Abstract
Opsonophagocytosis is a correlate of protection that measures the functional activity of vaccine-induced antibodies. A standardized opsonophagocytosis assay (OPA) should be used as part of the evaluation of current and future pneumococcal (Pnc) polysaccharide (Ps)-based vaccines. We enrolled five laboratories to evaluate a previously standardized viability OPA. Each laboratory was provided with a detailed OPA protocol, seven target Pnc strains (serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F), two quality control sera and 12 paired sera (blinded) from adult donors who received one dose of the 23-valent Pnc Ps vaccine. Laboratories sent their results to the Centers for Disease Control and Prevention for analysis. Sera were tested in duplicate (single run), and the results were averaged to yield a single OPA titer (≥50% killing) for each serum sample. The percentage of sera within one or two dilutions of the calculated median OPA titer was determined for each laboratory and for each serotype. In general, laboratories were capable of detecting OPA titers within one or two dilutions of the median for at least 75 and 88%, respectively, of the sera tested. The level of agreement with the median OPA titers varied depending on the participating laboratory (overall agreement = 0.8 [99% confidence interval = 0.75 to 0.85]). All OPA median titers reported for quality control sera were within one dilution of the expected titer. We conclude that this OPA can be done in multiple laboratories with a high degree of interlaboratory reproducibility.
Vaccine-induced protection to Streptococcus pneumoniae (pneumococcus) has been determined through vaccine efficacy trials for both polysaccharide (Ps) vaccines (1, 4, 22) and Ps-protein conjugate vaccines (2, 5, 8). Trials of these pneumococcal (Pnc) vaccine formulations have shown various efficacies for protection depending on the end point being measured and the population being studied. These trials are costly and difficult to perform given the large sample size. In addition, pneumococcus has 90 different capsular serotypes, with the majority of disease being caused by about 30 of these 90 serotypes. Distribution of these serotypes also varies with the geographical region, making the estimation of the burden of disease and the impact of vaccination rather difficult (3, 9, 10).
Efforts have been made for the identification and standardization of laboratory correlates of protection that can aid vaccine efficacy trials in the estimation of vaccine-induced protection. Currently, a highly standardized enzyme-linked immunosorbent assay (ELISA) is available (www.vaccine.uab.edu) for the evaluation of infant sera. Several modifications to the protocol described by Quartaert et al. (20) allowed for the measurement of Ps-specific antibodies in children and adults (6, 19, 18). Adults can have cross-reactive antibodies, which confound the measurements of immunoglobulin G (IgG) antibodies by ELISA, especially if absorption with a nonrelevant serotype is not performed prior to testing (6, 7, 26). These cross-reactive antibodies are more prevalent in prevaccination sera than in postvaccination sera (6). Due to the lower specificity previously observed with ELISA-based assays, efforts were centered on the identification of alternative assays that can measure the function of the anti-capsular antibodies and serve as correlates of protection against disease. In the case of pneumococcus, opsonophagocytosis is the primary mechanism for protection in the host (25). Therefore, standardization and validation of assays measuring opsonophagocytic activity are of high importance for Pnc vaccine evaluation.
This study describes the results of a multilaboratory evaluation of a viability opsonophagocytosis assay (OPA). The viability OPA was previously standardized for the use of HL-60 granulocytes as the effector cells (21). Functional assays are inherently variable. In this study, we calculate the interlaboratory variability of the viability OPA and we determine if this OPA is suitable for multilaboratory comparisons of functional antibodies induced by Ps-based Pnc vaccines.
MATERIALS AND METHODS
Study design.
