Abstract
Two hundred seventy one serum samples from South Korean patients were tested to detect antibodies against Anaplasma phagocytophilum (the human granulocytic ehrlichiosis agent) and Ehrlichia chaffeensis (the human monocytic ehrlichiosis agent) by indirect fluorescent-antibody assay (IFA) and the Western blot assay. These sera were collected from patients with symptoms of high fever. The rate of seropositivity for Orientia tsutsugamushi was 50.9% by IFA at the Public Health & Environmental Research Institute and National Institute of Health in South Korea. By IFA, 30 (11.1%) and 39 (14.4%) of the serum samples reacted with A. phagocytophilum and E. chaffeensis antigens, respectively. By the Western blot assays, 24 (8.9%) and 29 (10.7%) of the serum samples reacted with purified A. phagocytophilum and E. chaffeensis protein antigens, respectively. This report strengthens other evidence regarding the presence of A. phagocytophilum and E. chaffeensis infections in humans in South Korea.
Because of South Korean agricultural practices and Korean geography, various types of ticks and arthropod vectors are commonly present and every year transmit the agents of several vector-borne diseases, such as scrub typhus (tsutsugamushi disease), Lyme disease, and murine typhus (Rickettsia typhi) (8, 10, 14, 18-22). It is estimated that not less than 10,000 patients are treated for tick- or arthropod-borne diseases such as tsutsugamushi disease or spotted fever every year in South Korea.
Human anaplasmosis (formerly human granulocytic ehrlichiosis [HGE]) and human monocytic ehrlichiosis (HME) are emerging infectious diseases transmitted by ticks. The etiological agents of HGE and HME are Anaplasma phagocytophilum and Ehrlichia chaffeensis, respectively, and these have been classified as members of the family Anaplasmataceae (11, 13).
HME and HGE were first reported and described in the United States (2, 9). However, serologic evidence of A. phagocytophilum infection has been found broadly (3). Seroepidemiologic and molecular studies have shown that these causative agents are also present in Asia (6, 17). Nevertheless, the tick-borne diseases HGE and HME have not yet been reported in South Korea.
The diagnosis of these diseases depends on evaluation of clinical, laboratory, and epidemiological data. A. phagocytophilum and E. chaffeensis infections are characterized by the presence of intracytoplasmic inclusions called morulae within leukocytes of human or animal peripheral blood smears. However, since it is difficult to detect A. phagocytophilum and E. chaffeensis, examination of blood smears for morulae is not a sensitive approach for laboratory diagnosis. Thus, testing of serum for antibodies against A. phagocytophilum and E. chaffeensis is important.
In this study, we tested human patients in South Korea for antibodies against A. phagocytophilum and E. chaffeensis using the indirect fluorescent-antibody assay (IFA) and the Western blot assay.
MATERIALS AND METHODS
Human sera.
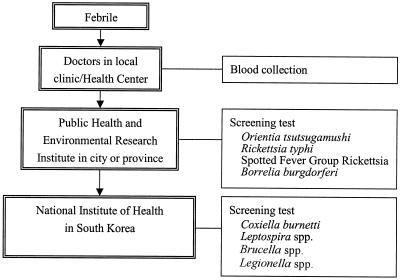
The Public Health & Environmental Research Institute (PHERI) and the National Institute of Health (NIH) in South Korea kindly provided unpaired serum specimens from 271 patients with symptoms of high fever. All serum specimens were initially tested for Orientia tsutsugamushi antibodies by IFA at PHERI or NIH (Fig. 1), and 138 (50.9%) were IFA positive for antibodies to the scrub typhus agent. Strains Karp, Kato, Giliam, and Boryong were used, with an IFA titer cutoff of 128 used for immunoglobulin G (IgG) and a cutoff of 10 used for IgM.
FIG. 1.
Flow diagram for diagnosis of ehrlichiosis or anaplasmosis from febrile patients by PHERI and NIH in South Korea.
Preparation of antigen by in vitro culture of E. chaffeensis and A. phagocytophilum.
