Abstract
Secretory immunity protects against mucosal transmission of viruses, as demonstrated with the oral poliovirus vaccine. In a previous study we showed that this immunity could be induced in mice by injection of a fusion peptide consisting of an unnatural peptide-like sequence (PADRE) and a viral epitope (ELDKWASLW). PADRE is a T-helper-cell epitope able to bind most major histocompatibility complex class II molecules of different haplotypes in mice and humans and to increase antibody responses. ELDKWA is a well-known consensual sequence of gp41 involved in a key structure of human immunodeficiency virus (HIV) type 1. Here, the antibody response to the native form of ELDKWA was mainly of the immunoglobulin A isotype and selectively occurred in mucosa. Adjuvants, such as cholera toxin and cytosine polyguanine, were useless and even competed with PADRE for the response. Interestingly, these antibodies were cross-reactive with the three major variants of the epitope, as shown both by direct enzyme-linked immunosorbent assay and by inhibition. This unconventional route of mucosal immunization allows control of the administered dose. The lack of adjuvant and the cross-reactivity of the antibodies increase the safety and the spectrum of the candidate vaccine, respectively. The drug-like nature of the construct suggests further improvements by synthesis of more antigenic sequences. The reasonable cost of short peptides at the industrial level and their purity make this approach of interest for future vaccines against mucosal transmission of HIV or other pathogens.
Vaccination is a method of choice for eradication of infectious diseases, including infection by HIV-1. Unfortunately, despite tremendous efforts using sophisticated methods of molecular biology, no clear protection against this disease has emerged from traditional approaches of vaccination of the systemic immune system in naive subjects. Additional methods of immunization against the virus thus are of interest to complement, potentiate, or replace the usual strategies. This is the case for vaccines inducing local immunity, which may protect against mucosa-transmitted viral diseases, as demonstrated by the efficacy of the oral polio vaccine in humans (30). Nevertheless, vaccination of the mucosal immune system rarely has been successful (6), leading to investigations of unconventional methods of inducing mucosal immunity by i.m. administration of a soluble Ag. This selected Ag was a fusion peptide consisting of a protective epitope of HIV-1 gp 41 (ELDKWA) with an unnatural major histocompatibility complex class II-binding peptide (PADRE).
In contrast with the systemic immune system, which is involved in protection against pathogens within the body, mucosal immunity forms a barrier preventing penetration of microorganisms through the epithelial border. Except for reducing the viral load in the gut reservoir (4), this immunity is rarely involved in recovery from disease. Conversely, it can decrease the inoculum to a harmless level (17). pIgA is the major isotype produced by mucosal plasma cells (8). It is actively transported across the epithelium with the help of the pIg receptor (22). The complex of pIgA with its receptor is cleaved from the apical membrane to form sIgA, which is then released in the mucosal lumen. The major functions of sIgA include immune exclusion and immune elimination. Immune exclusion (31) maintains the pathogen in the mucosal lumen by various mechanisms, such as agglutination, neutralization, and inhibition of interactions with the epithelium. Immune elimination also involves various mechanisms, such as clearance of intracellular or subepithelial pathogens by their transport to the lumen during transcytosis of the corresponding Abs (20). In the secretory lumen, the immune complexes formed with sIgA are eventually cleared by conveyance with the mucus stream. These mechanisms explain why a neutralizing activity of Abs in the mucosa is of interest but not an absolute requirement for protection against pathogens (9). However, since the secretory immune system acts only during the first step of infection, a preliminary contact with the Ag is a prerequisite for its role of immune barrier against transmission when the activity of natural Abs is overwhelmed (20, 27).
The mechanisms of induction and development of a mucosal immune response differ from those of systemic immunity. The first step consists of a translocation of the lumenal pathogen to Ag-presenting cells via epithelial M cells (18). The Ag-presenting cells are located in the mucosa-associated lymphoid tissues, where specific T and B lymphocytes are selected. Following a maturation cycle in the circulation, the B cells gain access to effector areas where Abs are produced by plasma cells dispersed throughout the subepithelial stroma. Induction of this humoral immunity is delayed, and the response is compartmentalized (5, 7, 21). Moreover, the persistence of the secretory immune response usually depends on that of the corresponding Ag. Therefore, most research programs deal with persisting Ags, such as live vaccines (6). Nonetheless, our previous results showed that a mucosal Ab response may persist for as long as 8 months following immunization of mice by injection of a soluble Ag, such as tetanus toxoid (11).
