarasota, Fla.) in 10 ml of phosphate-buffered saline (PBS) (pH 7.21) was inoculated into lactating udders with no clinical signs of mastitis from the teat sinus. As a negative control, one udder of each cow was inoculated with PBS alone. For preparation of milk PMN, one udder of each of four lactating Holstein cows was inoculated with 5 μg of SEC. For histopathological analysis, one udder of each of three lactating Holstein cows was inoculated with 100 μg of SEC. Cows were slaughtered at 28 h after inoculation. Mammary gland tissues were dissected from the central area of the upper body of the gland (mammary parenchymal tissue) and the area surrounding the gland cistern. Tissues were fixed in periodate-lysine-paraformaldehyde solution and embedded in paraffin. Sections (3 μm thick) were stained with hematoxylin-eosin.
Microscopic analysis of milk cells and preparation of milk PMN.
Milk cells were collected by the method described by Asai et al. (2). For microscopic analysis, milk cells (2 × 104 to 4 × 104) were deposited on a glass slide by using Cytospin (Shandon Scientific Ltd., Runcorn, Cheshire, England) and stained with Diff-Quick (International Reagents Corp., Kobe, Japan). To separate PMN, the milk cell suspension was layered on Lympholite-H (Cedarlane Laboratories Ltd., Hornby, Canada). After centrifugation at 1,200 × g for 25 min, cell pellet was washed several times with PBS and used as the milk PMN fraction. This procedure consistently yielded PMN with >90% purity as assessed with Diff-Quick stain.
Preparation of PBMC and PMN.
Heparinized blood was obtained from healthy Holstein cows. MNC were isolated by density gradient centrifugation across Lympholyte-H at 1,200 × g for 25 min. Cells at the interface were collected, rid of contaminating erythrocytes by hypotonic treatment, washed several times in PBS, and used as the fraction of peripheral blood mononuclear cells (PBMC). The cell pellet from the density gradient centrifugation was depleted of residual erythrocytes by hypotonic treatment, washed several times in PBS, and cultured for 2 h in culture medium (RPMI 1640 [Nissui Pharmaceutical Co., Ltd., Tokyo, Japan], 2 mM l-glutamine [Kanto Chemical Co., Inc., Tokyo, Japan], 20 U of penicillin per ml, 100 μg of streptomycin [Meiji Seika Kaisha, Ltd., Tokyo, Japan] per ml) supplemented with 10% fetal bovine serum (FBS) (Life Technologies, Rockville, Md.). Nonadherent cells were collected, washed in PBS, and used as the PMN fraction. This procedure consistently yielded PMN with >99% viability as determined by trypan blue dye exclusion and >97% purity as assessed with Diff-Quick stain.
Cultivation of PBMC.
PBMC (106/ml) were suspended in Hanks balanced salt solution (HBSS) supplemented with 0.1% bovine serum albumin (Sigma-Aldrich Fine Chemicals, St. Louis, Mo.) and cultured with or without SEC (1 μg/ml; Toxin Technology Inc.). After 24 h, the culture supernatant was collected. For the reverse transcription-PCR (RT-PCR) assay, cells were cultured for 2 h (for growth-regulated oncogenes [GROs] α, β, and γ) or 4 h (for gamma interferon [IFN-γ] and granulocyte-macrophage colony-stimulating factor [GM-CSF]).
PMN migration assay.
The migration of PMN was assayed with a 24-well cell culture insert (pore size, 3.0 μm; Becton Dickinson, San Jose, Calif.). Each stimulus was added to the lower well, and PMN (5 × 105 in 500 μl of HBSS supplemented with 0.1% bovine serum albumin) were added to the upper insert. Cell migration proceeded at 37°C for 30 min. At the end of the incubation, the insert was washed with PBS and stained with Diff-Quick. The membrane of the insert was mounted on a glass slide, and the PMN that had migrated were counted under a light microscope. A minimum of 300 total cells (migrated and nonmigrated cells) were counted. The extent of migration was expressed as the migration rate, which was calculated as 100 × number of migrated cells/total number of cells counted. Each stimulus was applied in duplicate.
Measurement of the concentrations of IL-8, LTB4, and PGE2.
The concentrations of IL-8, LTB4, and prostaglandin E2 (PGE2) in culture supernatants were measured with commercial enzyme-linked immunosorbent assay kits (IL-8, Genzyme Techne, Minneapolis, Minn.; LTB4 and PGE2, Cayman Chemical Co., Ann Arbor, Mich.).
RT-PCR assay.
Cells were lysed in 1 ml of Isogen (Nippon Gene, Toyama, Japan), and total RNA was extracted as described in the instruction manual. The total RNA was dissolved in 30 μl of diethyl pyrocarbonate-treated water (Nippon Gene) and incubated at 65°C for 10 min. cDNA synthesis was carried out with a first-strand cDNA synthesis kit (Amersham Bioscience Corp., Piscataway, N.J.). PCR was carried out with Ready-To-Go PCR beads (Amersham Bioscience Corp.) and a GeneAmp PCR system 9600 (Perkin-Elmer Corp., Norwalk, Conn.). Specific primer sequences are listed in Table 1. For GROs, 33 PCR cycles were done. The PCR conditions involved denaturation at 95°C for 30 s (the duration for the first cycle was 5 min), annealing at 63°C for 30 s, and extension at 72°C for 1 min. For IFN-γ and GM-CSF, 36 PCR cycles were done. The PCR conditions involved denaturation at 95°C for 30 s (the duration for the first cycle was 5 min), annealing at 55°C for 30 s, and extension at 72°C for 1 min. PCR products were electrophoresed by using 3% agarose (Nusieve 3:1 agarose; BMA, Rockland, Maine). After staining with ethidium bromide, amplified DNA bands were analyzed with a Chemi Imager (Alpha Innotech Corp., San Leadron, Calif.). The mRNA expression levels were expressed as relative units after normalization to the β-actin level.