The Webster strain of A. phagocytophilum (the HGE agent) was propagated in HL-60 cells (a human promyelocytic leukemia cell line) in RPMI 1640 medium (GIBCO-BRL) supplemented with 1% fetal bovine serum (GIBCO-BRL) and 2 mM l-glutamine (GIBCO-BRL) in an incubator at 37°C with 5% CO2 (15).
The E. chaffeensis Arkansas strain was propagated in DH82 cells (a dog macrophage cell line) in Dulbecco's minimal essential medium (GIBCO-BRL) supplemented with 10% fetal bovine serum (GIBCO-BRL) and 2 mM l-glutamine (GIBCO-BRL) in an incubator at 37°C with 5% CO2 (14).
Cell number and viability were checked manually by trypan blue staining. The infection rate was monitored by examination of cytocentrifuged (Cytospin 3 cytocentrifuge; Shandon, Pittsburgh, Pa.) preparations by Leuko-Stat staining (HEMA 3; Biochemical Science Inc., Swedesboro, N.J.).
IFA.
IFA was performed by a previously described procedure (26). Briefly, A. phagocytophilum-infected HL-60 cells and E. chaffeensis-infected DH82 cells were preloaded onto slides, fixed with acetone, and used as antigens. For antibody titrations, twofold serial dilutions of human serum were prepared, starting at a dilution of 1:80, in phosphate-buffered saline (PBS; pH 7.4) with 0.5% nonfat dry milk (PBSM). A fluorescein isothiocyanate-labeled goat anti-human IgG-IgA-IgM (IgGAM; Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) conjugate at a 1:50 dilution in PBSM was used as the secondary antibody. Before fluorescence microscopic examination, the antigen slides were counterstained with 0.005% Evans blue and mounted for examination.
Western blot assay.
The Western blot assay was performed by previously described procedures (1, 4, 12, 28). Antigens were prepared from A. phagocytophilum and E. chaffeensis purified by a gradient centrifugation method. Normal HL-60 cell and DH82 cells were used as negative antigen controls. Human serum was diluted 1:100 in PBSTM (1% normal goat serum diluted with 0.1 M PBS with 0.05% Tween 20 and 0.5% nonfat dry milk). Alkaline phosphatase-labeled goat anti-human IgGAM (Kirkegaard & Perry Laboratories, Inc.) was used as a secondary antibody at a 1:5,000 dilution in PBSTM. 5-Bromo-4-chloro-3-indolyl phosphate-nitro blue tetrazolium chloride was used as the substrate for alkaline phosphatase and color development.
Statistical analysis for independence of tests.
The independence of the test results for O. tsutsugamushi with regard to positive or negative IFA or Western blotting results for A. phagocytophilum and E. chaffeensis was assessed by the χ2 test.
RESULTS
IFA.
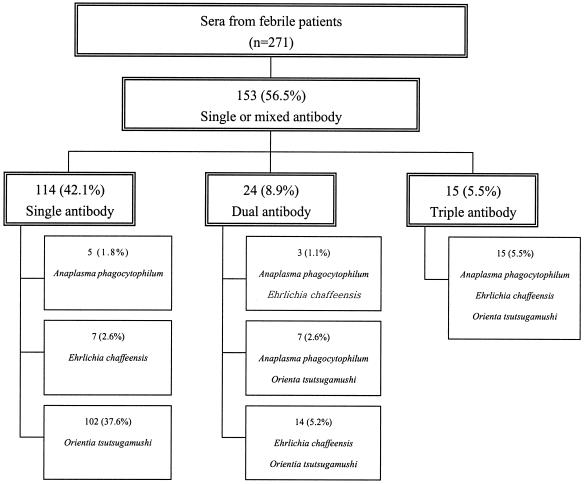
Of the 271 serum samples submitted, 30 (11.1%) and 39 (14.4%) reacted with A. phagocytophilum and E. chaffeensis in the IFA, respectively. The IFA titers of positive sera are shown in Tables 1 and 2. Among the serum samples that showed positive IFA reactions with A. phagocytophilum, 15 (5.5%) reacted with both O. tsutsugamushi and E. chaffeensis, 7 (2.6%) reacted only with O. tsutsugamushi, 3 (1.1%) reacted only with E. chaffeensis, and 5 (1.8%) reacted only with A. phagocytophilum. Among the 39 serum samples that were positive for E. chaffeensis by IFA, 14 (5.2%) also reacted with O. tsutsugamushi and 7 (2.6%) reacted only with E. chaffeensis (Fig. 2). Overall, the IFA results were independent from those obtained for O. tsutsugamushi when the results were analyzed by χ2 tests for A. phagocytophilum (P < 0.01) or E. chaffeensis (P < 0.002), or both (P < 0.001).
