Abstract
Canine parvovirus (CPV) is classified as a member of the feline parvovirus (FPV) subgroup. CPV isolates are divided into three antigenic types: CPV type 2 (CPV-2), CPV-2a, and CPV-2b. Recently, new antigenic types of CPV were isolated from Vietnamese leopard cats and designated CPV-2c(a) or CPV-2c(b). CPV-2c viruses were distinguished from the other antigenic types of the FPV subgroup by the absence of reactivity with several monoclonal antibodies (MAbs). To characterize the antigenicity of CPV-2c, a panel of MAbs against CPV-2c was generated and epitopes recognized by these MAbs were examined by selection of escape mutants. Four MAbs were established and classified into three groups on the basis of their reactivities: MAbs which recognize CPV-2a, CPV-2b, and CPV-2c (MAbs 2G5 and 20G4); an MAb which reacts with only CPV-2b and CPV-2c(b) (MAb 21C3); and an MAb which recognizes all types of the FPV subgroup viruses (MAb 19D7). The reactivity of MAb 20G4 with CPV-2c was higher than its reactivities with CPV-2a and CPV-2b. These types of specificities of MAbs have not been reported previously. A mapping study by analysis of neutralization-resistant mutants showed that epitopes recognized by MAbs 21C3 and 19D7 belonged to antigenic site A. Substitution of the residues in site B and the other antigenic site influenced the epitope recognized by MAb 2G5. It was suggested that the epitope recognized by MAb 20G4 was related to antigenic site B. These MAbs are expected to be useful for the detection and classification of FPV subgroup isolates.
Autonomous parvovirus is a small, nonenveloped virus. It possesses single-stranded DNA that codes for the two capsid proteins (VP1, VP2) and two nonstructural proteins (NS1, NS2). VP1 and VP2 are translated from alternatively spliced mRNAs, and the VP2 sequence is completely contained within VP1 (11). VP2 mainly composes the parvovirus capsid, and amino acid substitutions in the VP2 sequence are known to cause changes in antigenic properties (25, 30).
Among carnivores, the disease caused by parvovirus was thought to occur only in cats (in which the disease is caused by Feline panleukopenia virus [FPLV]) or raccoons until a similar disease caused by Mink enteritis virus (MEV) was observed in mink in the mid-1940s (35). Now these viruses have been classified into FPLV, MEV type 1 (MEV-1), MEV-2, and MEV-3 with a panel of monoclonal antibodies (MAbs) (22, 23).
In the late 1970s, parvovirus emerged in dogs and was named Canine parvovirus (CPV) or CPV type 2 (CPV-2) to distinguish it from an unrelated virus, the Canine minute virus (CPV-1). CPV-2 rapidly spread worldwide and initially killed thousands of dogs (3). Although CPV-2 is antigenically closely related to FPLV, they are distinguishable from each other with a panel of MAbs. Six conserved amino acid differences in VP2 were observed between FPLV and CPV-2 (14). At present, FPLV, MEV, and CPV-2 are classified as members of the feline parvovirus (FPV) subgroup (28).
Since the emergence of CPV-2, two new antigenic variants, designated CPV-2a and CPV-2b, have arisen consecutively (24, 26). These two variants have almost completely replaced CPV-2 in canine populations worldwide (5, 6, 24, 32). At least five conserved substitutions in VP2 are observed between CPV-2 and CPV-2a (26). CPV-2b has another two substitutions in VP2, the replacement of the Asn residue at position 426 (Asn-426) by Asp and the replacement of Ile-555 by Val (26). CPV-2b is distinguished from the other antigenic types in the FPV subgroup by the reactivities of two MAbs, MAb B4A2 (MAb I) and MAb B (26). These MAbs do not recognize CPV-2b, and the absence of reactivity is caused by the substitution of residue 426 in VP2 (26).