TABLE 1.
Specific primer sequences used for RT-PCR
| Target gene | Forward primer sequence (5′-3′) | Reverse primer sequence (5′-3′) | PCR product size (bp) | Reference or GenBank accession no. |
|---|---|---|---|---|
| GROα | TTGATACTCCAGCAGCCTCA | CCACTACTGTCTCTTGCCTT | 376 | U95812 |
| GROβ | CTCCGAGCTGAGACTCATGG | GGTTGTCACCTTCACGCTCT | 188 | U95813 |
| GROγ | TCAAGAACATCCAGAGCGTG | AGATGGCCTTAGGAGGTGGT | 268 | 8 |
| IFN-γ | AATGCAAGTAGCCCAGATG | GATCTGCAGATCATCCACCGG | 270 | 14 |
| GM-CSF | ATGTGGCTGCAGAACCTGCTTCTCC | CTTCTGGGCTGGTTCCCAGCAGTCA | 429 | 14 |
| β-Actin | ACGTCGCCTTGGACTTCGAGCAGG | GCTGGAAGGTGGACAGGGAGGCCAGGA | 405 | 14 |
Superoxide production assay.
PMN were suspended (106/ml) in culture medium supplemented with 2.5% FBS. After cultivation with stimulus (10% of SEC-stimulated PBMC culture supernatant [S-PBMC sup] or nonstimulated PBMC culture supernatant [N-PBMC sup]) in 96-well round-bottom culture plates (Becton Dickinson Labware, Franklin Lakes, N.J.) at 37°C with 5% CO2 for 24 h, the culture medium was removed and cells were washed with HBSS. Milk PMN were suspended in HBSS and dispensed in 96-well round-bottom culture plates (2 × 105 cells/well). These cells were preincubated for 15 min at 37°C in the presence or absence of superoxide dismutase (SOD) (140 U/ml; Wako Pure Chemical Industries, Ltd., Osaka, Japan), and then ferricytochrome c (2.4 mg/ml; Wako Pure Chemical Industries, Ltd.) and phorbol 12-myristate 13-acetate (PMA) (1.25 μg/ml; Sigma-Aldrich Fine Chemicals) were added. After further incubation for 60 min at 37°C, the reaction was stopped by placing the plate on ice, and the culture supernatant was collected. The absorbance at 550 nm was read with a microplate reader (Spectra Max; Molecular Devices, Sunnyvale, Calif.). The superoxide production was calculated by subtracting the value with SOD from the value without SOD and using an extinction coefficient for the change in ferricytochrome c to ferrocytochrome c of 21.1/mM/cm. Each stimulus was applied in triplicate.
Measurement of PMN survival rate.
PMN (106/ml) were suspended in culture medium supplemented with 2.5% FBS and cultured with stimulus (10% of S-PBMC sup or N-PBMC sup) at 37°C with 5% CO2 for 24 h. After cultivation, the PMN survival rate was measured by trypan blue dye exclusion. Results are presented as the survival rate (percent) calculated as follows: 100 × number of trypan blue-negative cells/total number of cells counted. Each stimulus was applied in triplicate.
DNA fragmentation assay.
PMN (106/ml) were suspended in culture medium supplemented with 2.5% FBS and cultured with stimulus (10% of S-PBMC sup or N-PBMC sup) at 37°C with 5% CO2 for 13 h. After cultivation, cells were lysed in 100 μl of lysis buffer (50 mM Tris-HCl [pH 7.5], 20 mM EDTA, and 1% NP-40), and the lysate was centrifuged at 1,600 × g for 5 min to select the low-molecular-weight DNA (apoptotic DNA fragments). Seventy microliters of lysis buffer, 20 μl of 10% sodium dodecyl sulfate, and 500 μg of RNase (DNase free; Nippon Gene) per ml were added to centrifugal supernatants. After incubation at 56°C for 2 h, proteinase K (75 U/ml; Wako Pure Chemical Industries, Ltd.) was added and further incubated at 56°C for 2 h. DNA was precipitated by the addition of 600 μl of ethanol followed by centrifugation at 10,000 × g for 5 min and dissolved in 100 μl of TE buffer (10 mM Tris-HCl [pH 8.0] and 1 mM EDTA). DNA fragments were detected by electrophoresis with 1.5% agarose (SeaKem GTG agarose; FMC BioProducts, Rockland, Maine) followed by staining with ethidium bromide.
Endotoxin level of SEC preparation.
The endotoxin level in the SEC preparation was determined with Endospecy ES-50 M Set (Associates of Cape Cod Inc., Falmouth, Mass.). The SEC preparation contained <0.001% (wt/wt) endotoxin.
Statistical analysis.
Data are presented as means ± standard deviations (SD). The results from the PMN migration assay, RT-PCR assay, superoxide production assay, and PMN survival assay were compared by using two-way analysis of variance followed by the Newman-Keuls test. The remaining results were compared by using Student's t test. P values of <0.05 were considered significant.
RESULTS
Histopathological analysis of SEC-inoculated mammary glands.