In addition, with the choice of the method of administration, the major problem in vaccine studies is selection of the Ag. In the context of a secretory antipeptide vaccine, the Abs must target an accessible epitope for immune exclusion or elimination. It has been demonstrated that the envelope protein gp41 is involved in the pathogenicity of the HIV infection, and many studies focus on a short amino acid sequence (aa 659 to 675) recognized by the human monoclonal Ab 2-F5 (23). This Ab protects against systemic (10) or mucosal transmission of the disease in simian models (2, 19). This protection, and the consensual sequence (ELDKWA) of the corresponding epitope (26), led us to select this peptide to investigate the induction of a mucosal immune response by our new method. Our group has induced both systemic and mucosal immune responses following i.m. injection of mice with a 17-aa peptide (EKNEQELLELDKWASLWN) coupled with tetanus toxoid (11). Recently, we obtained an Ab response in the mucosal organs by i.m. injection of a synthetic fusion peptide containing ELDKWA and PADRE (12). Interestingly, we showed that this mucosal immune response persists for a long time after the booster injection (11, 12).
In the present study, our aim was to improve this mucosal immune response to the i.m.-administered fusion peptide. We investigate two mucosal adjuvants, since we have already observed that Alum, the most commonly used systemic adjuvant, exerts an inhibitory effect on this response (12). We show that these adjuvants, CT and CpG, also suppress the response and are therefore not suitable for this immunization. In addition, we analyze the reactivity of the mucosal IgA Abs to ELDKWA with the three variants of this epitope and find them to be cross-reactive.
MATERIALS AND METHODS
Abbreviations.
i.m., intramuscular; Ag, antigen; Ab, antibody; Ig, immunoglobulin; IgA, immunoglobulin A; pIg and pIgA, polymeric Ig and IgA; sIgA, secretory IgA; PADRE, pan DR epitope; CT, cholera toxin; CpG, cytosine polyguanine; OVA, ovalbumin; HIV-1, human immunodeficiency virus type 1; aa, amino acid; PBS, phosphate-buffered saline; ELISA, enzyme-linked immunosorbent assay; OD, optical density; i.m., intramuscular; MAb, monoclonal antibody.
Immunization and collection of specimens.
Groups of five 2-month-old female BALB/c mice were i.m. injected in the thigh, four times at monthly intervals, with 0.2 ml of the PADRE-ELDKWA peptide in PBS. In some experiments, different concentrations of CT (Sigma Chemical, St. Louis, Mo.) or CpG ODN1826 (5′-TCC-ATG-ACG-TTC-CTG-ACG-TT-3′; MWG-Biotech, Courtaboeuf, France) were mixed with the peptide in the same final volume of 0.2 ml. The specimens were collected and treated by using the perfusion extraction method, PERFEXT, slightly modified (33). This method allowed measurement of the Ab response in local plasma cells, irrespective of the number of specific B cells. Briefly, the mice were exsanguinated 2 weeks following the last booster injection. The liver was fully bled, and the organs (spleen, jejunum, ileum, cecum, and colon) were freed from the peritoneum and the intestinal content. They were homogenized in the presence of protease inhibitors and extensively washed to remove the extracellular fluid. The pellet was suspended in a final dilution of about 1:10 and frozen at −20°C. Disruption of the cell membranes, to release intracellular Igs, was achieved by thawing overnight at 4°C in the presence of 2% (vol/vol) saponin (final dilution) and then cleared by centrifugation. Aliquots of the supernatants were stored at −20°C until use. Vaginal fluids were collected by washing with 0.1 ml of PBS and pooled. Mucus and cells from each pool were removed by centrifugation at 10,000 × g, and the pellets were controlled for the absence of red blood cells. The supernatants were stored at −80°.
Antigens.
