Abstract
Human sera collected from 28 consenting adult volunteers were used to define assay conditions for meningococcal vaccine clinical trial serology. Immunoassay parameters were optimized with these test sera and the standard reference serum, CDC1992. Coating conditions for serogroup Y and W135 polysaccharide antigens were found to influence the predicted serum immunoglobulin G (IgG) antibody concentrations. Sera that displayed IgG antibody binding profiles most unlike that of CDC1992 were influenced the most by coating conditions. Our results suggest that presentation of specific epitopes is influenced by antigen-coating concentrations for serogroup Y and W135 polysaccharides.
Invasive disease caused by Neisseria meningitidis continues to be a primary health concern worldwide. Meningococcal capsular polysaccharides (MnPSs) are the basis for serogroup identification and remain a primary target for vaccine development. Four serogroups are currently included in licensed vaccine formulations (18). Serogroup A (-6ManpNAc-α1-OPO3-) and C (-9-NeupNAc-α2-) MnPSs are homopolymeric carbohydrates, while serogroup Y (-6-Glcp-α1-4-NeupNAc-α2) and W135 (-6-Galp-α1-4-NeupNAc-α2) MnPSs are polymers of disaccharide repeating units with closely related primary structures (2, 3, 5, 16, 19-21). Bactericidal antibodies specific for the meningococcal capsule confer protection against systemic meningococcal illness in the absence of the so-called blocking antibodies (14).
Humoral responses to various antigens are often quantified by some variation of the enzyme-linked immunosorbent assay (ELISA) (9, 10). The most common ELISA procedures involve the direct adsorption of an antigen to a solid support, such as polystyrene in a 96-well format, although more exotic assays have been described (see reference 6 for a review). Established ELISA methods for measuring anti-MnPS antibodies in human sera have been in use for over a decade. These procedures generally involve the use of a binding agent such as methylated human serum albumin (mHSA) (22) to promote adsorption of the hydrophilic polysaccharides to the polystyrene surface of the assay plate (1, 7, 13, 23, 24). Published methods were used by the Centers for Disease Control and Prevention (CDC; Atlanta, Ga.) to create the standard reference serum CDC1992 (8, 17) from 14 postvaccination human sera and to assess this pooled standard against the two previous single-donor meningococcal reference sera, ECG (15) and PB-2 (11, 12).
The purpose of this study was to define and optimize our ELISA operating conditions for serogroup Y and W135 MnPSs by using pre- and postvaccination human sera and reference serum CDC1992. Our results provide a detailed analysis of MnPS antigen coating conditions for these ELISA methods.
(This work was presented in part at the 13th International Pathogenic Neisseria Conference in Oslo, Norway, 2002.)
Experimental vaccination.
Twenty-eight consenting, healthy,adult volunteers (ages 21 to 57 years) with no history of meningococcal disease were selected to take part in this study. Plasma was collected from these subjects prior to vaccination and converted to sera with a COBE Spectra apheresis system (approximately 1 liter per subject). Subjects received a single dose of meningococcal polysaccharide vaccine (Menomune; Aventis) (50 μg [each] of serogroups A, C, Y, and W135 in a 0.5-ml dose administered subcutaneously) on day 0, and sera were collected from vaccinated subjects at approximately week 4 by plasmapheresis as described above. This study complied with all relevant federal guidelines and institutional policies.
Analysis of sera from adult volunteers.
Functional bactericidal antibodies were measured in pre- and postvaccination sera as previously described (4, 23, 24). Likewise, the concentrations of MnPS-specific immunoglobulin G (IgG) antibodies (in micrograms per milliliter) in unknown and control sera were measured by using the reference standard, CDC1992, as previously described (8, 17). The results of the experimental vaccination are shown in Table 1. Overall, the geometric mean concentration (GMC) of anti-serogroup C MnPS IgG increased 148-fold. Likewise, the geometric mean serum bactericidal titer (bactericidal GMT) (serogroup C strain C11) (see Table 2) increased 212-fold overall. The GMC and bactericidal GMT of anti-serogroup A (strain F8238) rose 72- and 18-fold, respectively, although the overall GMT for these samples was nearly 3-fold higher than the GMT for serogroup C. This discrepancy can be attributed to the finding that, overall, anti-serogroup A bactericidal titers in prevaccination sera were higher than anti-serogroup C titers. The GMTs of anti-serogroup Y and W135 increased 196- and 69-fold, respectively, and prevaccination titers were within the range observed for serogroup C. Overall, the concentrations of anti-serogroup Y and W135 MnPS IgG increased 26- and 24-fold, respectively. Therefore, on the whole, the volunteers responded immunologically well to the MnPS vaccination.