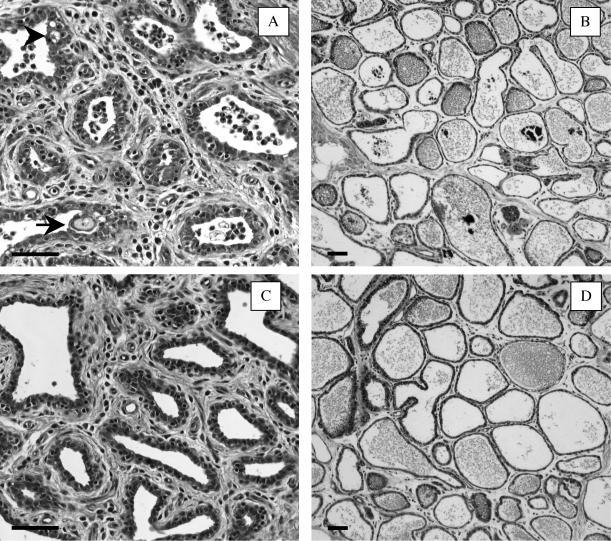
In SEC-inoculated mammary glands, the stromal area of the gland cistern exhibited edema. Leukocyte migration was observed in the stromal area and the ductular lumen (Fig. 1A). The ductular epithelium exhibited invagination and cytoplasmic vacuolation of epithelial cells (Fig. 1A). In PBS-inoculated mammary glands, although a small degree of leukocyte migration in the stromal area was observed, no leukocytosis in the ductular lumen and the epithelial cellular degeneration was observed (Fig. 1C). Microscopic analysis of milk cells demonstrated that the migrated leukocytes were predominantly PMN (Table 2). In SEC-inoculated mammary glands, 85% ± 7.5% of milk cells were PMN, which was significantly (P < 0.05) more than in PBS-inoculated mammary glands (68% ± 4.3%).
FIG. 1.
Photomicrographs of the gland cistern (A and C) and the mammary parenchymal (B and D) tissues dissected from SEC-inoculated (A and B) and PBS-inoculated (C and D) mammary glands. SEC (100 μg) or PBS alone was inoculated into lactating mammary glands, and tissues were collected at 28 h after inoculation. Each photomicrograph is typical of those for three experimental cows. The arrows indicate invaginations of the ductular epithelium. The arrowheads indicate cytoplasmic vacuolations of epithelial cells. Bars, 50 μm.
TABLE 2.
Percentage of each cell type in milk cell fractions prepared from SEC- or PBS-inoculated mammary glands
Mean ± SD from three separate experiments.
Mainly epithelial cells.
Significantly (P < 0.05) different from value for PBS.
In mammary parenchymal tissue from SEC-inoculated mammary glands, the interalveolar stroma exhibited edema (Fig. 1B). A small amount of leukocyte migration was observed in the interalveolar stroma and alveolar lumen. In PBS-inoculated mammary glands, no histopathological changes were observed in mammary parenchymal tissue (Fig. 1D).
Induction of PMN migration by S-PBMC sup in vitro.
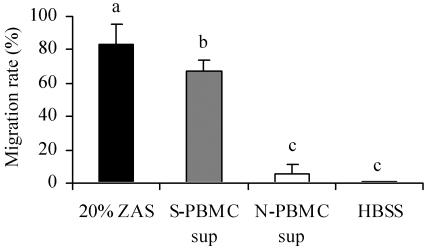
In the preliminary experiments, SEC itself did not induce the migration of PMN (data not shown). Therefore, we analyzed S-PBMC sup. As shown in Fig. 2, the S-PBMC sup induced significantly (P < 0.05) greater PMN migration than the N-PBMC sup. The migration rates with 20% zymosan-activated bovine serum as a positive control and HBSS as a negative control were 83% ± 13% and 0.52% ± 0.33%, respectively.
FIG. 2.
Induction of PMN migration by culture supernatant of PBMC. PMN migration was assayed with a cell culture insert. The migration rate was calculated as 100 × number of cells migrated/total number of cells counted. The results shown are means ± SD from five separate experiments. Values with different letters are significantly different (P < 0.05). ZAS, 20% zymosan-activated bovine serum.
Induction of various chemotactic factors by SEC.
To investigate the mechanism by which S-PBMC sup induces the migration of PMN, the concentrations of IL-8 and LTB4, which are strong chemotactic factors for PMN, in S-PBMC sup and N-PBMC sup were measured (Table 3). The concentration of IL-8 in S-PBMC sup was 170 ± 58 pg/ml, which was significantly (P < 0.05) higher than that in N-PBMC sup (3.6 ± 6.2 pg/ml). LTB4 was also detected at a significantly (P < 0.05) higher concentration in S-PBMC sup (7.2 ± 2.2 pg/ml) than in N-PBMC sup (3.3 ± 0.86 pg/ml).
TABLE 3.
Concentrations of IL-8 and LTB4 in S-PBMC sup and N-PBMC sup
| Supernatant | Concn (pg/ml)a
|
|
|---|---|---|
| IL-8 | LTB4 | |
| S-PBMC sup | 170 ± 58b | 7.2 ± 2.2b |
| N-PBMC sup | 3.6 ± 6.2 | 3.3 ± 0.86 |
Mean ± SD for four separate samples.
Significantly (P < 0.05) higher than value for N-PBMC sup.
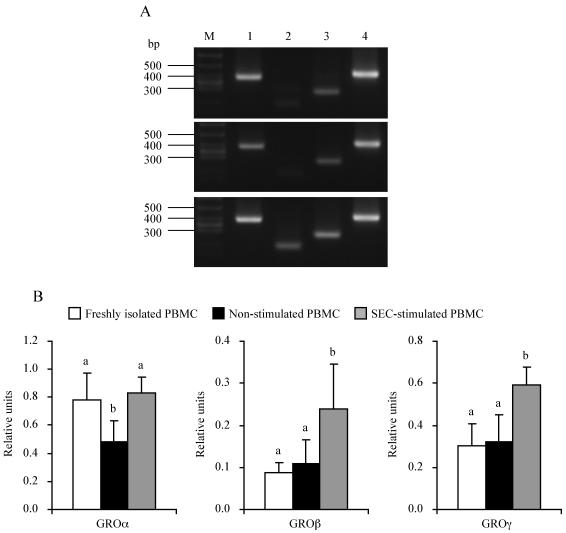
We also investigated the induction of other chemokines by using the RT-PCR method. After stimulation with SEC for 2 h, the expression levels of GROα, -β, and -γ mRNAs were significantly (P < 0.05) higher in SEC-stimulated PBMC than in nonstimulated PBMC (Fig. 3). Especially for GROβ and γ, the expression levels in SEC-stimulated PBMC were more than 2 times higher (P < 0.05) than those in freshly isolated PBMC. In the case of GROα, the expression level in SEC-stimulated PBMC was equal to that in freshly isolated PBMC.