Since the late 1980s, CPV-2a and CPV-2b have been isolated from domestic cats and wild felids worldwide (9, 10, 18, 19, 29, 33). Recently, Ikeda et al. (9, 10) isolated six CPV-2a- and CPV-2b-related viruses from leopard cats in Vietnam and Taiwan. Three of these isolates were shown to be new antigenic types of CPV by the absence of reactivity with several MAbs (10). They were designated CPV-2c and were further divided into two antigenic types, CPV-2c(a) and CPV-2c(b), by their reactivities with MAb B4A2, which distinguishes CPV-2a and CPV-2b. CPV-2c isolates have a common substitution in VP2, the replacement of Gly-300 by Asp. This residue is known to be variable among antigenic types: Ala in FPLV and CPV-2, Val in MEV-2, and Gly in CPV-2a and CPV-2b (31). The emergence of CPV-2c suggests that further new substitutions of residue 300 may occur. Therefore, it is important to characterize the epitopes related to the unique Asp-300 residue of CPV-2c and monitor the substitution of residue 300 in the field. Although MAbs are useful for the classification of the FPV subgroup and characterization of their epitopes, no CPV-2c-specific MAb has been reported.
In the study described here, we generated new MAbs against CPV-2c in order to detect and monitor antigenic changes in the FPV subgroup. The antigenic types recognized by those MAbs were determined by hemagglutination inhibition (HI) tests and the virus neutralization (VN) test. Furthermore, resistant mutants were selected and examined by sequencing analysis of the VP2 gene and the HI test to determine the epitopes recognized by those MAbs.
MATERIALS AND METHODS
Cells and viruses.
The viruses used in this study were as follows. FPLV TU-1 and No. 311 were isolated from Japanese domestic cats (17, 19). MEV-1 Abashiri (7) and MEV-2 M-1 (10) were isolated in Japan and China, respectively. CPV-2 Cp49 (1), Cp82031 (27), CPV-2a 97-003, and CPV-2b 97-008 (10) were isolated from Japanese domestic dogs. FPLV V142, CPV-2a V220, and CPV-2b V123 were isolated from domestic cats in Vietnam (16). CPV-2c(a) V139 and V140 and CPV-2c(b) V203 were isolated from Vietnamese leopard cats (Felis bengalensis) (9). Isolates 97-003 and 97-008 were propagated in Madin-Darby canine kidney (MDCK) cells. The other strains were propagated in Crandell feline kidney (CRFK) cells. CRFK cells, MDCK cells, the feline T-lymphoblastoid cell line FL74, and the canine fibroblastic cell line A72 were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and antibiotics. The feline thymic lymphoma cell line 3201 and the murine myeloma cell line P3U1 were grown in RPMI 1640 medium supplemented with 10% fetal calf serum and antibiotics.
Titration of viruses.
Viruses were titrated on FL74 cells as described previously (8). Briefly, FL74 cells (104 cells/100 μl) and 100 μl of viruses serially diluted 10-fold were mixed and incubated at 37°C for 7 days. The 50% tissue culture infective doses (TCID50s) were calculated as described elsewhere (4).
Generation of MAbs against CPV-2c.
CPV-2c strain V203 was used as the immunogen. The viruses were propagated in 3201 cells and purified by ultracentrifugation. BALB/c mice were intraperitoneally immunized twice and then intravenously once with purified viruses (108 TCID50s/mouse) at intervals of 3 weeks. The immunogen was prepared in Freund's complete adjuvant for the first immunization, in Freund's incomplete adjuvant for the second immunization, and in phosphate-buffered saline without any adjuvant for the last immunization. Three days after the last immunization, spleen cells were taken out of the mice and fused with P3U1 cells with polyethylene glycol 1500 (Roche, Mannheim, Germany). Hybridomas secreting antibodies against strain V203 were detected by HI tests and cloned by limiting dilution. Because a large amount of MAbs at high concentrations was necessary for selection of escape mutants, ascitic fluid was prepared by injecting the minimum number of pristane-primed BALB/c mice with hybridoma cells (108 cells/mouse). The immunoglobulin subtypes of the MAbs were determined with the Mouse Monoclonal Antibody Isotyping test kit (Serotec Ltd., Oxford, United Kingdom). All procedures were conducted in accordance with the University of Tokyo guidelines on the care and use of laboratory animals.
HI tests.
HI tests were performed as described previously (15). Phosphate-buffered saline (pH 6.0) with 0.1% bovine serum albumin was used as the HI buffer. Twenty-five microliters of ascitic fluid serially diluted twofold and viruses (4 hemagglutination units/25 μl) were mixed and incubated at room temperature for 1 h. Then, 50 μl of a 0.75% suspension of formalin-fixed porcine erythrocytes was added. The plates were set at 4°C overnight. The HI titer was determined as the reciprocal of the highest dilution that completely inhibited viral hemagglutination.