The peptides (Table 1) were synthesized by Neosystem (Strasbourg, France). Their purity and molecular mass were controlled by liquid chromatography and mass spectroscopy. The consensual immunizing Ag consisted of PADRE with the ELDKWA peptide (aa 659 to 664) extended with its adjacent C terminus sequence in gp41 SLW (aa 665 to 667). The Ags used for detection contained either the consensual sequence of gp41 or one of its three major variants. A consensual epitope (p145) of the streptococcal protein M served as a negative control. These five peptides were extended with a C terminus cysteine residue used to branch six chains to OVA by disulfide bridging, as already described (11, 12). This construct maintains the branched peptides under a native form necessary for ELISA and for inhibition studies.
TABLE 1.
Amino acid sequences involved in the selection, construction, injection, and investigation of the specificity of the vaccine
| Peptide name | Amino acid sequencea | Property |
|---|---|---|
| ELDKWA | ELDKWA | Consensual hexamer |
| PADREb | aKXVAAWTLKAAaZ | MHC class II binderf |
| PADRE-ELDKWA | aKXVAAWTLKAAaZELDKWASLW | Immunizing Ag |
| P659-675 | EKNEQELLELDKWASLW | Consensual 17-aa peptide |
| ELDKWA-OVAc | (EKNEQELLELDKWASLWC)6-OVA | Assayed consensual Ag |
| ELEKWA-OVAd | (EKNEQELLELEKWASLWC)6-OVA | Assayed E669 variant Ag |
| ELNKWA-OVA | (EKNEQELLELNKWASLWC)6-OVA | Assayed N669 variant Ag |
| ELDEWA-OVA | (EKNEQELLELDEWASLWC)6-OVA | Assayed E670 variant Ag |
| P145-OVAe | (LRRDLDASREAKKQVEKALEC)6-OVA | Assayed negative control |
Single-letter designation (ital.). Bold characters indicate the key-sequences of gp41 (ELDKWA) and of its variants, as well as that of the streptococcal peptide ASREAK.
PADRE, Pan DR epitope; a, d-alanine; X, l-cyclo-hexyl-alanine; Z, amino-caproic acid.
The additional carboxy-terminal cysteine (C) allows disulfide exchanges with the six cysteines of OVA.
The underlined residues correspond to the amino acid changes of the variant peptides.
Peptide 145 from the streptococcal protein M.
MHC, major histocompatibility complex.
Antibody assays.
Microtiter plates (Nunc, Roskilde, Denmark) were coated overnight at 4°C with 50 μl of ELDKWA-OVA (4 μg/ml) per well (Table 1). Washes were carried out with 0.1% Tween 20 in PBS. The free reactive groups were saturated with 100 μl of 0.5% gelatin in PBS per well. The wells were washed with PBS-Tween and incubated with 50 μl of diluted samples for 1 h at 37°C. Following additional washes and incubation with a peroxidase-labeled sheep Ab to the murine α or γ chain (Sigma), the enzyme was revealed with o-phenylene diamine. The reproducibility of the OD values was ∼10%. The level of IgA Abs was expressed in micrograms per milliliter by comparison with the curve of a calibrated reference pool present in the same plate. The calibration (in micrograms per milliliter of IgA Abs) of this pool of jejunum and ileum extracts, selected for a high level of IgA Abs to ELDKWA, was carried out by comparison with the IgA concentration curve of the MAb TEPC15 (Sigma). OD values lower than the exponential segment of the reference curve were considered not significant. The results of the pools of vaginal fluids were expressed as relative Ab levels both because of the variable dilution of this fluid and the variable efficiency of the washing. The relative level (in arbitrary units) was defined as the ratio of Ab activity to the concentration of total Ig (12). Because of their low levels, the IgG Abs were expressed only by OD values.
Statistical analysis.
The IgA Ab levels in the tissue extracts from mice immunized in the presence, or absence, of adjuvant were compared by using the two-tailed U test of Mann and Whitney. The comparison was carried out between the corresponding organs of the adjuvant-free group and of the adjuvant-treated groups. In the case of colon extracts, the sensitivity of the comparison was increased by joining the results for all CT-treated animals (n = 15) in one group, which was compared with the adjuvant-free group (n = 5).
Cross-reactivity and inhibition experiments.