FIG. 3.
GROα, -β, and -γ mRNA levels in SEC-stimulated and nonstimulated PBMC. PBMC were cultured with or without SEC (1 μg/ml) for 2 h. GROα, -β, and -γ mRNA levels were measured by the RT-PCR method. (A) Representative amplification of RT-PCR from freshly isolated (top), nonstimulated (middle), and SEC-stimulated (bottom) PBMC. RT-PCR products were detected by electrophoresis with 3.0% agarose followed by staining with ethidium bromide. Lane M, molecular size marker; lane 1, GROα (376 bp); lane 2, GROβ (188 bp); lane 3, GROγ (268 bp); lane 4, β-actin (405 bp). (B) Relative units after normalization to the β-actin mRNA level. The results shown are means ± SD from three separate experiments. Values with different letters are significantly different (P < 0.05).
Oxidative burst activity of milk PMN collected from SEC-inoculated mammary glands and S-PBMC sup-stimulated PMN.
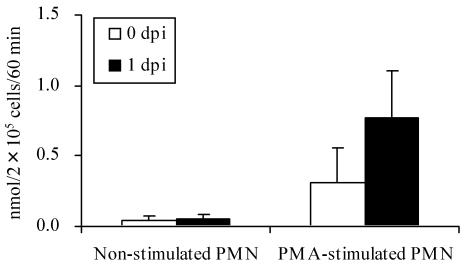
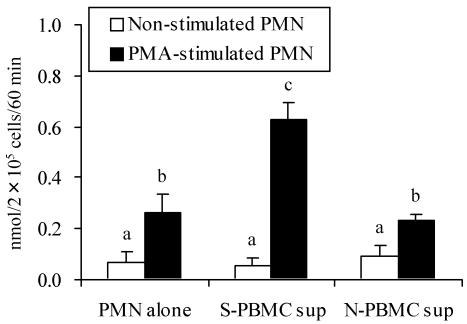
In the presence of PMA, milk PMN collected from SEC-inoculated mammary glands at 1 day postinoculation (dpi) produced more than 2 times the amount of superoxide than at 0 dpi (preinoculation) (Fig. 4). Next, the effect of S-PBMC sup on the oxidative burst activity of peripheral PMN was analyzed. After stimulation for 24 h with S-PBMC sup, PMN responded markedly to PMA, and significantly (P < 0.05) more superoxide was produced than with PMN alone or with N-PBMC sup-stimulated PMN (Fig. 5).
FIG. 4.
Superoxide production by milk PMN collected from SEC-inoculated mammary glands. Milk PMN were collected from SEC-inoculated mammary glands at 0 (preinoculation) and 1 dpi. Superoxide production by nonstimulated and PMA-stimulated cells was measured. The results shown are means ± SD for four separate glands.
FIG. 5.
Superoxide production by PMN stimulated with culture supernatant of PBMC. PMN were cultured with S-PBMC sup or N-PBMC sup at 37°C for 24 h. After cultivation, superoxide production by nonstimulated and PMA-stimulated cells was measured. The results shown are means ± SD from four separate experiments. Values with different letters are significantly different (P < 0.05).
Inhibition of PMN apoptosis by stimulation with S-PBMC sup.
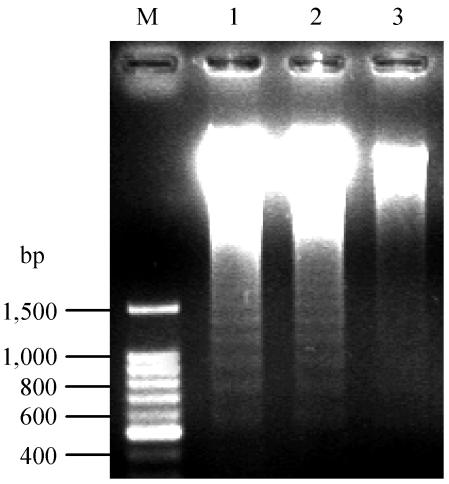
To investigate the differences in the response to PMA, the survival rates of PMN stimulated with S-PBMC sup or N-PBMC sup were measured. After cultivation for 24 h, the survival rate with S-PBMC sup (91% ± 5.6%; n = 5) was significantly (P < 0.05) higher than that with N-PBMC sup (54% ± 8.8%; n = 5) or with PMN alone (50.2% ± 6.4%; n = 5). To confirm that the long-time survival of PMN was due to the inhibition of apoptosis, a DNA fragmentation assay was carried out. No DNA fragmentation was detected in PMN stimulated with S-PBMC sup. On the other hand, PMN alone and PMN stimulated with N-PBMC sup showed DNA fragmentation (Fig. 6).
FIG. 6.
Detection of DNA fragmentation. PMN were cultured with S-PBMC sup or N-PBMC sup at 37°C for 13 h. After cultivation, cells were harvested and DNA was extracted. DNA fragments were detected by electrophoresis with 1.5% agarose followed by staining with ethidium bromide. Lane M, molecular size marker; lane 1, PMN alone; lane 2, N-PBMC sup-stimulated PMN; lane 3, S-PBMC sup-stimulated PMN. The data are typical of those from two separate experiments.