Paired sera from 12 healthy adults (58% male and 42% female, mean age = 39 years) receiving the 23-valent Pnc Ps vaccine (Pneumovax II; Merck Sharp and Dohme Ltd.) were collected at the Oxford Blood Transfusion Service, Oxford, United Kingdom. Subjects agreed upon the use of their sera for experimental purposes according to good clinical practice and informed consent guidelines. These quality control sera are currently available at the National Institute for Biological Standards and Control (Potters Bar, Hertfordshire, United Kingdom) for use in Pnc assay standardization. Sera were lyophilized in 2-ml aliquots and stored at −20°C until use by the participating laboratories. The Centers for Disease Control and Prevention (CDC; Atlanta, Ga.) provided detailed copies of a standardized viability OPA that uses HL-60 granulocytes as effector cells (21). CDC also provided instructions, worksheets, Pnc strains, and two positive controls. Pnc strains were obtained from Richard Facklam, Streptococcal Reference Laboratory, CDC. The Pnc strains were provided in lyophilized ampules to include serotypes 4 (DS 2382-94), 6B (DS 2212-94), 9V (DS 400-92), 14 (DS 2214-94), 18C (SP116), 19F (DS2217-94), and 23F (DS 2216-94). These serotypes are the top disease-causing serotypes in children living in developed countries, and they are also included in a recently licensed Pnc conjugate vaccine (Prevnar; Wyeth-Lederle). Participants were provided with a detailed protocol. Briefly, HL-60 cells (promyelocytic leukemia cells, CCL240; American Type Culture Collection, Rockville, Md., or its authorized distributor) were differentiated into polymorphonuclear leukocyte (PMN)-like cells by using 100 mM N,N-dimethyl formamide (DMF, 99.8% purity; Fisher Scientific, Fair Lawn, N.J., or its equivalent distributor) for a period of 5 days at 37°C and 5% CO2. The OPA was performed as previously described by Romero-Steiner et al. (21). In addition, CDC provided a detailed protocol of this OPA, which included HL-60 cell growth and differentiation instructions. Participating laboratories were asked to use HL-60 cells that have been actively growing to a density of 4 × 105 to 7 × 105 cells/ml prior to the differentiation with 100 mM DMF. Participants were given telephone and electronic assistance to ensure that the growth and differentiation of HL-60 cells were successful in their laboratories. Active growth of HL-60 cell stocks was accomplished by daily feeding or splitting of the tissue culture stock to maintain a balanced growth environment. The viability of undifferentiated and differentiated cells was recommended to be >90%, as judged by Trypan blue exclusion cell counts. Participants were asked to differentiate their HL-60 cells at an inoculum of 2 × 105 cells/ml with DMF. Prior to use of the differentiated cells, participants were requested to check the cell viability and the overall formation of elongated sickle cell-like cells as their primary indicators for optimal differentiation. Serum samples were tested in duplicate in the same assay plate with a starting in-well dilution of 1:8. Therefore, the lower limit of detection was an OPA titer of 1:8 and the lower limit of quantification was an OPA titer of 1:4. The reciprocal of the dilution with an average percent killing of ≥50% compared to the in-plate complement controls was reported as the OPA titer. Worksheets with OPA results were sent to the CDC for final analysis. All test plates included a positive control serum (R21654-34301107) and Sandoglobulin (purified IgG, lot no. 441x9605; Sandoz Pharmaceuticals Co., East Hanover, N.J.). Control serum and Sandoglobulin (6% [wt/vol] concentration) were provided by the CDC along with a list of their expected OPA titers (median ± dilution range) and initial testing dilution for each of the serotypes tested. Laboratory 3 modified the assay by automating the colony counting procedure. This modification is now widely used by many other laboratories for the determination of viability colony counts.
Statistical analysis.
The median OPA titer was calculated for each serum sample and serotype. Once median OPA titers were obtained, results from all laboratories were compared to the median values (median). The percentage of serum samples in agreement with the median titer and the percentage of serum samples within one or two dilutions from the median titer were calculated for each serotype and for each participating laboratory. The level of agreement to the median was determined in 2 by 2 tables with the formula a/(a + c), where a is the number of sera at or above the given titer by both the test laboratory and the median and c is the number of sera below the given titer by the test laboratory but at or above the given titer by the median. The 95 and 99% confidence intervals (CI) were calculated with the aid of the Sigma Plot software program, version 3.0 (Jandel Scientific, San Rafael, Calif.). OPA titers of <8 were reported as a titer of 4 for calculation purposes. Therefore, a titer of 8 was defined as the lower limit of quantification. Outliers were identified if OPA titers were more than 3 dilutions from the median titer. However, all data submitted were included in the analysis. No data points were edited or excluded.
The participating laboratories were as follows: Respiratory Diseases Branch, Immunology Section, CDC; Center for Biologics Evaluation and Research, U.S. Food and Drug Administration, Bethesda, Md.; U.S. Pneumococcal Reference Laboratory, University of Rochester, Rochester, N.Y., presently located at the University of Alabama, Birmingham; National Public Health Institute, Helsinki, Finland; and Glaxo SmithKline Biologicals, Rixensart, Belgium.
RESULTS
Median OPA titers.
Although OPA killing curves can generate continuous data with sigmoidal curve-fitting analysis, we elected to report all data in the form of OPA titers (discontinuous data). Titers were defined as the reciprocal of the twofold dilution that exhibited ≥50% killing compared to the complement control wells (no antibody source added). Table 1 gives the median OPA titers by serotype for the 24 standard serum samples (pre- and postvaccination). The expected median OPA titers for the quality control reference serum R21654-34301107 and Sandoglobulin lot no. 441x9605 were determined prior to the initiation of the study (n = 6 independent duplicate assays per serotype). The expected OPA titers for the reference serum were 128 (serotype 4), 256 (serotypes 6B, 9V, 14, 18C, and 19F) and 2,048 (serotype 23F), and the expected OPA titers for Sandoglobulin were 128 (serotype 4), 256 (serotype 9V), 512 (serotypes 18C and 19F), and 1,024 (serotypes 6B, 14, and 23F). The variability of the expected median OPA titers was within 1 dilution, except for Sandoglobulin (serotype 6B, ±2 dilutions). Observed OPA titers were determined by the participant laboratories (n = 6 OPA titers per serotype submitted by laboratories 1, 2, 4, and 5). Observed OPA titers were within one or two dilutions of the expected median OPA titer for both controls, except for the reference serum for serotypes 4 and 9 (≥3 dilutions apart). These two controls allowed for the assurance that HL-60 cell differentiation was optimal and/or other assay parameters were optimal for the calculation of OPA titers in test sera (12 paired sera).