Cross-reactivity to variant peptides was investigated with ELISA plates coated with OVA-linked peptides. Positive and negative controls were coated with ELDKWA-OVA or p145-OVA, respectively. Binding to these molecules was assayed with dilutions of the reference pool of IgA Abs. For inhibition experiments, dilutions of this pool were incubated with various concentrations of ELDKWA-OVA, or of its variants, for 1 h at 37°C. The remaining Ab activity in response to ELDKWA-OVA was then investigated with the ELISA. The percentage of inhibition was calculated by comparing the ODs at 490 nm of the Abs incubated with peptides or with PBS.
RESULTS
Decrease of locally synthesized IgA Abs in the presence of CT or CpG.
Uncleaved CT being nontoxic in adult mice, these animals remained healthy during the course of experiments. Although CT is a mucosal adjuvant that enhances the Ab response during transcutaneous immunization (3), it was found here to impair the IgA response to 1 μg of PADRE-ELDKWA/injection (P ≤ 0.01). This result was observed in all mucosal organs (Fig. 1) as well as in the pooled vaginal washes (Fig. 2). IgA Abs were not detected in serum nor in the spleen. Interestingly, the level of IgA Abs in the pooled vaginal washes was higher at the dose of 1 μg of CT/injection than at 0.1 and 0.01 μg. Six groups of mice immunized with doses of 0.1 or 0.01 μg of PADRE-ELDKWA together with CT (1, 0.1, or 0.01 μg) exhibited lower responses than those with 1 μg of PADRE-ELDKWA plus 1 μg of CT (data not shown). This confirms that 1 μg of PADRE-ELDKWA is the optimal dose, whatever the concentration of CT. No Ab response was observed in a control group of mice injected with 1 μg of CT alone. CpG also decreased the IgA Ab response to PADRE-ELDKWA (Fig. 3), but more severely, and no IgA Ab was detected in the vaginal fluid. Investigation of IgG Abs (Table 2) showed low-level and variable responses with no evidence of an inhibitory effect of the adjuvants.
FIG. 1.
In the range of 0.01 to 1 μg, CT inhibits the IgA Ab response to ELDKWA in mice injected with a constant dose of PADRE-ELDKWA (1 μg/injection). (A) Ag without CT. (B, C, and D) Ag plus dilutions of CT. ELISA was carried out against ELDKWA-OVA. The 1:20 dilution of tissue extracts corresponds to that of the serum (1:100). The Ab level was established as indicated in the text. The statistical analysis compared the Ab values in specimens from each group of CT-treated mice with those from the mice administered PADRE-ELDKWA alone. *, P ≤ 0.05; **, P ≤ 0.01.
FIG. 2.
In the range of 0.01 to 0.1 μg, CT inhibits the IgA Ab response to ELDKWA in the vaginal fluid of mice i.m. injected with 1 μg of PADRE-ELDKWA. The relative level of IgA Abs was established in pooled vaginal washes from five mice immunized with PADRE-ELDKWA in the presence or absence of CT.
FIG. 3.
In the range of 0.01 to 1 μg, CpG inhibits the IgA Ab response to 1 μg of ELDKWA in mice injected with PADRE-ELDKWA. **, P ≤ 0.01.
TABLE 2.
