Abstract
We tested a single-step serological assay against Coxiella burnetii and Bartonella species and found a sensitivity of 100%, and a positive predictive value of 98% for the diagnosis of blood culture-negative endocarditis (BCNE). This assay should be considered as a possible commercial test for the diagnosis of BCNE.
Infective endocarditis is a serious, life-threatening disease with highly variable clinical signs that make the condition a diagnostic challenge. A diagnosis is readily made if blood cultures are positive, but in 2.5 to 31% of all infective endocarditis cases, routine blood cultures are negative (1, 2, 8). In such situations, alternative diagnostic approaches are necessary, especially serology. There is only one recent report of a study that analyzed 63 cases of blood culture-negative endocarditis (BCNE) including the new etiologic agents (Coxiella burnetii and Bartonella sp.) and a modification of the Duke criteria (10). Nearly one-quarter of the 63 cases of BCNE described were caused by these agents (10). These bacteria represent the most common etiological agents of BCNE, with about 400 cases of C. burnetii endocarditis and more than 100 cases of Bartonella endocarditis reported to date (2). These serologic tests are included as diagnostic parameters for infective endocarditis in the modified Duke criteria (5, 11). An immunoglobulin G (IgG) titer of 1:1,600 against the phase I antigen of C. burnetii is required for the diagnosis of Q fever endocarditis (16), whereas a titer of ≥1:800 for IgG antibodies to either Bartonella henselae or B. quintana is used for the diagnosis of endocarditis due to Bartonella sp. (13). In this study, we assessed the ability of a one-step serological assay using a single serum dilution to diagnose BCNE due to either C. burnetii or Bartonella species.
Sera from 50 patients with BCNE due to either Bartonella spp. (15 patients with B. quintana and 5 with B. henselae endocarditis) or C. burnetii (30 patients) were used in this study. Bartonella or C. burnetii endocarditis was diagnosed by positive cell culture or by PCR amplification of DNA of the organisms from valve samples (6, 14). Control patients included 40 apparently healthy blood donors, 99 patients with blood culture-positive endocarditis caused by various bacteria (Table 1), 15 patients with cat scratch disease (17), 15 patients with acute Q fever (15), and 25 patients with a serological diagnosis of a bacterial or viral disease, i.e., 5 patients with cytomegalovirus infection, 5 patients with Epstein-Barr virus infection, 5 patients with hepatitis B, 5 patients with herpes simplex virus infection, and 5 patients with human immunodeficiency virus-positive serology (12). Sera were numbered from 1 to 244 before testing, which was performed blind by a technician. The presence of rheumatoid factor was determined with a latex agglutination assay (Rapitex, RF; Dade Behring) as recommended by the manufacturer. Antigens used for the serologic assay were prepared as previously described for B. henselae Houston-1 (3, 13) or for the C. burnetii phase I Nine Mile strain (7, 16). To avoid false-negative results because sera had not been added to slide wells, we also used a positive control of Staphylococcus aureus (ATCC 29213) antigen, the protein A of which is reactive with the Fc fraction of human immunoglobulins (4). The three different antigens were spotted by pen point (about 2 μl) onto each well of 20-spot slides (Dynex; Cell-Line Associates Inc., New Field, N.J.), fixed in acetone for 20 min, and used for detection of IgG antibodies by immunofluorescence assay as previously described (13, 16).
TABLE 1.