VN tests.
VN tests were performed as described previously (8). Briefly, 50 μl of heat-inactivated ascitic fluid serially diluted twofold and viruses (100 TCID50s/50 μl) were mixed and incubated at 37°C for 1 h. FL74 cells (104 cells/100 μl) were then added to this mixture. The cells were observed for 5 days, and the VN titers were determined as the reciprocal of the highest dilution that inhibited the viral cytopathic effect in at least two of four wells with the particular dilution.
Selection of neutralization-resistant mutants.
Neutralization-resistant mutants were selected as described previously (22), with minor modifications. In addition to strain V203, strain 97-008 was used as the parental virus because it also reacted with all four MAbs produced in this study, but the reactivity of strain 97-008 with MAb 20G4 was lower than that of strain V203 with the MAb. Parental viruses (5 × 108 TCID50s) diluted in 5 ml of Dulbecco's modified Eagle's medium were mixed with 5 or 50 μl of ascitic fluid, and the mixture was incubated at 37°C for 1 h. Then, the mixture was inoculated onto CRFK cells or A72 cells. After one to three passages, escape mutants were cloned by the limiting dilution method, as follows. Fifty microliters of viruses serially diluted 10-fold and 50 μl of diluted ascitic fluid (0.2 or 2 μl/ml) were mixed. Then, the CRFK cells (2 × 103 cells/100 μl) were added to the mixture. One week later, the supernatants of the cultures were screened by hemagglutination, and the viruses in the positive wells with the highest dilution were propagated in the cells in the presence of ascitic fluid (0.05 or 0.5 μl/ml) and cultured once without ascitic fluid. Neutralization-resistant mutants were designated “res” followed by the name of the MAb and the name of the parental virus strain. For example, the mutant that was resistant to MAb 2G5 and that was derived from strain V203 is designated res2G5/V203.
Sequencing of mutant VP2 genes.
The VP2 gene was amplified by PCR with the primer sets reported previously (10). After the amplified DNA fragments were purified from the agarose gel, they were used for the sequencing reaction with a Big Dye Terminator cycle sequencing kit (Applied Biosystems, Foster City, Calif.). The samples were resolved on an automated DNA sequencer (model 377; Applied Biosystems).
The sequences of the VP2 genes of the parental viruses were obtained from the DDBJ database, and the accession numbers are AB054224 for strain V203 and AB115504 for strain 97-008.
RESULTS AND DISCUSSION
Generation and characterization of MAbs.
Four hybridoma clones producing MAbs reactive to CPV-2c strain V203 were obtained. HI tests were performed to determine the antigenic types of the FPV subgroup recognized by these MAbs. The MAbs were classified into three groups: MAbs which recognize CPV-2a, CPV-2b, and CPV-2c (MAbs 2G5 and 20G4); an MAb which reacts with only CPV-2b and CPV-2c(b) (MAb 21C3); and an MAb which recognizes all the antigenic types of the FPV subgroup (MAb 19D7) (Table 1). The reactivity of MAb 20G4 with CPV-2c was 20 to 320 times higher than its reactivities with CPV-2a and CPV-2b. The results of the VN tests were concordant with those of the HI tests (Table 2). None of the four MAbs reacted with purified virus (strain V203) by immunoblotting analysis (data not shown), indicating that these MAbs recognize conformational epitopes.
TABLE 1.