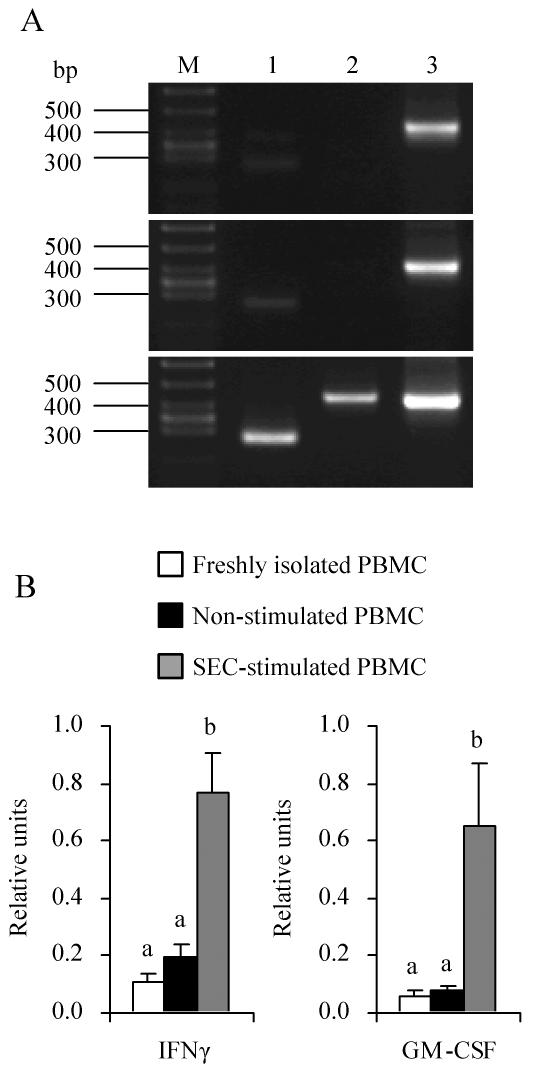
As stated above, the concentration of IL-8, which delays apoptosis of PMN (1), was significantly (P < 0.05) higher in S-PBMC sup than N-PBMC sup (Table 3). The concentration of PGE2, one of the arachidonic acid metabolites and an inhibitor of PMN apoptosis (9), was significantly (P < 0.05) higher in S-PBMC sup (1,400 ± 230 pg/ml; n = 3) than in N-PBMC sup (18 ± 1.9 pg/ml; n = 3). IFN-γ and GM-CSF, which have been reported to contribute to delayed apoptosis in SE-treated human PMN (21), were also induced by SEC (Fig. 7). The IFN-γ and GM-CSF mRNA levels were significantly (P < 0.05) higher in SEC-stimulated PBMC than in nonstimulated PBMC.
FIG. 7.
IFN-γ and GM-CSF mRNA levels in SEC-stimulated and nonstimulated PBMC. PBMC were cultured with or without SEC (1 μg/ml) for 4 h. IFN-γ and GM-CSF mRNA levels were measured by the RT-PCR method. (A) Representative amplification of RT-PCR from freshly isolated (top), nonstimulated (middle), and SEC-stimulated (bottom) PBMC. RT-PCR products were detected by electrophoresis with 3.0% agarose followed by staining with ethidium bromide. Lane M, molecular weight marker; lane 1, IFN-γ (270 bp); lane 2, GM-CSF (429 bp); lane 3, β-actin (405 bp). (B) Relative units after normalization to the β-actin mRNA level. The results shown are means ± SD from three separate experiments. Values with different letters are significantly different (P < 0.05).
DISCUSSION
We previously reported an acute and significant increase of SCC caused by intramammary inoculation with SEC (16). In this study, a histopathological analysis of SEC-inoculated mammary glands was performed. Interstitial inflammation, such as stromal edema and leukocyte migration in the stromal area and the ductular lumen, was observed. The inflammatory state was more severe in the gland cistern tissue than in the mammary parenchymal tissue dissected from SEC-inoculated mammary gland. These results agree with an earlier report that described histopathological responses of mammary gland to experimentally induced Staphylococcus aureus infection (22). Since SEC was inoculated from the teat sinus, we concluded that the gland cistern tissue was affected earlier and more easily by the toxin.
The predominant leukocytes that migrated into the mammary gland were PMN. This result consisted with the general knowledge that PMN are the first leukocytes to migrate in an inflammatory site. Therefore, we tested the induction of PMN migration by SEC in vitro and demonstrated that the migration was induced by S-PBMC sup. These results indicate that chemotactic factors were produced by SEC-stimulated PBMC. We detected a significantly higher IL-8 concentration in S-PBMC sup than in N-PBMC sup. IL-8 is the major neutrophil chemotaxin from macrophages stimulated with SEA (20). Moreover, chemotactic activity caused by IL-8 was detected in mastitic milk naturally infected with S. aureus (4). The concentration of IL-8 in S-PBMC sup (170 ± 58 pg/ml) was higher than that which induced the migration of PMN in vitro (>50 pg/ml) (24). Therefore, a contribution by IL-8 to PMN migration induced by S-PBMC sup was suggested. LTB4, which is another strong chemotactic factor, was also detected at significantly higher concentrations in S-PBMC sup than in N-PBMC sup. However, since the concentration of LTB4 in S-PBMC sup (7.2 ± 2.2 pg/ml) was more than 10 times less than that which physiologically induced PMN migration (>100 pg/ml) and that which was detected in mastitic milk experimentally infected with Klebsiella pneumoniae (>300 pg/ml) (23, 29), we concluded that LTB4 contributed slightly to the migration of PMN in vitro detected in this study.