Low values of IgG antibodies to ELDKWA in the organs and serum of mice immunized with PADRE-ELDKWA in the presence or absence of CT or CpG
| Adjuvant (concn [μg]) | OD490b of IgG in organs and seruma
|
|||||
|---|---|---|---|---|---|---|
| Spleen | Jejunum | Ileum | Cecum | Colon | Serum | |
| None | 0.06 (0-0.26) | 0.11 (0.07-0.14) | 0.10 (0.04-0.14) | 0.18 (0.15-0.45) | 0.12 (0.09-0.20) | 0.13 (0.01-0.19) |
| CT (1) | 0.15 (0.04-0.23) | 0.07 (0.06-0.07) | 0.21 (0.17-0.23) | 0.13 (0.11-0.24) | 0.08 (0.06-0.10) | 0.14 (0.06-0.18) |
| CT (0.1) | 0.20 (0.05-0.38) | 0.11 (0.06-0.12) | 0.20 (0.08-0.28) | 0.09 (0.04-0.17) | 0.14 (0.08-0.15) | 0.09 (0.04-0.12) |
| CT (0.01) | 0.03 (0.01-0.13) | 0.17 (0.10-0.25) | 0.15 (0.08-0.27) | 0.11 (0.05-0.14) | 0.19 (0.13-0.24) | 0.05 (0.04-0.06) |
| CPG (1) | 0.01 (0.00-0.07) | 0.15 (0.06-0.20) | 0.26 (0.05-0.39) | 0.07 (0.03-0.08) | 0.15 (0.09-0.28) | 0.03 (0.02-0.04) |
| CPG (0.1) | 0.00 (0.00-0.06) | 0.12 (0.08-0.26) | 0.14 (0.10-0.22) | 0.11 (0.02-0.14) | 0.15 (0.10-0.16) | 0.02 (0.02-0.09) |
| CPG (0.01) | 0.04 (0.00-0.05) | 0.16 (0.09-0.28) | 0.16 (0.11-0.32) | 0.02 (0.00-0.14) | 0.10 (0.10-0.19) | 0.04 (0.02-0.07) |
Median values and ranges.
OD at 490 nm.
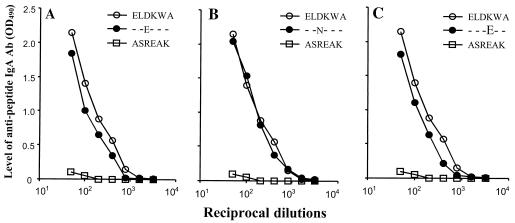
Reactivity of Abs to ELDKWA with the variant peptides.
A reactivity was observed when the pool of mucosal IgA Abs to ELDKWA was assayed with ELEKWA-OVA, ELNKWA-OVA, and ELDEWA-OVA (Fig. 4). The curves of activity were similar to that of the positive control ELDKWA-OVA. As expected, no reactivity was observed with the negative control p145-OVA, containing the ASREAK peptide from the streptococcal protein M.
FIG. 4.
Cross-reactivity of IgA Abs to ELDKWA with the variant peptides. Abs were from the pool of jejunum and ileum extracts of mice immunized with PADRE-ELDKWA. The ELISA plates were coated with ELDKWA-OVA, ELEKWA-OVA, ELNKWA-OVA, or ELDEWA-OVA. The negative control ASREAK peptide from the streptococcal protein M was assayed with p145-OVA-coated wells. The dilution curves of the consensual sequence and those of the variants were similar.
Inhibition of the Ag-Ab reaction.
Incubation of the IgA pool of Abs to ELDKWA with the four OVA-coupled HIV peptides dose dependently inhibited the reactivity of this pool with ELDKWA-OVA (Fig. 5). The inhibition curves were similar.
FIG. 5.
Inhibition of IgA Abs to ELDKWA following incubation with the OVA-linked peptides. The reference pool of IgA Abs was incubated with dilutions of ELDKWA-OVA or with the OVA-linked variants and assayed against ELDKWA-OVA. The concentration of inhibitor corresponds to that of the whole OVA peptide. The concentration of the ELDKWA epitope alone would be ∼15-fold lower.
DISCUSSION
We investigate here an alternative method of induction of IgA Abs to a protective epitope of HIV. We show that addition of mucosal adjuvants impairs the response and that the induced Abs to ELDKWA cross-react with the variants of this consensual epitope. We have already shown that the response is long lasting and indicated that the method may be suitable for mass vaccinations in humans.
To avoid anatomic and physiologic differences between humans and mice, the Ags were administered at a distance from the pharyngeal mucosa (8). Similarly, the transcytosis of serum-derived pIgA through murine hepatocytes (14) led us to investigate pIgA Abs in tissue extracts and not in the content of the gut lumen. We tried to improve the immune response by analyzing the effects of two different adjuvants: CT and CpG. CT is a major adjuvant of the mucosal IgA response, either under its complete form or under its B subunit (28). In humans, CT (but not its B subunit) is highly toxic and must be used as a genetically modified nontoxic derivative (25). This is the case for a recent oral anticholera vaccine (24). CpG is also a mucosal adjuvant (13). Impairment of the anti-ELDKWA responses (Fig. 1 to 3) is unusual and at variance with the increased serum IgG response to a fucopentose-PADRE conjugate in the presence of different adjuvants (1). We notice that the three adjuvants shown to suppress the mucosal IgA response anti-ELDKWA usually display different mechanisms that improve the immune response. Alum acts by a depot effect, leading to a long-lasting release of the Ag. CT enhances the penetration of the Ag through the gut epithelium and the skin and acts as both an immunogen and an adjuvant (28). CPG induces both specific and nonspecific immunostimulations via a Toll-like receptor (16).