TABLE 1.
Patient sexes and sex ages IFA titers, and Western blot assay results for sera reacted against A. phagocytophilum and O. tsutsugamushia
| Sample no. | Sex | Age (yr) |
A. phagocytophilum
|
IFA titer for O. tsutsugamushi | |
|---|---|---|---|---|---|
| IFA titer | Western blot result (kDa)b | ||||
| 2 | F | 36 | 160 | Neg | 128 |
| 6 | M | 77 | 320 | 44 | Neg |
| 10 | F | 78 | 640 | 44 | 128 |
| 19 | F | 49 | 320 | 44 | 128 |
| 29 | F | 50 | 320 | 44 | 512 |
| 37 | F | 58 | 160 | Neg | Neg |
| 55 | U | U | 640 | 44, 68 | Neg |
| 77 | U | U | 320 | 44, 68, 70 | Neg |
| 7 | F | 65 | 160 | Neg | 512 |
| 9 | U | U | 320 | 44, 60, 120 | 4,096 |
| 11 | U | U | 160 | 44 | 4,096 |
| 14 | U | U | 160 | 44 | 8,192 |
| 16 | U | U | 640 | 44 | Neg |
| 21 | U | U | 320 | 44, 68 | 4,096 |
| 43 | F | 71 | 320 | 44, 85 | 8,192 |
| 46 | M | 59 | 160 | Neg | 4,096 |
| 53 | F | 74 | 160 | 44, 60 | Neg |
| 58 | U | U | 320 | 44 | 8,192 |
| 67 | U | U | 320 | 44, 60, 85 | 4,096 |
| 71 | M | 84 | 320 | 44 | 4,096 |
| 80 | F | 44 | 160 | 44 | Neg |
| 87 | F | 88 | 160 | 44, 85 | 1,280 |
| 93 | F | 65 | 320 | 44, 70 | Neg |
| 106 | U | U | 80 | Neg | 4,096 |
| 111 | F | 67 | 320 | 44 | 16,384 |
| 126 | U | U | 640 | 44, 60, 85 | 4,096 |
| 136 | M | 69 | 320 | 44, 60, 85 | 4,096 |
| 156 | F | 70 | 320 | 44, 68 | 4,096 |
| 160 | M | 84 | 320 | NP | 4,096 |
| 167 | M | 59 | 320 | 44, 85 | 4,096 |
Abbreviations: F, female; M, male; U, unknown; NP, not performed; Neg, negative.
Approximate molecular size of reactive antigen.
TABLE 2.