Using an RT-PCR assay, we detected the induction of GRO mRNA expression, especially that of GROβ and -γ, in SEC-stimulated PBMC. GROs were originally identified as melanoma growth factors and belong to the C-X-C α-chemokine family (13). GROs activate leukocytes and induce chemotaxis of PMN (13). It was reported that the expression of GROs was induced by the stimulation of macrophages and monocytes with bacteria and these components in vitro (5, 25). Moreover, Fujiki et al. reported that GROs were induced in an interstitial pneumonia mouse model produced by intratracheal instillation of SEB (12). Tessier et al. also reported that MIP-2 and KC, the murine homologue of human GRO, were involved in acute inflammation induced by subcutaneous injection of SEs (34). From these observations, we concluded that GROβ and -γ as well as IL-8 contributed to the in vitro migration of PMN detected in this study.
Since the SEC preparations used in this study contained <0.001% (wt/wt) of endotoxin, the quantity of endotoxin inoculated into mammary glands was <1 ng. It was reported that the SCC in 1 ng of endotoxin-inoculated mammary gland changed by less than 2 × 105/ml (24). In an in vitro assay (the final concentration of SEC was 1 μg/ml), the concentration of endotoxin was <10 pg/ml, which is more than 10 times less than the minimum concentration that induced IL-8 mRNA expression in bovine macrophages (17). Therefore, we think that endotoxin contamination in SEC preparations contributed slightly to the results in this study.
In the gland cistern tissue from SEC-inoculated mammary glands, invagination and cytoplasmic vacuolation of epithelial cells were observed. Cytotoxic effects of migrated leukocytes are crucial for environmental tissue degeneration. Oxygen metabolites are strong cytotoxic agents produced by PMN that have migrated to inflammatory sites (36). Milk PMN collected from SEC-inoculated mammary glands at 1 dpi show higher oxidative burst activity than those collected at 0 dpi (preinoculation). Moreover, significantly more superoxide was produced by S-PBMC sup-stimulated PMN in vitro. It was reported that lipopolysaccharide stimulated PMN damage to mammary epithelial cells by producing superoxide (7). These observations may indicate that the superoxide produced by migrated PMN contributes to the epithelial cellular degeneration in SEC-inoculated mammary glands. On the other hand, a direct interaction between SEC and mammary epithelial cells cannot be ruled out. Few reports are available on the expression of conventional SE receptors (MHC class II molecules) on mammary epithelial cells. However, colonic epithelial cells stimulated with culture supernatant of SEB-stimulated PBMC up-regulated the expression of MHC class II molecules and directly interacted with SEB (19). Moreover, novel SE receptors were recently reported. SEB and SEC bound to human colon carcinoma cells and COS-1 cells, an African green monkey kidney fibroblast-like cell line, via the novel SE receptors, which are distinct from MHC class II molecules (10, 28). These reports raise the possibility that SEC directly affected mammary epithelial cells. Therefore, further analyses are needed to elucidate the causes of epithelial cellular degeneration in SEC-inoculated mammary glands.
PMN that migrate to inflammatory sites are primed by tumor necrosis factor alpha and GM-CFS and have potentiated oxidative burst activity (35). Although priming with these factors is effective for receptor-mediated stimulation, such as by formyl-methionyl-leucyl-phenylalanine and opsonized zymosan, the effect of priming is less than that of receptor-independent stimulation, such as with PMA (35). Since PMA was used as the stimulator of the oxidative burst, we speculated that priming is slightly involved in the increases of superoxide production by milk PMN collected from SEC-inoculated mammary glands. Therefore, we tested other means to increase oxidative burst activity and confirmed that the viability of S-PBMC sup-stimulated PMN was significantly greater than those of PMN alone and N-PBMC sup-stimulated PMN. A DNA fragmentation assay showed the inhibition of PMN apoptosis. These results suggest that the difference in cell viability is one of the causes of the difference in oxidative burst activity.
Moulding et al. reported that SEA, SEB, and toxic shock syndrome toxin 1 indirectly delay human PMN apoptosis via the production of T-lymphocyte-derived and monocyte-derived cytokines (21). Our result that S-PBMC sup-stimulated PMN show a significantly higher survival rate agrees with their report. Although we could not specify the inhibitor of PMN apoptosis, the concentrations of IL-8 and PGE2, which delay apoptosis (1, 9), were significantly higher in S-PBMC sup than in N-PBMC sup. IFN-γ and GM-CSF, which are reported to contribute to the delayed apoptosis of PMN induced by SEs (21), were induced in SEC-stimulated PBMC. These results suggest that these mediators derived from SEC-stimulated PBMC are responsible for long-time survival of PMN in vitro. On the other hand, Schuberth et al. reported superantigen-dependent accelerated death of bovine PMN in the presence of MNC (30). Although the experimental conditions, such as the concentrations of SEs and the methods of viable cell measurement, were different, it is unclear why the findings conflict. However, it was reported that various inflammatory mediators which can be induced by SEs have antiapoptotic effects on PMN (1, 9, 21). Moreover, we have already reported an increase of SCC in milk after the intramammary inoculation of SEC (16). Consequently, we believe that the results reported by us and by Moulding et al. are more reasonable than those reported by Schuberth et al.
In inflammation, the long-term survival of PMN increases the risk of tissue damage as well as the chance of pathogen elimination. Therefore, an appropriate exclusion of PMN from inflammatory sites is essential for the termination of inflammation. In this study, we showed PMN migration and epithelial cellular degeneration in SEC-inoculated mammary glands. In addition, SEC indirectly induces the PMN migration, the significantly larger amount of superoxide production, and the inhibition of PMN apoptosis via the stimulation of PBMC in vitro. These results suggest that SEC induces PMN activation by the stimulation of tissue MNC (lymphocytes and macrophages) in the mammary gland and acts as a causative agent of staphylococcal mastitis.