Reactivities of MAbs against parvoviruses by HI assay
| Antigenic type | Isolate | Reactivity with the following MAb (isotype)a:
|
|||
|---|---|---|---|---|---|
| 2G5 (IgG3) | 21C3 (IgG1) | 19D7 (IgG2a) | 20G4 (IgG2a) | ||
| FPLV | TU-1 | <1,000 | <100 | 32,000 | <100 |
| FPLV | No. 311 | <1,000 | <100 | 64,000 | <100 |
| FPLV | V142 | <1,000 | <100 | 32,000 | <100 |
| MEV-1 | Abashiri | <1,000 | <100 | 16,000 | <100 |
| MEV-2 | M-1 | <1,000 | <100 | 64,000 | <100 |
| CPV-2 | Cp49 | <1,000 | <100 | 32,000 | <100 |
| CPV-2 | Cp82031 | <1,000 | <100 | 32,000 | <100 |
| CPV-2a | 97-003 | 64,000 | <100 | 32,000 | 3,200 |
| CPV-2a | V220 | 64,000 | <100 | 32,000 | 6,400 |
| CPV-2b | 97-008 | 64,000 | 12,800 | 128,000 | 1,600 |
| CPV-2b | V123 | 64,000 | 6,400 | 256,000 | 6,400 |
| LCPV-2c(a)b | V139 | 128,000 | <100 | 32,000 | 512,000 |
| LCPV-2c(a) | V140 | 128,000 | <100 | 32,000 | 512,000 |
| LCPV-2c(b) | V203 | 64,000 | 3,200 | 128,000 | 128,000 |
The specificities of the MAbs for the antigenic types were as follows: MAb 2G5, CPV-2a, CPV-2b, CPV-2c(a), and CPV-2c(b); MAb 21C3, CPV-2b and CPV-2c(b); MAb 19D7, FPLV, MEV-1, MEV-2, CPV-2, CPV-2a, CPV-2b, CPV-2c(a), and CPV-2c(b); MAb 20G4, CPV-2a and CPV-2b < CPV-2c. IgG3, immunoglobulin G3.
LCPV, leopard cat CPV.
TABLE 2.
Reactivities of MAbs against parvoviruses by VN assay
| Antigenic type | Isolate | Reactivity with the following MAba:
|
|||
|---|---|---|---|---|---|
| 2G5 | 21C3 | 19D7 | 20G4 | ||
| FPLV | TU-1 | <1,000 | <100 | 16,000 | <100 |
| FPLV | No. 311 | <1,000 | <100 | 4,000 | <100 |
| FPLV | V142 | <1,000 | <100 | 6,400 | <100 |
| MEV-1 | Abashiri | <1,000 | <100 | 3,200 | <100 |
| MEV-2 | M-1 | <1,000 | <100 | 8,000 | <100 |
| CPV-2 | Cp49 | <1,000 | <100 | 8,000 | <100 |
| CPV-2 | Cp82031 | <1,000 | <100 | 8,000 | <100 |
| CPV-2a | 97-003 | 16,000 | <100 | 4,000 | 3,200 |
| CPV-2a | V220 | 16,000 | <100 | 1,600 | 3,200 |
| CPV-2b | 97-008 | 32,000 | 3,200 | 16,000 | 6,400 |
| CPV-2b | V123 | 16,000 | 3,200 | 16,000 | 6,400 |
| LCPV-2c(a)b | V139 | 32,000 | <100 | 4,000 | 256,000 |
| LCPV-2c(a) | V140 | 16,000 | <100 | 4,000 | 256,000 |
| LCPV-2c(b) | V203 | 16,000 | 6,400 | 16,000 | 25,600 |
See footnote a of Table 1 for the specificities of the MAbs.
LCPV, leopard cat CPV.
Selection of neutralization-resistant mutants.
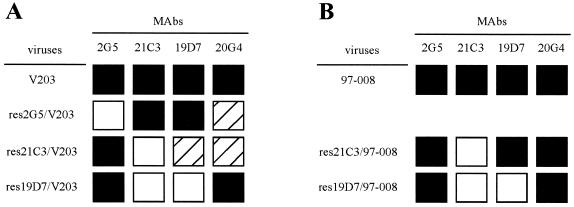
To analyze the epitopes recognized by MAbs, we attempted to select neutralization-resistant mutants by using strains V203 and 97-008 as parental viruses. Two mutants for each MAb and each parental strain were subjected to the following analyses: the reactivities of the MAbs with mutants by HI tests (Fig. 1) and amino acid substitutions in the VP2 protein by sequencing analysis (Table 3). The VP2 genes of the parental viruses used for selection were sequenced and confirmed to have no mutation before the selection.
FIG. 1.
HI reactivities of MAbs with parental viruses and resistant mutants. The reactivities of resistant mutants were compared with those of their parental viruses. Relative reactivity was calculated as follows: HI titer of the mutant/HI titer of the parental virus. ▪, relative reactivity of >1/16; ▨, relative reactivity between 1/16 and 1/80; □, relative reactivity <1/80. (A) Resistant mutants derived from strain V203; (B) resistant mutants derived from strain 97-008. The res20G4/V203, res2G5/97-008, and res20G4/97-008 mutants were not obtained.