Patient sexes and sex ages, IFA titers, and Western blot assay results for sera reacted against E. chaffeensis and O. tsutsugamushia
| Sample no. | Sex | Age (yr) |
Ehrlichia chaffeensis
|
IFA titer for O. tsutsugamushi | |
|---|---|---|---|---|---|
| IFA titer | Western blot result (kDa)b | ||||
| 10 | F | 78 | 320 | 28, 32 | 128 |
| 20 | M | 76 | 320 | 28 | 128 |
| 42 | F | 62 | 320 | 26, 28, 32 | Neg |
| 75 | U | U | 160 | 28 | Neg |
| 77 | U | U | 320 | 28, 32 | Neg |
| 91 | F | 71 | 320 | 28, 32 | 128 |
| 98 | F | 74 | 320 | 28, 32 | 128 |
| 7 | F | 65 | 160 | 28 | 512 |
| 14 | U | U | 160 | Neg | 8,192 |
| 16 | U | U | 160 | 28 | Neg |
| 21 | U | U | 320 | 28, 35, 70, 80 | 4,096 |
| 32 | F | 67 | 320 | 28 | 1,024 |
| 33 | U | U | 160 | 28 | 1,024 |
| 38 | U | U | 160 | Neg | Neg |
| 43 | F | 71 | 160 | 28 | 8,192 |
| 46 | M | 59 | 320 | 26 | 4,096 |
| 47 | F | 62 | 320 | 26 | 2,048 |
| 54 | F | 49 | 320 | 28, 35 | 32,768 |
| 57 | F | 42 | 160 | 28 | Neg |
| 58 | U | U | 320 | 26, 28, 32 | 8,192 |
| 67 | U | U | 320 | 28, 35 | 4,096 |
| 69 | U | U | 160 | 28 | 4,096 |
| 71 | M | 84 | 160 | 28 | 4,096 |
| 86 | U | U | 320 | 28, 32 | 4,096 |
| 87 | F | 88 | 160 | 28 | 1,280 |
| 93 | F | 65 | 160 | 28 | Neg |
| 99 | U | U | 160 | Neg | 512 |
| 106 | U | U | 160 | 28 | 4,096 |
| 108 | U | U | 160 | Neg | Neg |
| 109 | F | 84 | 80 | Neg | Neg |
| 111 | F | 67 | 320 | 28, 32 | 16,384 |
| 115 | U | U | 320 | Neg | 4,096 |
| 136 | M | 69 | 320 | 28, 32 | 4,096 |
| 147 | F | 66 | 160 | Neg | 2,048 |
| 148 | M | 57 | 160 | Neg | Neg |
| 154 | U | U | 160 | NP | 2,048 |
| 156 | F | 70 | 320 | 28, 68, 100 | 4,096 |
| 166 | F | 75 | 160 | NP | 512 |
| 167 | M | 59 | 320 | 28, 30 | 4,096 |
Abbreviations: F, female; M, male; U, unknown; NP, not performed; Neg, negative.
Approximate molecular size of reacting antigen.
FIG. 2.
E. chaffeensis, A. phagocytophilum, and O. tsutsugamushi antibodies in human sera collected from patients in Jeonnam and Jeonbuk, South Korea, in 2001 and 2002. n, number of patients examined.
Western blot assay.
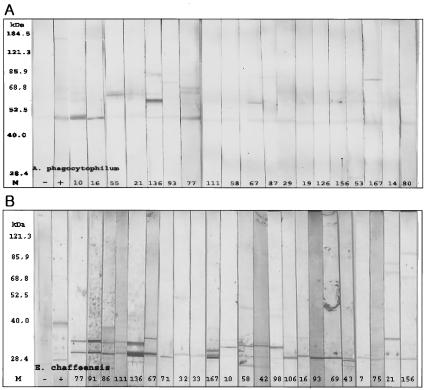
The Western blot assay was performed with the sera with positive reactions by IFA. The results of the Western blot assay for representative serum samples that reacted with purified A. phagocytophilum or E. chaffeensis antigen are shown in Fig. 3A and B. The IFA titers of sera with positive reactions by the Western blot assays were higher than those of sera with negative reactions by the Western blot assays. Among the 30 and 39 serum specimens reactive with A. phagocytophilum and E. chaffeensis in the IFA, respectively, 24 (80.0%) and 29 (74.4%) reacted only with purified A. phagocytophilum and E. chaffeensis antigens, respectively, and not with negative control antigens prepared from uninfected HL-60 cells or DH-82 cells (Tables 1 and 2). When purified A. phagocytophilum antigen was used, all 24 serum specimens reacted with an approximately 44-kDa antigen, presumably major surface protein 2 (Msp2). The reaction patterns of these sera could be divided into three groups; 11 serum specimens reacted only with the 44-kDa antigen, 8 reacted with the 44-kDa antigen plus one antigen between 60 and 85 kDa, and 5 reacted with the 44-kDa antigen plus two antigens between 60 and 160 kDa (Fig. 3A). When purified E. chaffeensis antigen was used, the reaction patterns of 32 serum specimens could also be divided into three groups, with each group minimally defined by reaction with an antigen of approximately 28 kDa; 15 serum specimens reacted only with an antigen of approximately 28 kDa, 10 reacted with two antigens between 28 and 35 kDa, and 4 reacted with three or four antigens between 26 and 100 kDa (Fig. 3B). A weak 120-kDa antigen was detected in only three serum specimens. Overall, the Western blotting results were independent from those obtained for O. tsutsugamushi when the results were analyzed by χ2 tests for A. phagocytophilum (P < 0.05) or E. chaffeensis (P < 0.002), or both (P < 0.001).