Acknowledgments
The pathological observations of Hiroyuki Okada, Department of Veterinary Pathology, Rakuno Gakuen University; the histopathological analytical assistance of Hisashi Aso, Laboratory of Functional Morphology, Department of Animal Production Science, Faculty of Agriculture, Tohoku University; the experimental advice of Kouji Taniyama, Kenzo Kai, and Yumiko Komine; and the technical assistance of Takako Watanabe, Michiko Itasaka, Megumi Kai, and the technical staff of Miyagi Agricultural College Research Farm are gratefully acknowledged.
REFERENCES
- 1.Akgul, C., D. A. Moulding, and S. W. Edwards. 2001. Molecular control of neutrophil apoptosis. FEBS Lett. 487:318-322. [DOI] [PubMed] [Google Scholar]
- 2.Asai, K., K. Kai, H. Rikiishi, S. Sugawara, Y. Maruyama, T. Yamaguchi, M. Ohta, and K. Kumagai. 1998. Variation in CD4+ T and CD8+ T lymphocyte subpopulations in bovine mammary gland secretions during lactating and non-lactating periods. Vet. Immunol. Immunopathol. 65:51-61. [DOI] [PubMed] [Google Scholar]
- 3.Baggioloni, M., and B. Dewald. 1985. The neutrophil. Int. Arch. Allergy Appl. Immunol. 76(Suppl. 1):13-20. [DOI] [PubMed] [Google Scholar]
- 4.Barber, M. R., and T. J. Yang. 1998. Chemotactic activities in nonmastitic and mastitic mammary secretions: presence of interleukin-8 in mastitic but not nonmastitic secretions. Clin. Diagn. Lab. Immunol. 5:82-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker, S., J. Quay, H. S. Koren, and J. S. Haskill. 1994. Constitutive and stimulated MCP-1, GROα, β, and γ expression in human airway epithelium and bronchoalveolar macrophages. Am. J. Physiol. 266:L278-L286. [DOI] [PubMed] [Google Scholar]
- 6.Blowey, R., and P. Edmondson. 1995. Mastitis control in dairy herds: an illustrated and practical guide. Farming Press Books, Ipswich, United Kingdom.
- 7.Boulanger, V., X. Zhao, and P. Lacasse. 2002. Protective effect of melatonin and catalase in bovine neutrophil-induced model of mammary cell damage. J. Dairy Sci. 85:562-569. [DOI] [PubMed] [Google Scholar]
- 8.Coussens, P. M., and W. Nobis. 2002. Bioinformatics and high throughput approach to create genomic resources for the study of bovine immunobiology. Vet. Immunol. Immunopathol. 86:229-244. [DOI] [PubMed] [Google Scholar]
- 9.Daffern, P. J., M. A. Jagels, and T. E. Hugil. 1999. Multiple epithelial cell-derived factors enhance neutrophil survival. Regulation by glucocorticoids and tumor necrosis factor-α. Am. J. Respir. Cell Mol. Biol. 21:259-267. [DOI] [PubMed] [Google Scholar]
- 10.Dohlsten, M., G. Hedlund, S. Segren, P. A. Lando, T. Herrmann, A. P. Kelly, and T. Kalland. 1991. Human histocompatibility complex class II-negative colon carcinoma cells present staphylococcal superantigens to cytotoxic T lymphocytes: evidence for a novel enterotoxin receptor. Eur. J. Immunol. 21:1229-1233. [DOI] [PubMed] [Google Scholar]
- 11.Ferens, W. A., W. C. Davis, M. J. Hamilton, Y. H. Park, C. F. Deobald, L. Fox, and G. Bohach. 1998. Activation of bovine lymphocytes subpopulations by staphylococcal enterotoxin C. Infect. Immun. 66:573-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujiki, M., T. Shinbori, M. Suga, H. Miyakawa, and M. Ando. 1999. Role of T cells in bronchoalveolar space in the development of interstitial pneumonia induced by superantigen in autoimmune-prone mice. Am. J. Respir. Cell Mol. Biol. 21:675-683. [DOI] [PubMed] [Google Scholar]
- 13.Geiser, T., B. Dewald, M. H. Ehrengruber, I. Clark-Lewis, and M. Baggiolini. 1993. The interleukin-8-related chemotactic cytokines GROα, GROβ, and GROγ activate human neutrophil and basophil leukocytes. J. Biol. Chem. 268:15419-15424. [PubMed] [Google Scholar]
- 14.Ito, T., and M. Kodama. 1996. Demonstration by reverse transcription-polymerase chain reaction of multiple cytokine mRNA expression in bovine alveolar macrophages and peripheral blood mononuclear cells. Res. Vet. Sci. 60:94-96. [DOI] [PubMed] [Google Scholar]
- 15.Johnson, H. M., B. A. Torres, and J. M. Soos. 1996. Superantigens: structure and relevance to human disease. Proc. Soc. Exp. Med. 212:99-109. [DOI] [PubMed] [Google Scholar]
- 16.Kuroishi, T., K. Komine, K. Kai, M. Itagaki, J. Kobayashi, M. Ohta, S. Kamata, and K. Kumagai. 2003. Concentrations and specific antibodies of staphylococcal enterotoxin-C and toxic shock syndrome toxin-1 in bovine mammary gland secretions, and inflammatory responses by the intramammary inoculation of these toxins. J. Vet. Med. Sci. 65:899-906. [DOI] [PubMed]
- 17.Lafleur, R. L., M. S. Abrahamsen, and S. K. Maheswaran. 1998. The biphasic mRNA expression pattern of bovine interlukin-8 in Pasteurella haemolytica lipopolysaccharide-stimulated alveolar macrophages is primarily due to tumor necrosis factor alpha. Infect. Immun. 66:4087-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsen, H. D., F. M. Aarestrup, and N. E. Jensen. 2002. Geographical variation in the presence of genes encoding superantigenic exotoxins and β-hemolysin among Staphylococcus aureus isolated from bovine mastitis in Europe and USA. Vet. Microbiol. 85:61-67. [DOI] [PubMed] [Google Scholar]