TABLE 3.
Amino acid substitutions in VP2 proteins of escape mutants
| Straina | Amino acid at position:
|
|||||
|---|---|---|---|---|---|---|
| 5 | 296 | 300 | 437 | 438 | 440 | |
| V203 | Ala | Gln | Asp | Gly | Gly | Thr |
| res2G5/V203 | Ala | Glub | His | Gly | Gly | Thr |
| res21C3/V203 | Ala | Gln | His | Glu | Gly | Thr |
| res19D7/V203 | Ala | Gln | His | Gly | Gly | Lys |
| 97-008 | Ala | Gln | Gly | Gly | Gly | Thr |
| res21C3/97-008 | Pro | Gln | Gly | Glu | Gly | Thr |
| res19D7/97-008 | Ala | Gln | Gly | Gly | Arg | Thr |
Although isolation was attempted twice with CRFK cells and once with A72 cells, no mutants resistant to MAb 20G4 were obtained. The epitope recognized by MAb 20G4 might be highly conserved. The substitutions in this epitope may make CPV-2b and CPV-2c(b) unviable.
Epitope mapping with MAbs 21C3 and 19D7.
The reactivities of MAbs 21C3 and 19D7 were affected by a single substitution, the replacement of Gly-438 by Arg in res19D7/97-008 (Table 3; Fig. 1B). This shows that the epitopes recognized by these two MAbs are related to each other. The specificity of MAb 21C3 (Table 1) suggested that the reactivity of this MAb was affected by the substitution of residue 426, which previous studies have shown to be responsible for the CPV-2b-specific antigenic property (26). Residue 426 is located on the tip of the threefold spike in the capsid structure (34) and is a component of antigenic site A (30). Since residues 437, 438, and 440 are located on the same structure (34) and were found to be substituted in the mutants resistant to MAbs 21C3 and 19D7 (Table 3), the epitopes recognized by MAbs 21C3 and 19D7 are thought to belong to site A.
Interestingly, res21C3/V203 showed partial resistance to MAb 19D7, but res21C3/97-008 did not (Fig. 1). Res21C3/V203 has an additional substitution of residue 300 (Table 3), which is on the shoulder of the threefold spike and which belongs to antigenic site B (30). This result may indicate that the substitution in site B affects the antigenicity of site A. This is consistent with the findings of Parker and Parrish (20), who reported that the replacement of Ala-300 by Asp reduced the HI reactivity of a site A-specific MAb.
Res21C3/97-008 had a substitution in common with res21C3/V203, the replacement of Gly-437 by Glu, indicating that this substitution is important for resistance to MAb 21C3 (Fig. 1; Table 3). In addition to this substitution, res21C3/97-008 had a unique replacement of Ala-5 by Pro (Table 3). As MAb 21C3 had no reactivity in immunoblotting analysis (data not shown), this MAb was thought to recognize the conformational epitope. Because residue 5 is a component of linear epitopes (12, 13), the substitution of this residue may not influence the reactivity of MAb 21C3. Therefore, the substitution of residue 437 may solely influence the reactivity with MAb 21C3.
Epitope mapping with MAb 2G5.
Res2G5/V203 had replacements of Gln-296 by Glu and Asp-300 by His (Table 3). Residue 296 is located in the vicinity of residue 300 (34) but is not known to belong to site B. Whether resistance to MAb 2G5 needed both of these substitutions was not determined because mutants which had the sole substitution could not be obtained. However, as the reactivity of res2G5/V203 with MAbs 19D7 and 21C3, which recognize the epitopes of site A, was not influenced (Fig. 1A), it was clear that the epitope recognized by MAb 2G5 was independent of site A.
Epitope mapping with MAb 20G4.
