Abstract
Three recombinant antigens of Leishmania chagasi (= L. infantum) were expressed in prokaryotic systems and evaluated (using a panel of dog sera characterized by parasitological and serological immunofluorescent antibody test [IFAT] techniques) as diagnostic markers of infection. The whole open reading frame encoding K9, the gene fragment encoding the repetitive sequence of K26, and the 3′-terminal gene fragment encoding a single 39-amino-acid subunit of the kinesin-related protein K39 (K39sub) were amplified from L. infantum DNA and cloned into a pGEX-2T expression vector in frame with glutathione S-transferase (GST). The sensitivity and specificity of enzyme-linked immunosorbent assays (ELISAs) using K26 as an antigen (evaluated with sera from 20 parasitologically positive and 20 parasitologically negative dogs) were both 100% (95% confidence interval [CI] = 83.2 to 100). When K9 and K39sub were used, sensitivity was 95% (95% CI = 75.1 to 99.9) and specificity was 100% (95% CI = 83.2 to 100). Using 182 field sera, a good agreement was found between the recombinant K26 ELISA and IFAT (K = 0.92; 95% CI = 0.86 to 0.98) results and between the K9 and K39sub ELISA (used in parallel) and IFAT (K = 0.87; 95% CI = 0.80 to 0.95) results. The results demonstrate that each antigen carries immunodominant epitopes and that their combination may further increase the sensitivity of currently available serological tests.
Leishmaniases comprise a broad spectrum of human and animal diseases caused by infection of the mononuclear phagocyte system with protozoan parasites of the genus Leishmania. These agents are transmitted by the bite of phlebotomine sandflies in tropical, subtropical, and temperate zones of the world. Although humans are the sole reservoir hosts for some Leishmania species, in most cases other animals play a major role in the maintenance of infections. In countries of the Mediterranean basin, Middle East, and Latin America, wild canids and domestic dogs are the main reservoirs of zoonotic visceral leishmaniasis (ZVL), a severe disease caused by Leishmania infantum (synonym: Leishmania chagasi) (9). Over the past decade, the frequency of ZVL increased due to climate changes and human factors (5, 10).
The diagnosis of L. infantum infection in dogs (canine leishmaniasis) is important in veterinary practice and in surveillance of ZVL. The immunofluorescent antibody test (IFAT) is routinely used for the detection of specific antibodies. IFAT is difficult to standardize and to interpret, however, and it is too laborious for screening large numbers of sera.
Immunoenzymatic assays such as the enzyme-linked immunosorbent assay (ELISA) are easier to standardize and more practical as routine laboratory tools. The performance of ELISA tests is greatly affected by the quality of the antigens used, however, and test specificity limitations are the main drawback when crude antigen preparations are used.
Recombinant technology, together with the characterization of specific immunodominant antigens at the genetic level, allowed the development of a second generation of diagnostic immunoassays, and the recent validation of a recombinant K39 ELISA as a diagnostic marker for canine leishmaniasis represents a good example (11). The rK39 (recombinant K39) antigen is a repetitive immunodominant B-cell epitope of the 230-kDa kinesin-related protein of L. chagasi (3, 13). In Leishmania spp., K39 antigen is mainly expressed in the amastigote stage and elicits a strong immunoresponse in both asymptomatic and clinically infected dogs.
In addition to K39, two other antigens of L. chagasi (K9 and K26) have been genetically characterized (1): K26 shows a central 14-amino-acid repeat region which is specific to L. chagasi and L. donovani and a flanking region homologous to K9. Since these two antigens had not been evaluated as diagnostic markers for canine leishmaniasis, in this work we characterized the recombinant K9 and the recombinant repeat region of K26 expressed in Escherichia coli. In addition, since it was not clear whether the single repetition unit of K39 antigen carried an immunodominant epitope, the 39-amino-acid subunit of this kinesin-related protein was expressed in a similar way. The three recombinant antigens were then employed in a multiple-well ELISA to screen a panel of well-characterized dog sera.