- 19.Liu, Z. X., S. Sugawara, N. Hiwatashi, M. Noguchi, H. Rikiishi, K. Kumagai, and T. Toyota. 1997. Accessory cell function of human colonic epithelial cell line HT-29 for bacterial superantigens. Clin. Exp. Immunol. 108:384-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller, E. J., S. Nagao, F. K. Carr, J. M. Noble, and A. B. Cohen. 1996. Interleukin-8 (IL-8) is a major neutrophil chemotaxin from human alveolar macrophages stimulated with staphylococcal enterotoxin A (SEA). Inflamm. Res. 45:386-392. [DOI] [PubMed] [Google Scholar]
- 21.Moulding, D. A., C. Walter, C. A. Hart, and S. W. Edwards. 1999. Effects of staphylococcal enterotoxins on human neutrophil functions and apoptosis. Infect. Immun. 67:2312-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nickerson, S. C., and C. W. Heald. 1981. Histopathologic response of the bovine mammary gland to experimentally induced Staphylococcus aureus infection. Am. J. Vet. Res. 42:1351-1355. [PubMed] [Google Scholar]
- 23.Palmer, R. M. J., R. J. Stepney, G. A. Higgs, and K. E. Eakins. 1980. Chemokinetic activity of arachidonic and lipooxygenase products on leukocytes of different species. Prostaglandins 20:411-418. [DOI] [PubMed] [Google Scholar]
- 24.Persson, K., I. Larsson, and C. Hallén Sandgren. 1993. Effects of certain inflammatory mediators on bovine neutrophil migration in vivo and in vitro. Vet. Immunol. Immunopathol. 37:99-112. [DOI] [PubMed] [Google Scholar]
- 25.Ragno, S., M. Romano, S. Howell, D. J. C. Pappin, P. J. Jenner, and M. J. Colston. 2001. Changes in gene expression in macrophages infected with Mycobacterium tuberculosis: a combined transcriptomic and proteomic approach. Immunology 104:99-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riollet, C., P. Rainard, and B. Poutrel. 2000. Cells and cytokines in inflammatory secretions of bovine mammary gland. Adv. Exp. Med. Biol. 480:247-258. [DOI] [PubMed] [Google Scholar]
- 27.Riollet, C., P. Rainard, and B. Poutrel. 2000. Differential induction of complement fragment C5a and inflammatory cytokines during intramammary infections with Escherichia coli and Staphylococcus aureus. Clin. Diagn. Lab. Immunol. 7:161-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers, T. J., and L. Zhang. 1997. Structural basis for the interaction of superantigen with the alternative superantigen-binding receptor p85. Mol. Immunol. 34:263-272. [DOI] [PubMed] [Google Scholar]
- 29.Rose, D. M., S. N. Giri, S. J. Wood, and J. S. Cullor. 1989. Role of leukotriene B4 in the pathogenesis of Klebsiella pneumoniae-induced bovine mastitis. Am. J. Vet. Res. 50:915-918. [PubMed] [Google Scholar]
- 30.Schuberth, H. J., C. Krueger, A. Hendricks, D. Bimczok, and W. Leibold. 2000. Superantigen-dependent accelerated death of bovine neutrophilic granulocytes in vitro is mediated by blood mononuclear cells. Immunobiology 202:493-507. [DOI] [PubMed] [Google Scholar]
- 31.Shuster, D. E., M. E. Kehrli, Jr., P. Rainard, and M. Paape. 1997. Complement fragment C5a and inflammatory cytokines in neutrophil recruitment during intramammary infection with Escherichia coli. Infect. Immun. 65:3286-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Surette, M. E., R. Palmantier, J. Gosselin, and P. Borgeat. 1993. Lipopolysaccharides prime whole human blood and isolated neutrophils for the increased synthesis of 5-lipooxygenase products by enhancing arachidonic acid availability: involvement of the CD14 antigen. J. Exp. Med. 178:1347-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutra, L., and B. Poutrel. 1994. Virulence factors involved in the pathogenesis of bovine intramammary infections due to Staphylococcus aureus. J. Med. Microbiol. 40:78-89. [DOI] [PubMed] [Google Scholar]
- 34.Tessier, P. A., P. H. Naccache, K. R. Diener, R. P. Gladue, K. S. Neote, I. Clark-Lewis, and S. R. McColl. 1998. Induction of acute inflammation in vivo by staphylococcal superantigens. II. Critical role for chemokines, ICAM-1, and TNF-α. J. Immunol. 161:1204-1211. [PubMed] [Google Scholar]
- 35.Utsumi, T., J. Klostergaard, K. Akimaru, K. Edashige, E. F. Sato, and K. Utsumi. 1992. Modulation on TNF-α-priming and stimulation-dependent superoxide generation in human neutrophils by protein kinase inhibitors. Arch. Biochem. Biophys. 294:271-278. [DOI] [PubMed] [Google Scholar]
- 36.Weiss, S. J. 1989. Tissue destruction by neutrophils. N. Engl. J. Med. 320:365-376. [DOI] [PubMed] [Google Scholar]
- 37.Yokomizo, Y., Y. Mori, Y. Shimojo, S. Shimizu, H. Sentsui, M. Kodama, and H. Igarashi. 1995. Proliferative response and cytokine production of bovine peripheral blood mononuclear cells induced by the superantigens staphylococcal enterotoxins and toxic shock syndrome toxin-1. J. Vet. Med. Sci. 57:299-305. [DOI] [PubMed] [Google Scholar]