Since Gly-300 in site B of CPV-2a and CPV-2b was the only difference from the amino acid sequence in VP2 of CPV-2c (Asp-300) (10), this difference was considered to induce the lower reactivities of CPV-2a and CPV-2b with MAb 20G4 than that of CPV-2c (Tables 1 and 2). Furthermore, the reactivity of CPV-2c(a) with MAb 20G4 was relatively higher than that of CPV-2c(b), especially in VN tests (Tables 1 and 2). Because the amino acid residue at position 426 in VP2 was the sole difference between CPV-2c(a) (Asn-426) and CPV-2c(b) (Asp-426) (10), it was suggested that residue 426 in site A might also affect the reactivity of MAb 20G4. Interestingly, CPV-2a and CPV-2b reacted similarly with MAb 20G4 (Tables 1 and 2), irrespective of the difference at residue 426. Therefore, the higher reactivity of CPV-2c(a) with MAb 20G4 relative to that of CPV-2c(b) might be induced by the combination of substitutions of residues 426 and 300. Although res2G5/V203 and res21C3/V203 were partially resistant to MAb 20G4, res19D7/V203 maintained high levels of reactivity with MAb 20G4 (Fig. 1A; Table 3). Since all these mutants had His-300 in common (Table 3), the different reactivity with MAb 20G4 might also be due to the combination of substitutions of residue 300 with those of other residues. Further studies will be necessary to determine which residues influence the reactivity of CPV-2c with MAb 20G4.
Usefulness of new MAbs for antigenic typing of FPV subgroup isolates.
MAb 19D7 would be useful for the detection of viruses of the FPV subgroups because it recognized all the FPV subgroups, including the new antigenic type of CPV (CPV-2c). Since the mutants resistant to MAb 19D7 were viable (Fig. 1; Table 3), viruses that are not recognized by MAb 19D7 could exist in nature. Such viruses, however, have not been found in a preliminary study of the recent field isolates in Japan and Vietnam (data not shown).
MAb 21C3 is the first MAb that recognizes CPV-2b as well as CPV-2c(b) but not the other antigenic types. CPV-2b has been distinguished from the other antigenic types by two MAbs, MAb B4A2 (MAb I) and MAb B, which do not react with CPV-2b (26). Recently, natural variants of CPV, which have a replacement of Asn-426 by Glu, were reported (2). These variants did not react with MAb B4A2 (2). Tests with MAb 21C3 may help determine whether they belong to the CPV-2b type or a new antigenic type.
CPV-2c(b) strain V203 can infect domestic cats and be shed in the feces (20), and the replacement of residue 300 by Asp increases the stability of the virus in the environment (21). These lines of evidences suggest that CPV-2c has the potential to spread in the cat population in the future, although only three CPV-2c type isolates have so far been isolated from leopard cats in Vietnam (10). Since MAb 20G4 was able to identify the CPV-2c type in the FPV subgroup by the HI assay (Table 1), the antibody may be useful for research on the distribution of CPV-2c in the cat population.
In conclusion, the MAbs produced in this study will contribute to the detection and classification of parvoviruses in carnivore populations.
Acknowledgments
This study was supported in part by grants from Heiwa Nakajima Foundation and from the Ministry of Education, Culture, Sports, Science and Technology of Japan. K. Nakamura is supported by research fellowships from the Japanese Society for the Promotion of Science for Young Scientists.
REFERENCES
- 1.Azetaka, M., T. Hirasawa, S. Konishi, and M. Ogata. 1981. Studies on canine parvovirus isolation, experimental infection and serologic survey. Jpn. J. Vet. Sci. 43:243-255. [DOI] [PubMed] [Google Scholar]
- 2.Buonavoglia, C., V. Martella, A. Pratelli, M. Tempesta, A. Cavalli, D. Buonavoglia, G. Bozzo, G. Elia, N. Decaro, and L. E. Carmichael. 2001. Evidence for evolution of canine parvovirus type 2 in Italy. J. Gen. Virol. 82:3021-3025. [DOI] [PubMed] [Google Scholar]
- 3.Carmichael, L. E., and L. N. Binn. 1981. New enteric viruses in the dog. Adv. Vet. Sci. Comp. Med. 25:1-37. [PubMed] [Google Scholar]
- 4.Cunningham, C. H. 1973. Quantal and enumerative titration of virus in cell cultures, p. 527-532. In P. F. Kruse, Jr., and M. K. Patterson, Jr. (ed.), Tissue culture: methods and applications. Academic Press, Inc., New York, N.Y.