Etiological agents of the 99 control cases of endocarditis
| Bacteria | No. of cases |
|---|---|
| Streptococcus sp. | 24 |
| Staphylococcus aureus | 22 |
| Streptococcus bovis | 18 |
| Coagulase-negative staphylococci | 13 |
| Enterococcus sp. | 12 |
| Escherichia coli | 3 |
| Gemella morbillorum | 2 |
| Acinetobacter baumanii | 1 |
| Actinobacillus | 1 |
| Campylobacter fetus | 1 |
| Granulicatella elegans | 1 |
| Haemophilus segnis | 1 |
| Total | 99 |
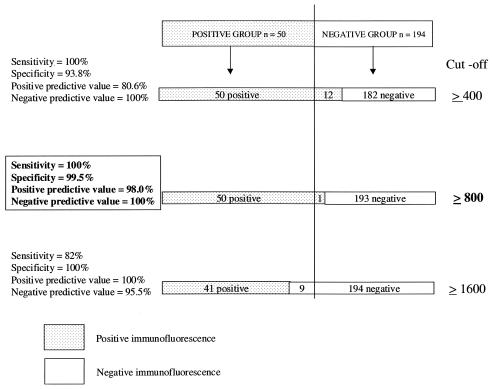
The etiologies of the 99 blood culture-positive endocarditis cases are presented in Table 1. The sera of 11 of these 99 patients contained rheumatoid factor, as determined by latex agglutination. As a result of preliminary studies, we only used the phase I antigen of C. burnetii in our testing and only measured IgG against C. burnetii and Bartonella sp. to avoid false positives due to rheumatoid factor (false-positive IgM when specific IgG antibodies are present). All of the 244 serum samples tested were positive at a cutoff titer of 1:800 against S. aureus, demonstrating that serum had been added to each slide well. The sensitivity, specificity, positive predictive value, and negative predictive value for the different cutoffs used are presented in Fig. 1. At the different cutoff titers used (1:400, 1:800, and 1:1,600), all of the sera from blood donors, cat scratch disease patients, and patients seropositive for bacterial or viral diseases not related to Bartonella or Coxiella species were negative. We found that the sensitivity was 100% and the specificity was 99.5% at a cutoff titer of 1:800 for the detection of Bartonella and C. burnetii IgG antibodies in patients with endocarditis. At this cutoff titer, the only false positive was a patient suffering from acute Q fever with no evidence of endocarditis. All of the 99 patients suffering from endocarditis due to bacteria other than Bartonella sp. and C. burnetii were negative. At a cutoff titer of 1:400, the specificity was lower (93.8%), with 12 false positives (eight patients with acute Q fever and four with blood culture-positive endocarditis). Conversely, at a cutoff titer of 1:1,600, the sensitivity was 82%, with nine false negatives (nine patients with endocarditis due to Bartonella sp.). Since high titers of antibodies against either C. burnetii or Bartonella sp. can discriminate between patients with endocarditis and those with acute or benign clinical manifestations of infection, our assay enabled us to avoid the problem of interpretation of the results when antibody titers are low (12, 15). Determination of IgG levels was more reproducible than determination of total immunoglobulin levels, which can contain rheumatoid factor and lead to false-positive results. For each assay, addition of S. aureus antigen to our test wells enabled us to be sure that sera had been added to the wells in the test. With a cutoff titer of 1:800, we found no cross-reactions between C. burnetii and Bartonella antigens as previously reported (9). We only found one false positive in a patient with acute Q fever but no evidence of endocarditis. This result is in accordance with the fact that some patients with acute Q fever may have antibodies to phase I antigen greater than or equal to 1:800 (15).
FIG. 1.
Sensitivity, specificity, positive predictive value, and negative predictive value for the different cutoffs used. The positive group represents patients with endocarditis due to either C. burnetii (n = 30) or Bartonella sp. (n = 20). The negative group comprises 40 apparently healthy blood donors, 99 patients with blood culture-positive endocarditis of known etiology, 15 patients with cat scratch disease, 15 patients with acute Q fever, and 25 patients seropositive for bacterial or viral diseases not related to Bartonella or Coxiella species.
Our assay is adapted to detect specific clinical entities and can be used in all patients suffering from endocarditis and should be considered in the future as a possible commercial test for the diagnosis of BCNE due to Bartonella spp. or C. burnetii.
Acknowledgments
We thank Patrick Kelly for reviewing the manuscript.