TABLE 1.
Summary of analysis for pre- and postvaccination seraa
| Sero- group | IgG concn (μg/ml) [range (GMC)]
|
Bactericidal titer [range (GMT)]
|
||
|---|---|---|---|---|
| Prevaccination | Postvaccination | Prevaccination | Postvaccination | |
| A | 0.05-4.49 (0.2) | 0.9-356.4 (14.3) | 2-2,048 (221) | 128-32,768 (3,898) |
| C | 0.05-1.33 (0.1) | 2.8-54.4 (14.8) | 2-1,024 (7) | 64-8,192 (1,485) |
| W135 | 0.05-6.37 (0.15) | 0.05-331.21 (5.9) | 2-2,048 (27) | 128-32,768 (1,855) |
| Y | 0.05-4.32 (0.14) | 0.08-60.67 (3.76) | 2-1,024 (11) | 64-32,768 (2,152) |
n = 28 samples.
TABLE 2.
Bacterial strains used in this study
| Strain | Serogroup | Source | Reference |
|---|---|---|---|
| F8238 | A | CDC | 23 |
| C11 | C | CDC | 15 |
| S1975 | Y | CDC | 23 |
| M00-242317 | W135 | PHLSa | This study |
PHLS, Public Health Laboratory Service, Manchester, United Kingdom.
Serum IgG antibody binding profiles.
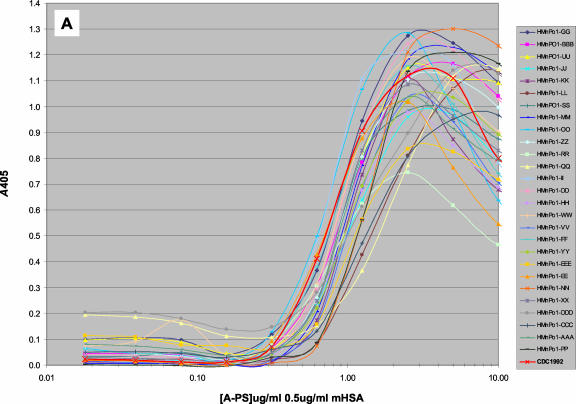
Standard antigen titration assays (10 to 0.01 μg/ml in antigen dilution buffer with various mHSA concentrations) were performed to determine the optimal coating conditions for serogroup A, C, Y, and W135 MnPSs, with mHSA as the binding agent. The profiles of the anti-serogroup A IgG response were qualitatively similar among all sera tested and emulated that of standard reference serum CDC1992 (Fig. 1A). The optimal coating concentration was determined to be within 1 to 3 μg/ml for serogroup A MnPS and 0.5 μg/ml for mHSA in phosphate-buffered saline (PBS) for nearly all samples tested. All constituents of these assays have been optimized based on these coating conditions. Likewise, optimal binding for serogroup C MnPS was consistent for all sera tested (data not shown).
FIG. 1.
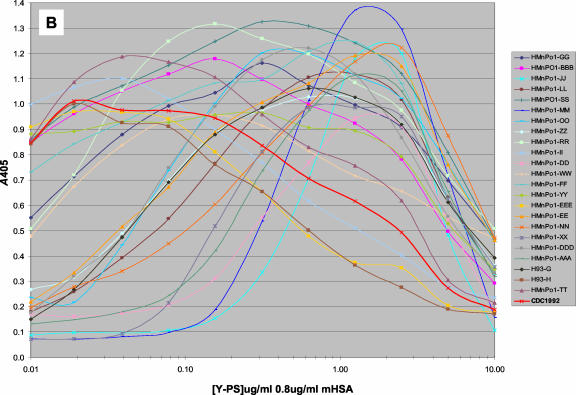
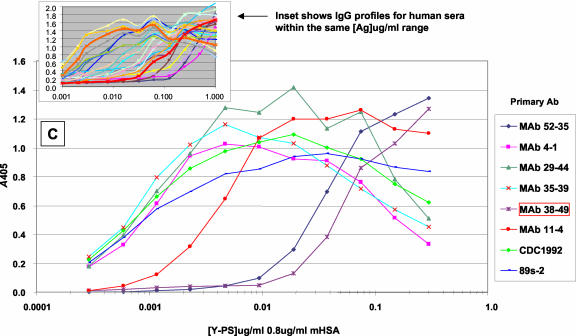
IgG binding profiles for different sera against MnPSs at various polysaccharide (PS) concentrations and with the concentration of mHSA held constant. Serum IgG binding profiles for serogroup A MnPS (A), serogroup Y MnPS (B), and various monoclonal antibodies against serogroup Y MnPS (C) are shown. See Table 3 for production antigens and specificities. All MAbs were prediluted 1,000-fold in antibody dilution buffer. Reference sera CDC1992 and 89s-2 were prediluted 1,000- and 500-fold, respectively. The inset in panel C shows IgG profiles for human sera at the same concentration range. Data represent the mean results for three data points. Ag, antigen.