FIG. 3.
Western blot assay of electrophoretically separated gradient-purified A. phagocytophilum (A) and E. chaffeensis (B) antigens reacted with IFA-positive sera. Lanes: M, protein size marker; −, negative control; +, positive control; the lane numbers represent sample numbers. (A) Twenty-five serum specimens (83.3%) reacted with an A. phagocytophilum antigen of approximately 44 kDa presumed to be Msp2. The reaction patterns were divided into three groups: 11 serum specimens reacted only with the 44-kDa antigen, 8 reacted with the 44-kDa antigen and another antigen between 60 and 85 kDa, and 5 reacted with the 44-kDa antigen and two other antigens between 60 and 160 kDa. (B) Twenty-nine (74.4%) serum specimens reacted with purified E. chaffeensis antigens. The reaction patterns of 32 serum specimens were also divided into three groups: 15 reacted only with antigens of 28 kDa, 10 reacted with two antigens between 28 and 35 kDa, and 4 reacted with three or four antigens between 26 and 100 kDa.
DISCUSSION
Although IFA has both advantages and disadvantages, it is the most widely used method for the identification of E. chaffeensis and A. phagocytophilum antibodies in both acute- and convalescent-phase human sera after infection. In this study, we used IFA as a screening test and the Western blot assay for confirmation.
Among all of the serum specimens submitted for this study, 138 showed positive reactions for O. tsutsugamushi by IFA. The relatively higher rate of IFA positivity for O. tsutsugamushi seems to reflect the fact that all serum specimens were collected from patients with febrile responses typical for scrub typhus. However, this may also indicate cross-reactivity between A. phagocytophilum, E. chaffeensis, and O. tsutsugamushi. It is well recognized that serum samples from patients with HGE cross-react with E. chaffeensis (24). Bunnell et al. (5) suggested that suspected cross-reactions between A. phagocytophilum and Borrelia burgdorferi antigens in a murine model were not significant enough to confuse the results of specific serological tests. In the present study, 102 serum specimens were IFA positive only for O. tsutsugamushi, suggesting that cross-reactions between E. chaffeensis or A. phagocytophilum and O. tsutsugamushi are also not significant and that HGE or HME may exist as mixed infections or subsequent to infection with O. tsutsugamushi in patients with acute fever. Moreover, the independence of the results obtained by both IFA and the Western blot tests indicates that the concurrent positive reactions are unlikely to be due to cross-reactions. It would be useful to assess seroconversion or fourfold increases in titers between paired serum specimens, which was not possible for the single serum samples tested in the present study.
Although IFA is the most sensitive method, it can give inconsistent results because of antigenic diversity and various technical factors among different laboratories. For example, use of the MRK strain of A. phagocytophilum (Ehrlichia equi) as the antigen for IFA revealed a high degree of variability even if tests were performed in the same laboratory (1). Western blot assays are generally thought to provide more detailed information than IFA about the specific reactive antigens. Therefore, we confirmed the IFA results by Western blot assays using purified A. phagocytophilum and E. chaffeensis antigens. All sera that reacted against A. phagocytophilum in Western blot assays recognized an antigen of approximately 44 kDa that is presumed to represent MspII. The reaction patterns of those sera could be further divided into three groups, depending on the overall number of antigens detected, further enforcing the likelihood of the addition of specificity with Western blot assays. Likewise, when purified E. chaffeensis antigens were used in a Western blot assay, all sera reacted against an antigen of approximately 28 kDa that is presumed to represent the p28 major membrane protein family. These reaction patterns could also be divided into three groups: the first group reacted only with an antigen of approximately 28 kDa; the second group reacted with two antigens that showed bands between 28 and 35 kDa, which may reflect detection of the E. chaffeensis p28 family of membrane proteins; and the third group reacted with three or four antigens which showed bands between 26 and 120 kDa, also reflecting the detection of higher-molecular-size antigens, such as the immunodominant protein gp120 (29) and conserved heat shock proteins of questionable specificity for E. chaffeensis.