- 5.Gamoh, K., M. Senda, Y. Shimazaki, H. Makie, Y. Inoue, and O. Itoh. 2003. Chronological antigenic survey of canine parvovirus in Japan. Vet. Rec. 152:142-143. [DOI] [PubMed] [Google Scholar]
- 6.Greenwood, N. M., W. S. K. Chalmers, W. Baxendale, and H. Thompson. 1996. Comparison of isolates of canine parvovirus by monoclonal antibody and restriction enzyme analysis. Vet. Rec. 138:495-496. [DOI] [PubMed] [Google Scholar]
- 7.Higashihara, T., H. Izawa, M. Onuma, H. Kodama, and T. Mikami. 1981. Mink enteritis in Japan. I. Isolation and characterization of the causative virus and its pathogenicity in cat. Jpn. J. Vet. Sci. 43:841-851. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda, Y., T. Miyazawa, K. Kurosawa, R. Naito, S. Hatama, C. Kai, and T. Mikami. 1998. New quantitative methods for detection of feline parvovirus (FPV) and virus neutralizing antibody against FPV using a feline T lymphoid cell line. J. Vet. Med. Sci. 60:973-974. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda, Y., T. Miyazawa, K. Nakamura, R. Naito, Y. Inoshima, K. C. Tung, W. M. Lee, M. C. Chen, T. F. Kuo, J. A. Lin, and T. Mikami. 1999. Serosurvey for selected virus infections of wild carnivores in Taiwan and Vietnam. J. Wildl. Dis. 35:578-581. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda, Y., M. Mochizuki, R. Naito, K. Nakamura, T. Miyazawa, T. Mikami, and E. Takahashi. 2000. Predominance of canine parvovirus (CPV) in unvaccinated cat populations and emergence of new antigenic types of CPVs in cats. Virology 278:13-19. [DOI] [PubMed] [Google Scholar]
- 11.Jongeneel, C. V., R. Sahli, G. K. McMaster, and B. Hirt. 1986. A precise map of splice junctions in the mRNAs of minute virus of mice, an autonomous parvovirus. J. Virol. 59:564-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langeveld, J. P. M., J. I. C. Casal, C. Vela, K. Dalsgaard, S. H. Smale, W. C. Puijk, and R. H. Meloen. 1993. B-cell epitopes of canine parvovirus: distribution on the primary structure and exposure on the viral surface. J. Virol. 67:765-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.López de Turiso, J. A., E. Cortés, A. Ranz, J. García, A. Santz, C. Vela, and J. I. Casal. 1991. Fine mapping of canine parvovirus B cell epitopes. J. Gen. Virol. 72:2445-2456. [DOI] [PubMed] [Google Scholar]
- 14.Martyn, J. C., B. E. Davidson, and M. J. Studdert. 1990. Nucleotide sequence of feline panleukopenia virus: comparison with canine parvovirus identifies host-specific differences. J. Gen. Virol. 71:2747-2753. [DOI] [PubMed] [Google Scholar]
- 15.Mathys, A., R. Mueller, C. Pederson, and G. H. Theilen. 1983. Hemagglutination with formalin-fixed erythrocytes for detection of canine parvovirus. Am. J. Vet. Res. 44:150-151. [PubMed] [Google Scholar]
- 16.Miyazawa, T., Y. Ikeda, K. Nakamura, R. Naito, M. Mochizuki, Y. Tohya, D. Vu, T. Mikami, and E. Takahashi. 1999. Isolation of feline parvovirus from peripheral blood mononuclear cells of cats in northern Vietnam. Microbiol. Immunol. 43:609-612. [DOI] [PubMed] [Google Scholar]
- 17.Mochizuki, M., S. Konishi, M. Ajiki, and T. Akaboshi. 1989. Comparison of feline parvovirus subspecific strains using monoclonal antibodies against a feline panleukopenia virus. Jpn. J. Vet. Sci. 51:264-272. [DOI] [PubMed] [Google Scholar]
- 18.Mochizuki, M., R. Harasawa, and H. Nakatani. 1993. Antigenic and genomic variabilities among recently prevalent parvoviruses of canine and feline origin in Japan. Vet. Microbiol. 38:1-10. [DOI] [PubMed] [Google Scholar]