A small subset of postvaccination sera displayed antibody binding profiles that deviated sharply from that of CDC1992 for serogroups Y (Fig. 1B) and W135 (data not shown). The optimal antigen-coating concentration for these assays varied depending on the serum tested. These results were confirmed (R. Borrow [Vaccine Evaluation Laboratory, Public Health Laboratory Service, Manchester, United Kingdom], personal communication) with an independent serum panel. The assay parameters were examined in an attempt to resolve the disparity in antibody binding profiles for these sera. Serum profiles were found to be qualitatively independent of assay plate manufacturer; antigen and antibody dilution buffers (PBS, pH 7; carbonate-bicarbonate buffer, pH 9; or succinate-buffered saline, pH 6); various incubation times and temperatures; and the source of mHSA, MnPS, and secondary antibody conjugate. The final mHSA concentration influenced the peak colorimetric signal (A405 − A690) but did not influence the relative IgG response profiles for these sera. Our data suggest that specific Y and W135 MnPS epitopes are optimally accessible at different antigen concentrations under these test conditions.
Experiments with monoclonal antibodies (MAbs) were conducted to test this hypothesis. The advantages of using MAbs instead of serum are that (i) each MAb recognizes a unique epitope and (ii) there are no other antibodies present, specific or cross-reactive, to compete for binding to the adsorbed antigen and influence the measured antibody binding profiles. Serogroup Y antigen titration assays (1 to 0.001 μg/ml in antigen dilution buffer with various mHSA concentrations) were performed with MAbs generated in mice against either serogroup Y or W135 MnPS (Table 3). The MAb binding profiles were shown to be analogous to our observations with human sera (Fig. 1C). The binding profiles for MAbs 4-1, 29-44, and 35-39 parallel the IgG binding profile for reference serum CDC1992. In contrast, MAb 52-35 (generated against serogroup W135 MnPS) and MAb 38-49 (specific for O-acetylated serogroup Y MnPS) showed optimal binding to serogroup Y MnPS at relatively higher coating concentrations. These data support the hypothesis that MAb-specific epitopes are optimally accessible at different points on the antigen titration curve.
TABLE 3.
MAbs used in this study
| MAb | Production antigen | Specificitya | Reference |
|---|---|---|---|
| 4-1 | Y | Y OAc+ and Y OAc− conserved region | 21a |
| 29-44 | Y | Y OAc+ and Y OAc− conserved region | This study |
| 35-39 | Y | Y OAc+ and Y OAc− conserved region | This study |
| 38-49 | Y | Y OAc+ only | 21a |
| 11-4 | Y | Y OAc+ and Y OAc− conserved region | This study |
| 52-35 | W135 | Y and W135 OAc+ and Y OAc− conserved region | This study |
OAc, O-acetyl.
MnPS coating recommendations.