Such variable reactions in Western blot assays have been demonstrated before with both A. phagocytophilum and E. chaffeensis. Unver et al. (25) reported that IFA-positive sera of HME patients reacted not only with the p28 family of major surface proteins but also with several other native antigens of E. chaffeensis, while Chen et al. (9a) showed variable degrees of genus, species, and strain specificity. However, further studies are needed to verify these Western blot assay results, perhaps by using variant A. phagocytophilum and E. chaffeensis isolates that may further validate and standardize this serological approach.
In order for these serological results to make sense, several ecological factors need to be considered. O. tsutsugamushi is transmitted through the bites of trombiculid mites, but not through the bites of ticks. However, the concurrence of antibodies to several vector-borne bacteria could be predicted, since both the trombiculid mites and ticks that transmit Ehrlichia and Anaplasma spp. share many of the same habitats. Likewise, an association of specific ticks and Ehrlichia or Anaplasma spp. is expected. A. phagocytophilum has been detected in Ixodes persulcatus ticks and in the blood of humans after tick bites in China (6, 30), consistent with the results of the present report. Although the I. persculcatus group that transmits A. phagocytophilum is present in South Korea, the classical tick vector for E. chaffeensis, Amblyomma americanum, is not. However, E. chaffeensis or a similar species has been detected in Amblyomma testudinarium and Haemaphysalis yeni ticks in South China (7), Boophilus microplus ticks in Tibet (27), and Ixodes ovatus ticks in Japan (23); and we have found evidence of both E. chaffeensis and A. phagocytophilum in both Haemaphysalis longicornis and I. persulcatus ticks from South Korea (17a). Although a definitive role of these causative agents and potential tick vectors in human infections cannot be assumed from these data, the presence of the causative agents and potential tick vectors with the capacity to bite humans suggests that the serological data reflect a previously unrecognized but emerging problem in South Korea and perhaps more broadly across Asia.
Many rickettsial diseases have been studied in South Korea, and the geographical distribution of O. tsutsugamushi was recently investigated. However, only one report has described the incidence of Ehrlichia or Anaplasma sp. infection in humans (16). To confidently confirm human exposure and the presence of HME and HGE in South Korea, much more study is needed, including the isolation and identification of Ehrlichia or Anaplasma strains from infected humans.
Acknowledgments
We thank Chan-Moon Jin, Mi-Young Shin, and Mi-Yeoun Park of PHERI and NIH in Jeonbuk and Jeonnam, Korea, for providing sera.
This study was supported by a grant of the Korea Health 21 R & D Project, Ministry of Health & Welfare of Korea (HMP-00-B-20200-0003), and by grant RO1-AI41213 from the U.S. National Institutes of Health to J.S.D. J.P. and K.-S.C. were supported in part by a grant from the Korea Science and Engineering Foundation.