REFERENCES
- 1.Berbari, E. F., F. R. Cockerill, and J. M. Steckelberg. 1997. Infective endocarditis due to unusual or fastidious microorganisms. Mayo Clin. Proc. 72:532-542. [DOI] [PubMed] [Google Scholar]
- 2.Brouqui, P., and D. Raoult. 2001. Endocarditis due to rare and fastidious bacteria. Clin. Microbiol. Rev. 14:177-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drancourt, M., J. Mainardi, P. Brouqui, F. Vandenesch, A. Carta, F. Lehnert, J. Etienne, F. Goldstein, J. Acar, and D. Raoult. 1995. Bartonella (Rochalimaea) quintana endocarditis in three homeless men. N. Engl. J. Med. 332:419-423. [DOI] [PubMed] [Google Scholar]
- 4.Forsgren, A., and J. Sjoquist. 1966. “Protein A” from S. aureus. I. Pseudo-immune reaction with human gamma-globulin. J. Immunol. 97:822-827. [PubMed] [Google Scholar]
- 5.Fournier, P. E., J. P. Casalta, G. Habib, T. Messana, and D. Raoult. 1996. Modification of the diagnostic criteria proposed by the Duke Endocarditis Service to permit improved diagnosis of Q fever endocarditis. Am. J. Med. 100:629-633. [DOI] [PubMed] [Google Scholar]
- 6.Fournier, P. E., H. Lelievre, S. J. Eykyn, J. L. Mainardi, T. J. Marrie, F. Bruneel, C. Roure, J. Nash, D. Clave, E. James, C. Benoit-Lemercier, L. Deforges, H. Tissot-Dupont, and D. Raoult. 2001. Epidemiologic and clinical characteristics of Bartonella quintana and Bartonella henselae endocarditis: a study of 48 patients. Medicine (Baltimore) 80:245-251. [DOI] [PubMed] [Google Scholar]
- 7.Fournier, P. E., T. J. Marrie, and D. Raoult. 1998. Diagnosis of Q fever. J. Clin. Microbiol. 36:1823-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houpikian, P., and D. Raoult. 2003. Diagnostic methods. Current best practices and guidelines for identification of difficult-to-culture pathogens in infective endocarditis. Cardiol. Clin. 21:207-217. [DOI] [PubMed] [Google Scholar]
- 9.La Scola, B., and D. Raoult. 1996. Serological cross reactions between Bartonella quintana, Bartonella henselae, and Coxiella burnetii. J. Clin. Microbiol. 34:2270-2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamas, C. C., and S. J. Eykyn. 2003. Blood culture negative endocarditis: analysis of 63 cases presenting over 25 years. Heart 89:258-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li, J. S., D. J. Sexton, N. Mick, R. Nettles, V. G. J. Fowler, T. Ryan, T. Bashore, and G. R. Corey. 2000. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. 30:633-638. [DOI] [PubMed] [Google Scholar]
- 12.Maurin, M., J. M. Rolain, and D. Raoult. 2002. Comparison of in-house and commercial slides for detection of immunoglobulins G and M by immunofluorescence against Bartonella henselae and Bartonella quintana. Clin. Diagn. Lab. Immunol. 9:1004-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raoult, D., P. E. Fournier, M. Drancourt, T. J. Marrie, J. Etienne, J. Cosserat, P. Cacoub, Y. Poinsignon, P. Leclercq, and A. M. Sefton. 1996. Diagnosis of 22 new cases of Bartonella endocarditis. Ann. Intern. Med. 125:646-652. [DOI] [PubMed] [Google Scholar]
- 14.Raoult, D., P. Houpikian, H. Tissot Dupont, J. M. Riss, J. Arditi-Djiane, and P. Brouqui. 1999. Treatment of Q fever endocarditis: comparison of two regimens containing doxycycline and ofloxacin or hydroxychloroquine. Arch. Int. Med. 159:167-173. [DOI] [PubMed] [Google Scholar]
- 15.Raoult, D., H. Tissot-Dupont, C. Foucault, J. Gouvernet, P. E. Fournier, E. Bernit, A. Stein, M. Nesri, J. R. Harle, and P. J. Weiller. 2000. Q fever 1985-1998—Clinical and epidemiologic features of 1,383 infections. Medicine 79:109-123. [DOI] [PubMed] [Google Scholar]
- 16.Tissot-Dupont, H., X. Thirion, and D. Raoult. 1994. Q fever serology: cutoff determination for microimmunofluorescence. Clin. Diagn. Lab. Immunol. 1:189-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeaiter, Z., P. E. Fournier, and D. Raoult. 2002. Genomic variation of Bartonella henselae strains detected in lymph nodes of patients with cat scratch disease. J. Clin. Microbiol. 40:1023-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]