Serogroup Y (-6-Glcp-α1-4-NeupNAc-α2) and W135 (-6-Galp-α1-4-NeupNAc-α2) antigens are structurally similar MnPSs composed of disaccharide repeating units. These antigens are somewhat more complex than serogroup A (-6ManpNAc-α1-OPO3-) and C (-9NeupNAc-α2-) MnPSs, and likewise the IgG binding profiles for Y and W135 MnPSs are shown to be more heterogeneous in our assays. A subset of postvaccination sera showed binding profiles that diverge from that of reference serum CDC1992. This circumstance creates a problem for the serogroup Y and W135 ELISAs. The concentration of MnPS-specific IgG antibodies present in sera is calculated by the log or log-linear regression method or by the four-parameter logistic method in our assays, and these calculations are based on a standard curve for reference serum CDC1992, which is generated on the same assay plate. Since the profile for the reference serum does not parallel the antibody binding profiles for a subset of sera, the calculated anti-MnPS IgG concentrations for these sera are dependent on antigen coating conditions. The serum most divergent from CDC1992 showed roughly a 10-fold difference in the optimal antigen-coating concentration and an 8- to 10-fold difference in the calculated IgG concentrations (in micrograms per milliliter) as a function of coating concentration.
Ultimately, higher MnPS and mHSA coating concentrations (e.g., 5 μg/ml each in PBS) provide more reliable results in our ELISA and mask inconsistencies associated with various mHSA preparations, which affect these assays at lower mHSA concentrations (Pearson's correlation coefficient [r] of 0.98 for both serogroup Y and W135 MnPSs). Furthermore, these coating conditions are most similar to those used by the CDC Immunology Section. Therefore, it is recommended that testing laboratories resolve optimal assay parameters based on the published (CDC) coating conditions for Y and W135 MnPS antigens whenever possible.
Acknowledgments
We extend our deepest appreciation to George Carlone and Cheryl Elie (CDC Immunology Section) for their contributions to this body of work.
REFERENCES
- 1.Arakere, G., and C. E. Frasch. 1991. Specificity of antibodies to O-acetyl-positive and O-acetyl-negative group C meningococcal polysaccharides in sera from vaccinees and carriers. Infect. Immun. 59:4349-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharjee, A. K., H. J. Jennings, C. P. Kenny, A. Martin, and I. C. Smith. 1976. Structural determination of the polysaccharide antigens of Neisseria meningitidis serogroups Y, W-135, and BO1. Can. J. Biochem. 54:1-8. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharjee, A. K., H. J. Jennings, C. P. Kenny, A. Martin, and I. C. Smith. 1975. Structural determination of the sialic acid polysaccharide antigens of Neisseria meningitidis serogroups B and C with carbon 13 nuclear magnetic resonance. J. Biol. Chem. 250:1926-1932. [PubMed] [Google Scholar]
- 4.Borrow, R., and G. M. Carlone. 2001. Serogroup B and C serum bactericidal assays, p. 289-304. In M. C. J. Maiden (ed.), Meningococcal vaccines: methods and protocols. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 5.Bundle, D. R., I. C. Smith, and H. J. Jennings. 1974. Determination of the structure and conformation of bacterial polysaccharides by carbon 13 nuclear magnetic resonance. Studies on the group-specific antigens of Neisseria meningitidis serogroups A and X. J. Biol. Chem. 249:2275-2281. [PubMed] [Google Scholar]
- 6.Butler, J. E. 2000. Solid supports in enzyme-linked immunosorbent assay and other solid-phase immunoassays. Methods 22:4-23. [DOI] [PubMed] [Google Scholar]
- 7.Carlone, G. M., C. E. Frasch, G. R. Siber, S. Quataert, L. L. Gheesling, S. H. Turner, B. D. Plikaytis, L. O. Helsel, W. E. DeWitt, W. F. Bibb, et al. 1992. Multicenter comparison of levels of antibody to the Neisseria meningitidis group A capsular polysaccharide measured by using an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 30:154-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elie, C. M., P. K. Holder, S. Romero-Steiner, and G. M. Carlone. 2002. Assignment of additional anticapsular antibody concentrations to the Neisseria meningitidis group A, C, Y, and W-135 meningococcal standard reference serum CDC1992. Clin. Diagn. Lab. Immunol. 9:725-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engvall, E., K. Jonsson, and P. Perlmann. 1971. Enzyme-linked immunosorbent assay. II. Quantitative assay of protein antigen, immunoglobulin G, by means of enzyme-labelled antigen and antibody-coated tubes. Biochim. Biophys. Acta 251:427-434. [DOI] [PubMed] [Google Scholar]