DNA was extracted from promastigotes of the L. infantum reference strain MHOM/TN/80/IPT1 (zymodeme MON-1) by conventional procedures. Primers were synthesized for the amplification of the whole open reading frame encoding the K9 antigen (GenBank accession number AF131227; forward [fw] positions 21 to 44, reverse [rv] positions 239 to 263), the gene fragment encoding the repetitive sequence of K26 (GenBank accession number AF131228; fw 106 to 129, rv 655 to 678), and the 3′-terminal gene fragment encoding a single 39-amino-acid unit of K39 (K39sub hereafter) (GenBank accession number L07879; fw 2798 to 2820, rv 2899 to 2914). Oligonucleotides contained restriction sites for BamHI (sense) and EcoRI (antisense) at the 5′ terminus to facilitate cloning. Amplification of the target genes was carried out in a 50-μl reaction volume containing 10 mM Tris-HCl (pH 8.8), 50 mM KCl, 2 mM MgCl2, 200 μM deoxynucleoside triphosphates, 10 pmol of each primer, 200 ng of the template, and 1 U of Taq DNA polymerase (Invitrogen). PCR was performed in 35 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. Amplified products of the expected length were purified, digested with appropriate restriction enzymes, and cloned into pGEX-2T expression vector (Amersham Bioscience) in frame with glutathione S-transferase (GST). Plasmid preparations from positive clones were sequenced with an ABI 310 and cycle sequencing method to confirm the specificity of the cloned fragments.
To express recombinant protein, early log-phase cultures of positive clones were induced for 2 h with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) under agitation. Bacterial cells were recovered by centrifugation and lysed by conventional physicochemical methods. Recombinant fusion proteins were recovered in the soluble fraction and purified by affinity chromatography (12) using a fast performance liquid chromatography (FPLC) system (Amersham Bioscience). The expected molecular mass for each fusion protein was 35 kDa for GST-K9, 46 kDa for GST-K26, and 31 kDa for GST-K39sub. To avoid false-positive reactions to the carrier moiety, recombinant GST was expressed and purified under the same conditions and used in the ELISA procedure as a negative antigen. The purity and yield of each antigen were estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Bradford methods (2).
A panel of 249 dog sera was used in this study: 20 sera were from parasitologically positive dogs in which infection was detected using Giemsa-stained smears of lymph node aspirate or parasite culture and IFAT (group I), and 20 sera were from dogs living in areas of endemicity and proven by the same techniques to be parasitologically negative (group II). These sera were used as standards in a preliminary phase to evaluate the cutoff value, sensitivity, and specificity of individual antigens. A total of 182 IFAT-characterized sera were from dogs living in well-established areas of endemicity (n = 109) and dogs living in a recently identified low-prevalence focus of infection (n = 73). An additional 27 sera were collected during a longitudinal study of 9 naive dogs exposed to natural infection and found at the end of the test period to be parasitologically positive.
For ELISA, microplates were coated with a positive antigen (GST-K9, GST-K26, or GST-K39sub) and a fusion partner (GST) at concentrations of 134, 176, or 119 and 100 ng, respectively. Dog sera were diluted 1:40 in phosphate-buffered saline-0.05% Tween 20-1% yeast extract and incubated for 1 h at 37°C (100 μl/well). After four washes, 10 ng of peroxidase-labeled protein A (diluted in the same buffer) was added (100 μl/well) and the plates were incubated as described above. After a final wash, ABTS [2,2′azinobis(3-ethylbenzthiazolinesulfonic acid)] was added and absorbance at 405 nm was measured after 10 min. Net absorbance was determined for each serum and each recombinant protein by subtracting the absorbance value from that of GST as the negative antigen. A subset of IFAT-positive sera (or sera from parasitologically positive dogs which were found to be negative in the K39sub ELISA) were retested in ELISA with the rK39 antigen (carrying repetitive immunodominant epitopes) according to procedures previously described (11).