REFERENCES
- 1.Asanovich, K. M., J. S. Bakken, J. E. Madigan, M. Aguero-Rosenfeld, G. P. Wormser, and J. S. Dumler. 1997. Antigenic diversity of granulocytic Ehrlichia isolates from humans in Wisconsin and New York and a horse in California. J. Infect. Dis. 176:1029-1034. [DOI] [PubMed] [Google Scholar]
- 2.Bakken, J. S., J. S. Dumler, S. M. Chen, M. R. Eckman, L. L. Van Etta, and D. H. Walker. 1994. Human granulocytic ehrlichiosis in the upper Midwest United States. A new species emerging? JAMA 272:212-218. [PubMed] [Google Scholar]
- 3.Bjoersdorff, A., J. Berglund, B. E. Kristiansen, C. Soderstrom, and I. Eliasson. 1999. Varying clinical picture and course of human granulocytic ehrlichiosis. Twelve Scandinavian cases of the new tick-borne zoonosis are presented. Lakartidningen 96:4200-4204. [PubMed] [Google Scholar]
- 4.Brouqui, P., C. Lecam, J. Olson, and D. Raoult. 1994. Serologic diagnosis of human monocytic ehrlichiosis by immunoblot analysis. Clin. Diagn. Lab. Immunol. 1:645-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunnell, J. E., L. A. Magnarelli, and J. S. Dumler. 1999. Infection of laboratory mice with the human granulocytic ehrlichiosis agent does not induce antibodies to diagnostically significant Borrelia burgdorferi antigens. J. Clin. Microbiol. 37:2077-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao, W. C., Q. M. Zhao, P. H. Zhang, J. S. Dumler, X. T. Zhang, L. Q. Fang, and H. Yang. 2000. Granulocytic ehrlichiae in Ixodes persulcatus ticks from an area in China where Lyme disease is endemic. J. Clin. Microbiol. 38:4208-4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao, W. C., Y. M. Gao, P. H. Zhang, X. T. Zhang, Q. H. Dai, J. S. Dumler, L. Q. Fang, and H. Yang. 2000. Identification of Ehrlichia chaffeensis by nested PCR in ticks from Southern China. J. Clin. Microbiol. 38:2778-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, W. H., J. S. Kang, W. K. Lee, M. S. Choi, and J. H. Lee. 1990. Serological classification by monoclonal antibodies of Rickettsia tsutsugamushi isolated in Korea. J. Clin. Microbiol. 28:685-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, S. M., J. S. Dumler, J. S. Bakken, and D. H. Walker. 1994. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 32:589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Chen, S. M., L. C. Cullman, and D. H. Walker 1997. Western immunoblotting analysis of the antibody responses of patients with human monocytotropic ehrlichiosis to different strains of Ehrlichia chaffeensis and Ehrlichia canis. Clin. Diagn. Lab. Immunol. 4:731-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chong, Y. 1989. Application of serologic diagnosis of tsutsugamushi disease (scrub typhus) in Korea where the disease was recently recognized to be endemic. Yonsei Med. J. 30:111-117. [DOI] [PubMed] [Google Scholar]
- 11.Dumler, J. S., and J. S. Bakken. 1995. Ehrlichial diseases of humans: emerging tick-borne infections. Clin. Infect. Dis. 20:1102-1110. [DOI] [PubMed] [Google Scholar]
- 12.Dumler, J. S., K. M. Asanovich, J. S. Bakken, P. Richter, R. Kimsey, and J. E. Madigan. 1995. Serologic cross-reactions among Ehrlichia equi, Ehrlichia phagocytophila, and human granulocytic Ehrlichia. J. Clin. Microbiol. 33:1098-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumler, J. S., and J. S. Bakken. 1998. Human ehrlichioses: newly recognized infections transmitted by ticks. Annu. Rev. Med. 49:201-213. [DOI] [PubMed] [Google Scholar]
- 14.Felek, S., A. Unver, R. W. Stich, and Y. Rikihisa. 2001. Sensitive detection of Ehrlichia chaffeensis in cell culture, blood, and tick specimens by reverse transcription-PCR. J. Clin. Microbiol. 39:460-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodman, J. L., C. Nelson, B. Vitale, J. E. Madigan, J. S. Dumler, T. J. Kurtti, and U. G. Munderloh. 1996. Direct cultivation of the causative agent of human granulocytic ehrlichiosis. N. Engl. J. Med. 334:209-215. [DOI] [PubMed] [Google Scholar]