The linear conjugation of PADRE with a B-cell epitope allows activation and Ag presentation of adjacent cells. PADRE may also be involved in the rejection of tumors when associated with a CD8-reactive foreign peptide (34). An interesting problem is the contrast between the selective induction of a mucosal response and the low level of serum Abs. This contrast may be due both to PADRE and to ELDKWA, since injection of soluble Ags containing various haptens and carriers leads to different responses in serum as well as in mucosa (11).
The cross-reactivity between the consensual epitope ELDKWA and variant peptides is of major interest for using a single Ag to protect against most HIV-1 clades. In contrast with the gp41 mutations induced by the synthetic peptide enfuvirtide (T-20), which render the virus resistant to this treatment (15, 29), the variations in the ELDKWA sequence may not impair the protection by sIgA Abs. Only three variants, corresponding to a change of aa 670 or 671, were described in primary isolates or in strains obtained by immune selection (26, 32). They were shown to be cross-reactive (35), as demonstrated with serum IgG Abs from rabbits that were immunized in the footpads with bovine serum albumin-linked peptides emulsified in Freund's complete adjuvant. At variance, our results were obtained in the context of the study of a future vaccine suitable for humans. Our mucosal IgA Abs were assayed with a long native peptide, which was coated to the ELISA plates via its branching to OVA. Moreover, the response was multiregional, as shown by IgA Abs in areas unrelated with the inoculation site.
A passive immunity can be transmitted with the MAb 2-F5 and other MAbs to gp41, indicating that under some conditions Abs may be sufficient for immune protection. Our results provide a basis for studying this response in primates and for investigating other epitopes.
Acknowledgments
We thank Sylvio Iscaki for helpful discussion and critical review of the manuscript.
This work was supported by Agence Nationale de Recherches sur le SIDA (ANRS) and Institut National de la Santé et de la Recherche Médicale (INSERM). Nipa Decroix is a recipient of a fellowship from ANRS. Perayot Pamonsinlapatham is working on a collaborative project between INSERM UR430 (France) and the Faculty of Pharmacy, Silpakorn University (Thailand).
REFERENCES
- 1.Alexander, J., M. F. Del Guercio, A. Maewal, L. Qiao, J. Fikes, R. W. Chesnut, J. Paulson, D. R. Bundle, S. DeFrees, and A. Sette. 2000. Linear PADRE T helper epitope and carbohydrate B cell epitope conjugates induce specific high titer IgG antibody responses. J. Immunol. 164:1625-1633. [DOI] [PubMed] [Google Scholar]
- 2.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. [DOI] [PubMed] [Google Scholar]
- 3.Beignon, A.-S., J.-P. Briand, S. Muller, and C. D. Partidos. 2002. Immunization onto bare skin with synthetic peptides: immunomodulation with a CpG-containing oligonucleotide and effective priming of influenza virus-specific CD4+ T cells. Immunology 105:204-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belyakov, I. M., Z. Hel, B. Kelsall, V. A. Kuznetsov, J. D. Alhers, J. Nacsa, D. I. Watkins, T. M. Allen, A. Sette, J. Altman, R. Woodward, P. D. Markham, J. D. Clemens, G. Franchini, W. Strober, and J. A. Berzofsky. 2001. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat. Med. 7:1320-1326. [DOI] [PubMed] [Google Scholar]