IFAT (used as a reference test) was carried out according to standard protocols (7).
The sensitivity and specificity of ELISA tests using GST-K9, GST-K26, or GST-K39sub as the antigen were estimated on sera of group I and II taken as positive and negative controls, respectively. An additional panel of 182 sera, 45 of which were previously found to be IFAT positive (titer > 80), 33 of which were previously found to be IFAT indeterminate (titer of 40 or 80), and 104 of which were previously found to be IFAT negative (titer < 40), were also tested. The sensitivity and specificity of the ELISAs (compared to those of the IFATs) were estimated with 45 IFAT-positive sera (titer > 1:40) and with 104 IFAT-negative sera (titer < 1:40). A total of 33 indeterminate sera (IFAT titer = 1:40) were also tested. Exact binomial 95% confidence intervals (CI) of estimates were obtained using Epi Info software (version 6.4) (4). Concordance between the ELISA and IFAT results was evaluated using R software and Kappa (K) statistics (8). The ELISA absorbance values of the longitudinal sera were represented in box plots.
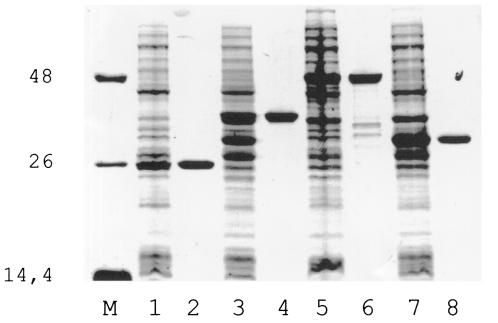
All fusion proteins were highly expressed, found in the soluble fraction, and successfully purified by affinity chromatography (Fig. 1).
FIG. 1.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis results showing expression and purification of recombinant antigens in E. coli. M, molecular-weight standard; lanes 1, 3, 5, and 7, total bacterial lysates expressing GST, GST-K9, GST-K26, and GST-K39sub, respectively; lanes 2, 4, 6, and 8, affinity-purified GST, GST-K9, GST-K26, and GST-K39sub, respectively.
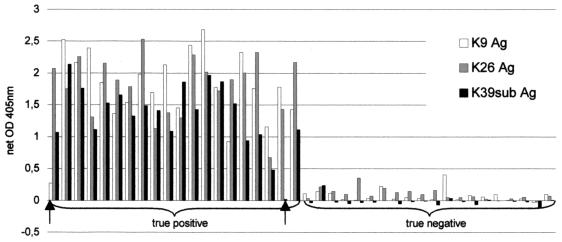
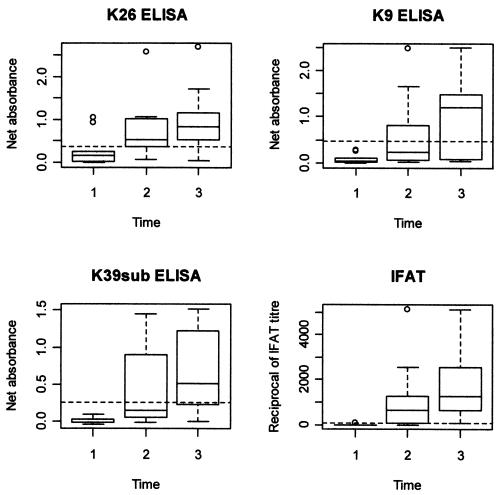
ELISA absorbance values for group I and II sera (as determined using the three antigens) are shown in Fig. 2. All sera were correctly classified by using antigen K26 (sensitivity and specificity = 100%; 95% CI = 83.2 to 100) and a cutoff value equal to the mean of the group II serum values plus three standard deviations (SDs). Both the K39sub and K9 antigens yielded one false-negative and one false-positive result (sensitivity and specificity = 95.0%; CI = 75.1 to 99.9) when the cutoff specified above was used; when the cutoff value was raised to a value equal to the mean value for group II sera plus four SDs, specificity was 100% whereas sensitivity remained 95%. When K9 and K39sub were used in parallel (and defining as positive the sera reacting with at least one antigen), all samples were correctly classified (cutoff value = the mean value for group II sera plus four SDs). The agreement between the K26 ELISA and IFAT results was good (K = 0.92; CI = 0.86 to 0.98) and was slightly higher than the agreement between the K9 and K39sub ELISA (used in parallel) and IFAT (K = 0.87; CI = 0.80 to 0.95) results. The sensitivity and specificity of K26 ELISA relative to IFAT were 92.1% (CI = 82.4 to 97.4) and 98.6% (CI = 95.1 to 99.8). Testing of K9 and K39 in parallel yielded relative sensitivity and specificity values of 88.9% (CI = 78.4 to 95.4) and 97.2% (CI = 93.0 to 99.2), respectively. A total of 14 out of 33 indeterminate sera were positive by K26 (42.4%; CI = 25.5 to 60.1), whereas 11 were positive by K9 and subK39 tested in parallel (33.3%; CI = 18.0 to 51.8). The ELISA absorbance values of the sera of nine dogs tested three consecutive times suggested that testing with K26 was more sensitive than that with K9 and K39sub in the early stage of infection (Fig. 3).