- 16.Heo, E. J., J. H. Park, J. R. Koo, M. S. Park, M. Y. Park, J. S. Dumler, and J. S. Chae. 2002. Serologic and molecular detection of Ehrlichia chaffeensis and Anaplasma phagocytophila (human granulocytic ehrlichiosis agent) in Korean patients. J. Clin. Microbiol. 40:3082-3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inokuma, H., G. Nane, T. Uechi, Y. Yonahara, P. Brouqui, M. Okuda, and T. Onishi. 2001. Survey of tick infestation and tick-borne ehrlichial infection of dogs in Ishigaki Island, Japan. J. Vet. Med. Sci. 63:1225-1227. [DOI] [PubMed] [Google Scholar]
- 17a.Kim, C. M., M. S. Kim, M. S. Park, J. H. Park, and J. S. Chae. 2003. Identification of Ehrlichia chafeensis, Anaplasma phagocytophilum, and A. bovis in Haemophysalis longicornis and Ixodes persulcatus ticks from Korea. Vector-Borne Zoonotic Dis. 3:17-26. [DOI] [PubMed] [Google Scholar]
- 18.Kim, T. H., E. H. Choi, M. G. Lee, and S. K. Ahn. 1999. Serologically diagnosed Lyme disease manifesting erythema migrans in Korea. J. Korean Med. Sci. 14:85-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, H. W., and van der Groen. 1989. Hemorrhagic fever with renal syndrome. Prog. Med. Virol. 36:62-102. [PubMed] [Google Scholar]
- 20.Park, K. H., W. H. Chang, and T. G. Schwan. 1993. Identification and characterization of Lyme disease spirochetes, Borrelia burgdorferi sensu lato, isolated in Korea. J. Clin. Microbiol. 31:1831-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park, S. K., S. H. Lee, Y. K. Rhee, S. K. Kang, K. J. Kim, M. C. Kim, K. W. Kim, and W. H. Chang. 1989. Leptospirosis in Chonbuk Province of Korea in 1987: a study of 93 patients. Am. J. Trop. Med. Hyg. 41:345-351. [PubMed] [Google Scholar]
- 22.Seong, S. Y., M. S. Choi, and I. S. Kim. 2001. Orientia tsutsugamushi infection: overview and immune responses. Microbes Infect. 3:11-21. [DOI] [PubMed] [Google Scholar]
- 23.Shibata, S., M. Kawahara, Y. Rikihisa, H. Fujita, Y. Watanabe, C. Suto, and T. Ito. 2000. New Ehrlichia species closely related to Ehrlichia chaffeensis isolated from Ixodes ovatus ticks in Japan. J. Clin. Microbiol. 38:1331-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Unver, A., S. Felek, C. D. Paddock, N. Zhi, H. W. Horowitz, G. P. Wormser, L. C. Cullman, and Y. Rikihisa. 2001. Western blot analysis of sera reactive to human monocytic ehrlichiosis and human granulocytic ehrlichiosis agents. J. Clin. Microbiol. 39:3982-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Unver, A., Y. Rikihisa, N. Ohashi, L. C. Cullman, R. Buller, and G. A. Storch. 1999. Western and dot blotting analyses of Ehrlichia chaffeensis indirect fluorescent-antibody assay-positive and negative human sera by using native and recombinant E. chaffeensis and E. canis antigens. J. Clin. Microbiol. 37:3888-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walls, J. J., M. Aguero-Rosenfeld, J. S. Bakken, J. L. Goodman, D. Hossain, R. C. Johnson, and J. S. Dumler. 1999. Inter- and intralaboratory comparison of Ehrlichia equi and human granulocytic ehrlichiosis (HGE) agent strains for serodiagnosis of HGE by the immunofluorescent-antibody test. J. Clin. Microbiol. 37:2968-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen, B., R. Jian, Y. Zhang, and R. Chen. 2002. Simultaneous detection of Anaplasma marginale and a new Ehrlichia species closely related to Ehrlichia chaffeensis by sequence analyses of 16S ribosomal DNA in Boophilus microplus ticks from Tibet. J. Clin. Microbiol. 40:3286-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong, S. J., G. S. Brady, and J. S. Dumler. 1997. Serologic responses to Ehrlichia equi, Ehrlichia chaffeensis, and Borrelia burgdorferi in patients from New York State. J. Clin. Microbiol. 35:2198-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu, X. J., P. Crocquet-Valdes, and D. H. Walker. 1997. Cloning and sequencing of the gene for a 120-kDa immunodominant protein of Ehrlichia chaffeensis. Gene 15:149-154. [DOI] [PubMed] [Google Scholar]
- 30.Zhao, Q., W. Cao, J. Li, P. Zhang, S. Chen, K. Cao, D. Gao, H. Yang, and X. Zhang. 2002. Detection and sequential analysis of granulocytic ehrlichia 444-Epank gene. Zhonghua Liu Xing Bing Xue Za Zhi 23:286-288. [PubMed] [Google Scholar]