- 5.Berneman, A., L. Bélec, V. A. Fischetti, and J. P. Bouvet. 1998. The specific patterns of human immunoglobulin G antibodies in serum differ from those in autologous secretions. Infect. Immun. 66:4163-4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouvet, J. P., N. Decroix, and P. Pamonsinlapatham. 2002. Stimulation of local antibody production: parenteral or mucosal vaccination? Trends Immunol. 23:209-213. [DOI] [PubMed] [Google Scholar]
- 7.Brandtzaeg, P., I. N. Farstad, and G. Haraldsen. 1999. Regional specialization in the mucosal immune system: primed cells do not always home along the same tract. Immunol. Today 20:267-277. [DOI] [PubMed] [Google Scholar]
- 8.Brandtzaeg, P., I. N. Farstad, F. E. Johansen, H. C. Morton, I. N. Norderhaug, and T. Yamanaka. 1999. The B-cell system of human mucosae and exocrine glands. Immunol. Rev. 67:45-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burns, J. W., M. Siadat-Pajouh, A. A. Krishnaney, and H. B. Greenberg. 1996. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science 272:104-107. [DOI] [PubMed] [Google Scholar]
- 10.Conley, A. J., J. A. Kessler, L. J. Boots, P. M. McKenna, W. A. Schleif, E. A. Emini, G. E. Mark, H. Katinger, E. K. Cobb, S. M. Lunceford, S. R. Rouse, and K. K. Murthy. 1996. The consequence of passive administration of an anti-human immunodeficiency virus type 1 neutralizing monoclonal antibody before challenge of chimpanzees with a primary virus isolate. J. Virol. 70:6751-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Decroix, N., H. Hocini, C. P. Quan, B. Bellon, M. D. Kazatchkine, and J. P. Bouvet. 2001. Induction in mucosa of IgG and IgA antibodies against parenterally administered soluble immunogens. Scand. J. Immunol. 53:401-409. [DOI] [PubMed] [Google Scholar]
- 12.Decroix, N., C. P. Quan, P. Pamonsinlapatham, and J. P. Bouvet. 2002. Mucosal immunity by intramuscular administration of free peptides in-line with PADRE: IgA antibodies to the ELDKWA epitope of HIV gp41. Scand. J. Immunol. 56:59-65. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson, K., and J. Holmgren. 2002. Recent advances in mucosal vaccines and adjuvants. Curr. Op. Immunol. 14:666-672. [DOI] [PubMed] [Google Scholar]
- 14.Giffroy, D., A. Langendries, M. Maurice, F. Daniel, B. Lardreux, P. J. Courtoy, and J.-P. Vaerman. 1998. In vivo stimulation of polymeric Ig receptor transcytosis by circulating polymeric IgA in rat liver. Int. Immunol. 10:347-354. [DOI] [PubMed] [Google Scholar]
- 15.Hanna, S. L., C. Yang, S. M. Owen, and R. B. Lal. 2002. Variability of critical epitopes within HIV-1 heptad repeat domains for selected entry inhibitors in HIV-infected populations worldwide. AIDS 16:1603-1608. [DOI] [PubMed] [Google Scholar]
- 16.Horner, A. A., N. Cinman, A. Ronaghy, and E. Raz. 2000. Mucosal adjuvanticity of immunostimulatory DNA sequences. Springer Semin. Immunopathol. 22:133-146. [DOI] [PubMed] [Google Scholar]
- 17.Kato, H., R. Kato, K. Fujihashi, and J. R. McGhee. 2001. Role of microbial antibodies in viral infections. Curr. Top. Microbiol. Immunol. 260:201-228. [DOI] [PubMed] [Google Scholar]
- 18.Kraehenbuhl, J. P., and M. R. Neutra. 2000. Epithelial M cells: differentiation and function. Annu. Rev. Cell Dev. Biol. 16:301-332. [DOI] [PubMed] [Google Scholar]
- 19.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 20.Mazanec, M. B., J. G. Nedrud, C. S. Kaetzel, and M. E. Lamm. 1993. A three-tiered view of the role of IgA in mucosal defense. Immunol. Today 14:430-435. [DOI] [PubMed] [Google Scholar]