FIG. 2.
ELISA net absorbance values for K9, K26, and K39sub recombinant antigen true positives (group I) and 20 true negatives (group II) for 20 dog sera; cutoff values are 0.46, 0.36, and 0.25, respectively. True-positive sera which tested as negative with K9 or K39sub are shown by arrows. OD, optical density; Ag, antigen.
FIG. 3.
Box plots of net absorbances of sera from nine naive dogs exposed to natural infection; the results of experiments with three recombinant antigens and IFAT are shown. Sera were collected before or immediately after exposure (lanes 1) and at 4 to 7 months (lanes 2) and 9 to 12 months (lanes 3) after exposure. Bases of boxes represent the first quartiles of absorbances, horizontal lines within boxes represent the medians, and top lines represent the third quartiles. Dashed lines are cutoff values of absorbances (ELISA) and IFAT titers (≥1:80).
Our results demonstrated that each antigen carries highly reactive and diagnostically relevant epitopes. The sensitivity of K26 as the antigen was greater than that of K9 and K39sub. However, K26 was the only antigen carrying a repetitive sequence. This not only resulted in a higher density of immunodominant epitope but also in a complete subset of overlapping epitopes which might be lost if a single repetition were to be used. Regarding K39, one parasitologically positive (true positive) dog serum gave negative results in both K39sub and standard rK39 ELISAs (11). This suggests a complementary effect of K9 and K26 epitopes on the overall sensitivity of K39 as the antigen.
The K26 ELISA results showed the best agreement with the IFAT results, while (in general) K9 and K39sub gave similar performances only when used in parallel. Moreover, K26 ELISAs classified as positive a higher proportion of IFAT-indeterminate sera. It was previously noted that IFAT results are minimally reproducible at threshold titers ranging from 1:20 to 1:160 (6, 11). K26 ELISAs may be useful in such situations and may also detect early antibody responses, as shown by the results seen with prospectively tested sera. It should be noted, however, that two sera showed a weak reaction (possibly caused by an imperfect purification of the K26 antigen) to K26 at the first sampling time.
Although results for the clinical or asymptomatic stages of dog infections were not recorded in this study, the reactivity to K9 and K26 was not dissimilar from that reported with rK39 (11) in that specific antibodies were usually detected in asymptomatic infections.
Interestingly, the performance of K26 in all groups of sera was not influenced by the use of either of the other two antigens. However, since all three antigens proved to be highly specific and independent in their antibody reactivity characteristics, use of the three antigens in combination could result in an increased signal-to-noise ratio.