- 10.Engvall, E., and P. Perlman. 1971. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 8:871-874. [DOI] [PubMed] [Google Scholar]
- 11.Frasch, C. E., and J. D. Robbins. 1978. Protection against group B meningococcal disease. III. Immunogenicity of serotype 2 vaccines and specificity of protection in a guinea pig model. J. Exp. Med. 147:629-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frasch, C. E., J. M. Zahradnik, L. Y. Wang, L. F. Mocca, and C. M. Tsai. 1988. Antibody response of adults to an aluminum hydroxide-adsorbed Neisseria meningitidis serotype 2b protein-group B polysaccharide vaccine. J. Infect. Dis. 158:710-718. [DOI] [PubMed] [Google Scholar]
- 13.Gheesling, L. L., G. M. Carlone, L. B. Pais, P. F. Holder, S. E. Maslanka, B. D. Plikaytis, M. Achtman, P. Densen, C. E. Frasch, H. Käyhty, et al. 1994. Multicenter comparison of Neisseria meningitidis serogroup C anti-capsular polysaccharide antibody levels measured by a standardized enzyme-linked immunosorbent assay. J. Clin. Microbiol. 32:1475-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gotschlich, E. C., I. Goldschneider, and M. S. Artenstein. 1969. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J. Exp. Med. 129:1367-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gotschlich, E. C., T. Y. Liu, and M. S. Artenstein. 1969. Human immunity to the meningococcus. 3. Preparation and immunochemical properties of the group A, group B, and group C meningococcal polysaccharides. J. Exp. Med. 129:1349-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holder, P. K., S. E. Maslanka, L. B. Pais, J. Dykes, B. D. Plikaytis, and G. M. Carlone. 1995. Assignment of Neisseria meningitidis serogroup A and C class-specific anticapsular antibody concentrations to the new standard reference serum CDC1992. Clin. Diagn. Lab. Immunol. 2:132-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jennings, H. J. 1990. Capsular polysaccharides as vaccine candidates. Curr. Top. Microbiol. Immunol. 150:97-127. [DOI] [PubMed] [Google Scholar]
- 19.Lemercinier, X., and C. Jones. 1996. Full 1H NMR assignment and detailed O-acetylation patterns of capsular polysaccharides from Neisseria meningitidis used in vaccine production. Carbohydr. Res. 296:83-96. [DOI] [PubMed] [Google Scholar]
- 20.Liu, T. Y., E. C. Gotschlich, F. T. Dunne, and E. K. Jonssen. 1971. Studies on the meningococcal polysaccharides. II. Composition and chemical properties of the group B and group C polysaccharide. J. Biol. Chem. 246:4703-4712. [PubMed] [Google Scholar]
- 21.Liu, T. Y., E. C. Gotschlich, E. K. Jonssen, and J. R. Wysocki. 1971. Studies on the meningococcal polysaccharides. I. Composition and chemical properties of the group A polysaccharide. J. Biol. Chem. 246:2849-2858. [PubMed] [Google Scholar]
- 21a.Longworth, E., P. Fernsten, T. L. Mininni, U. Vogel, H. Claus, S. Gray, E. Kaczmarski, and R. Borrow. 2002. O-acetylation status of the capsular polysaccharides of serogroup Y and W135 meningococci isolated in the UK. FEMS Immunol. Med. Microbiol. 32:119-123. [DOI] [PubMed]
- 22.Mandell, J. D., and A. D. Hershey. 1960. A fractionating column for analysis of nucleic acids. Anal. Biochem. 1:66-77. [DOI] [PubMed] [Google Scholar]
- 23.Maslanka, S. E., L. L. Gheesling, D. E. Libutti, K. B. J. Donaldson, H. S. Harakeh, J. K. Dykes, F. F. Arhin, S. J. N. Devi, C. E. Frasch, J. C. Huang, P. Kriz-Kuzemenska, R. D. Lemmon, M. Lorange, C. C. A. M. Peeters, S. Quataert, J. Y. Tai, G. M. Carlone, and the Multilaboratory Study Group. 1997. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin. Diagn. Lab. Immunol. 4:156-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sikkema, D. J., K. E. Friedman, B. Corsaro, A. Kimura, S. W. Hildreth, D. V. Madore, and S. A. Quataert. 2000. Relationship between serum bactericidal activity and serogroup-specific immunoglobulin G concentration for adults, toddlers, and infants immunized with Neisseria meningitidis serogroup C vaccines. Clin. Diagn. Lab. Immunol. 7:764-768. [DOI] [PMC free article] [PubMed] [Google Scholar]