- 19.Mochizuki, M., M. Horiuchi, H. Hiragi, M. C. San Gabriel, N. Yasuda, and T. Uno. 1996. Isolation of canine parvovirus from a cat manifesting clinical signs of feline panleukopenia. J. Clin. Microbiol. 34:2101-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura, K., M. Sakamoto, Y. Ikeda, E. Sato, K. Kawasaki, T. Miyazawa, Y. Tohya, E. Takahashi, T. Mikami, and M. Mochizuki. 2001. Pathogenic potential of canine parvovirus type 2a and 2c in domestic cats. Clin. Diagn. Lab. Immunol. 8:663-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker, J. S. L., and C. R. Parrish. 1997. Canine parvovirus host range is determined by the specific conformation of an additional region of the capsid. J. Virol. 71:9214-9222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parrish, C. R., and L. E. Carmichael. 1983. Antigenic structure and variation of canine parvovirus type-2, feline panleukopenia virus, and mink enteritis virus. Virology 129:401-414. [DOI] [PubMed] [Google Scholar]
- 23.Parrish, C. R., J. R. Gorham, T. M. Schwartz, and L. E. Carmichael. 1984. Characterization of antigenic variation among mink enteritis virus isolates. Am. J. Vet. Res. 45:2591-2599. [PubMed] [Google Scholar]
- 24.Parrish, C. R., P. H. O'Connell, J. F. Evermann, and L. E. Carmichael. 1985. Natural variation of canine parvovirus. Science 230:1046-1048. [DOI] [PubMed] [Google Scholar]
- 25.Parrish, C. R., and L. E. Carmichael. 1986. Characterization and recombination mapping of an antigenic and host range mutation of canine parvovirus. Virology 148:121-132. [DOI] [PubMed] [Google Scholar]
- 26.Parrish, C. R., C. F. Aquadro, M. L. Strassheim, J. F. Evermann, J. Y. Sgro, and H. O. Mohammed. 1991. Rapid antigenic-type replacement and DNA sequence evolution of canine parvovirus. J. Virol. 65:6544-6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Senda, M., N. Hirayama, O. Itoh, and H. Yamamoto. 1988. Canine parvovirus: strain difference in haemagglutination activity and antigenicity. J. Gen. Virol. 69:349-354. [DOI] [PubMed] [Google Scholar]
- 28.Siegl, G., R. C. Bates, K. I. Berns, B. J. Carter, D. C. Kelly, E. Kurstak, and P. Tattersall. 1985. Characteristics and taxonomy of Parvoviridae. Intervirology 23:61-73. [DOI] [PubMed] [Google Scholar]
- 29.Steinel, A., L. Munson, M. Vuuren, and U. Truyen. 2000. Genetic characterization of feline parvovirus sequences from various carnivores. J. Gen. Virol. 81:345-350. [DOI] [PubMed] [Google Scholar]
- 30.Strassheim, M. L., A. Gruenberg, P. Veijalainen, J. Y. Sgro, and C. R. Parrish. 1994. Two dominant neutralizing antigenic determinants of canine parvovirus are found on the threefold spike of the virus capsid. Virology 198:175-184. [DOI] [PubMed] [Google Scholar]
- 31.Truyen, U., A. Gruenberg, S. F. Chang, B. Obermaier, P. Veijalainen, and C. R. Parrish. 1995. Evolution of the feline-subgroup parvoviruses and the control of canine host range in vivo. J. Virol. 69:4702-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Truyen, U., J. F. Evermann, E. Vieler, and C. R. Parrish. 1996. Evolution of canine parvovirus involved loss and gain of feline host range. Virology 215:186-189. [DOI] [PubMed] [Google Scholar]
- 33.Truyen, U., G. Platzer, and C. R. Parrish. 1996. Antigenic type distribution among canine parvoviruses in dogs and cats in Germany. Vet. Rec. 138:365-366. [DOI] [PubMed] [Google Scholar]
- 34.Tsao, J., M. S. Chapman, M. Agbandje, W. Keller, K. Smith, H. Wu, M. Luo, T. J. Smith, M. G. Rossmann, R. W. Compans, and C. R. Parrish. 1991. The three-dimensional structure of canine parvovirus and its functional implications. Science 251:1456-1463. [DOI] [PubMed] [Google Scholar]
- 35.Wills, C. G. 1952. Notes on infectious enteritis of mink and its relationship to feline enteritis. Can. J. Comp. Med. 16:419-420. [PMC free article] [PubMed] [Google Scholar]