Thus, the use of the three antigens in a single-well test, or the expression of the immunodominant epitopes in a single fusion protein, may further enhance both the specificity (compared with that of crude antigen preparations) and the sensitivity (which is usually higher in multiple-epitope formats such as IFAT test) of tests.
Acknowledgments
We thank Marina Gramiccia for providing the L. infantum reference strain.
This work was supported by AGROLABO s.p.a.
REFERENCES
- 1.Bhatia, A., N. S. Daifalla, S. Jen, R. Badaro, S. G. Reed, and Y. A. Skeiky. 1999. Cloning, characterization and serological evaluation of K9 and K26: two related hydrophilic antigens of Leishmania chagasi. Mol. Biochem. Parasitol. 102:249-261. [DOI] [PubMed] [Google Scholar]
- 2.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 3.Burns, J. M., Jr., W. G. Shreffler, D. R. Benson, H. W. Ghalib, R. Badaro, and S. G. Reed. 1993. Molecular characterization of a kinesin-related antigen of Leishmania chagasi that detects specific antibody in African and American visceral leishmaniasis. Proc. Natl. Acad. Sci. USA 90:775-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dean, A. G., J. A. Dean, D. Coulombier, K. A. Brendel, D. C. Smith, A. H. Burton, R. C. Dicker, K. Sullivan, R. F. Fagan, and T. G. Arner. 1995. Epi Info, version 6: a word processing, database and statistics program for public health on IBM-compatible microcomputers. Centers for Disease Control and Prevention, Atlanta, Ga.
- 5.Desjeux, P. 2001. The increase in risk factors for leishmaniasis worldwide. Trans. R. Soc. Trop. Med. Hyg. 95:239-243. [DOI] [PubMed] [Google Scholar]
- 6.Dye, C., E. Vidor, and J. Deneure. 1993. Serological diagnosis of leishmaniasis: on detecting infection as well as disease. Epidemiol. Infect. 103:647-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gradoni, L., and M. Gramiccia. 2000. Leishmaniasis, p. 803-812. In OIE (ed.), Manual of standards for diagnostic tests and vaccines. Office International des Epizooties (OIE), Paris, France.
- 8.Ihaka, R., and R. Gentelman. 1996. R: a language for data analysis and graphics. J. Comput. Graph. Stat. 5:299-314. [Google Scholar]
- 9.Maurício, L. L., J. R. Stothard, and M. A. Miles. 2000. The strange case of Leishmania chagasi. Parasitol. Today 16:188-189. [DOI] [PubMed] [Google Scholar]
- 10.Reithinger, R., and C. R. Davies. 2002. Canine leishmaniasis: novel strategies for control. Trends Parasitol. 18:289-290. [DOI] [PubMed] [Google Scholar]
- 11.Scalone, A., R. De Luna, G. Oliva, L. Baldi, G. Satta, G. Vesco, W. Mignone, C. Turilli, R. R. Mondesire, D. Simpson, A. R. Donoghue, G. R. Frank, and L. Gradoni. 2002. Evaluation of the Leishmania recombinant K39 antigen as a diagnostic marker for canine leishmaniasis and validation of a standardized enzyme-linked immunosorbent assay. Vet. Parasitol. 104:275-285. [DOI] [PubMed] [Google Scholar]
- 12.Smith, D. B., and K. S. Johnson. 1988. Single-step purification of polypeptides expressed in Escherichia coli as fusion with glutathione S-transferase. Gene 67:31-40. [DOI] [PubMed] [Google Scholar]
- 13.Zijlstra, E. E., N. S. Daifalla, P. A. Kager, E. A. Khalil, A. M. El-Hassan, S. G. Reed, and H. W. Ghalib. 1998. rK39 enzyme-linked immunosorbent assay for diagnosis of Leishmania donovani infection. Clin. Diagn. Lab. Immunol. 5:717-720. [DOI] [PMC free article] [PubMed] [Google Scholar]