- 21.Moldoveanu, Z., M. W. Russell, H. Y. Wu, W. Q. Huang, R. W. Compans, and J. Mestecky. 1995. Compartmentalization within the common mucosal immune system. Adv. Exp. Med. Biol. 371A:97-101. [DOI] [PubMed] [Google Scholar]
- 22.Mostov, K. E. 1994. Transepithelial transport of immunoglobulins. Annu. Rev. Immunol. 12:63-84. [DOI] [PubMed] [Google Scholar]
- 23.Muster, T., F. Steindl, M. Purtscher, A. Trkola, A. Klima, G. Himmler, F. Ruker, and H. Katinger. 1993. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 67:6642-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naficy, A. B., D. D. Trach, N. T. Ke, N. T. Chuc, A. Sorkin, N. R. Rao, T. H. Sy, V. D. Thiem, D. G. Cahn, R. T. Mahoney, J. Holmgren, B. Invanoff, and J. D. Clemens. 2001. Cost of immunization with a locally produced oral cholera vaccine in Viet Nam. Vaccine 19:3720-3725. [DOI] [PubMed] [Google Scholar]
- 25.Pizza, M., M. M. Giuliani, M. R. Fontana, E. Monaci, G. Douce, G. Dougan, K. H. Mills, R. Rappuoli, and G. Del Giudice. 2001. Mucosal vaccines: non toxic derivatives of LT and CT as mucosal adjuvants. Vaccine 19:2534-2541. [DOI] [PubMed] [Google Scholar]
- 26.Purtscher, M., A. Trkola, A. Grassauer, P. Schulz, A. Klima, S. Döpper, G. Gruber, A. Buchacher, T. Muster, and H. Katinger. 1996. Restricted antigenic variability of the epitope recognized by the neutralizing gp41 antibody 2F5. AIDS 10:587-593. [DOI] [PubMed] [Google Scholar]
- 27.Quan, C. P., A. Berneman, R. Pirčs, S. Avrameas, and J. P. Bouvet. 1997. Natural polyreactive secretory immunoglobulin A autoantibodies as a possible barrier to infection in humans. Infect. Immun. 65:3997-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rappuoli, R., M. Pizza, G. Douce, and G. Dougan. 1999. Structure and mucosal adjuvanticity of cholera and Escherichia coli heat-labile enterotoxins Immunol. Today 20:493-500. [DOI] [PubMed] [Google Scholar]
- 29.Roman, F., D. Gonzalez, C. Lambert, S. Deroo, A. Fischer, T. Baurith, T. Staub, R. Boulme, V. Arendt, F. Schneider, R. Hemmer, and J. C. Schmit. 2003. Uncommon mutations at residue positions critical for enfuvirtide (T-20) resistance in enfuvirtide-naive patients infected with subtype B and non-B HIV-1 strains. J. Acquir. Immune Defic. Syndr. 32:134-139. [DOI] [PubMed] [Google Scholar]
- 30.Sabin, A. B., and L. R. Boulger. 1973. History of Sabin attenuated poliovirus oral live vaccine strains. J. Biol. Stand. 1:115-118. [Google Scholar]
- 31.Stokes, C. R., J. F. Southill, and M. W. Turner. 1975. Immune exclusion is a function of IgA. Nature 255:745-746. [DOI] [PubMed] [Google Scholar]
- 32.Trkola, A., A. B. Pomales, H. Yuan, B. Korber, P. J. Maddon, G. P. Allaway, H. Katinger, C. F. I. Barbas, D. R. Burton, D. D. Ho, and J. P. Moore. 1995. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J. Virol. 69:6609-6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villavedra, M., H. Carol, J. Holmgren, and C. Czerkinsky. 1997. “PERFEXT”: a direct method for quantitative assessment of cytokine production in vivo at the local level. Res. Immunol. 148:257-266. [DOI] [PubMed] [Google Scholar]
- 34.Wei, W. Z., S. Ratner, T. Shibuya, G. Yoo, and A. Jani. 2001. Foreign antigenic peptides delivered to the tumor as targets of cytotoxic T cells. J. Immunol. Methods 258:141-150. [DOI] [PubMed] [Google Scholar]
- 35.Xiao, Y., X.-N. Dong, and Y.-H. Chen. 2000. Induction of high levels of antibodies recognizing the neutralizing epitope ELDKWA and the D- or K-position-mutated epitopes by candidate epitope vaccines against HIV-1. Int. Arch. Allergy Immunol. 122:287-292. [DOI] [PubMed] [Google Scholar]