TABLE 1.
Median OPA titers for 24 quality control adult sera
| Serum sample no.a | Median OPA titer (50% killing) for S. pneumoniae serotype:
|
||||||
|---|---|---|---|---|---|---|---|
| 4 | 6B | 9V | 14 | 18C | 19F | 23F | |
| 380329 | 4 | 4 | 4 | 64 | 4 | 4 | 4 |
| 381039 | 16 | 4 | 32 | 256 | 8 | 64 | 8 |
| 380318 | 4 | 4 | 4 | 4 | 4 | 16 | 4 |
| 380773 | 256 | 16 | 8 | 2,048 | 4 | 128 | 4 |
| 380342 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| 381399 | 4 | 4 | 4 | 4 | 8 | 4 | 4 |
| 380292 | 4 | 4 | 4 | 16 | 4 | 4 | 4 |
| 380807 | 64 | 4 | 128 | 4,096 | 128 | 16 | 8 |
| 380376 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| 380847 | 4 | 4 | 4 | 1,024 | 32 | 4 | 4 |
| 380372 | 4 | 128 | 32 | 8 | 4 | 4 | 4 |
| 380964 | 32 | 128 | 128 | 1,024 | 32 | 16 | 4 |
| 380360 | 64 | 64 | 4 | 64 | 4 | 4 | 4 |
| 380828 | 128 | 64 | 256b | 32b | 256 | 64 | 4 |
| 380330 | 4 | 16 | 4 | 4 | 4 | 16 | 4 |
| 380638 | 32 | 64 | 64 | 4 | 64 | 256 | 8 |
| 380386 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| 380859 | 4 | 16 | 64 | 512 | 4 | 16 | 4 |
| 380327 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| 380808 | 64 | 4 | 64 | 256 | 128 | 128 | 128 |
| 380351 | 4 | 4 | 4 | 4 | 64 | 4 | 4 |
| 380824 | 2,048 | 64 | 64 | 512 | 64 | 16 | 1,024 |
| 380298 | 4 | 4 | 4 | 512 | 8 | 4 | 4 |
| 380860 | 128 | 256 | 128 | 2,048 | 256 | 512 | 128 |
Each prevaccination serum sample is followed by its corresponding postvaccination serum sample. Other numerical designations for these sera are available at the U.S. Pneumococcal Reference Laboratory website (www.vaccine.uab.edu).
Serum sample for which 3 of the participating laboratories reported OPA titers with a difference of ≥3 serum dilutions for serotypes 9V and 14.
Interlaboratory variability.
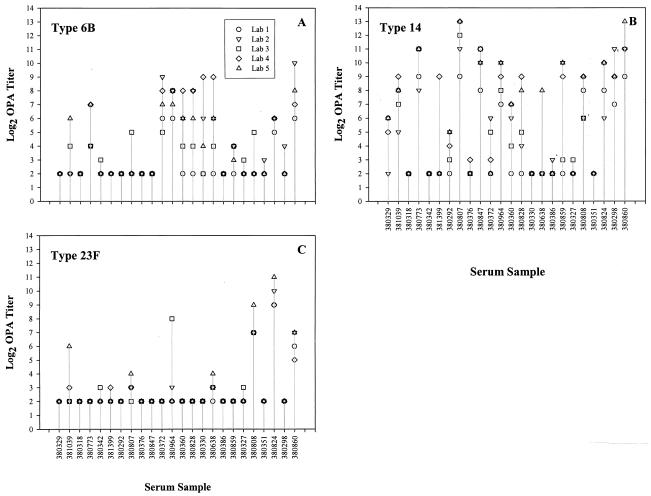
The percentage of sera within one and two dilutions from the median titer were calculated for each serotype and for each participating laboratory (Table 2). In general, participating laboratories were capable of detecting OPA titers within one or two dilutions of the median for at least 75 and 88%, respectively, of the sera tested. Figure 1 shows the reported OPA titers for serotypes 6B, 14, and 23F. These serotypes are examples of low, medium, and high interlaboratory agreement among the OPA titers reported by the participating laboratories. OPA titers tended to be more variable (≥3 dilutions from the median OPA titer) in sera with higher OPA titers.
TABLE 2.
Percentage of sera reported by each participating laboratory within one and two dilutions of the calculated median OPA titer
| S. pneumoniae serotype | % of sera within 1 and 2 dilutions of median OPA titer from laboratory no.a:
|
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| 4 | 88, 100 | 79, 88 | 100, 100 | 96, 96 | 96, 100 |
| 6B | 75, 88 | 83, 100 | 56, 72 | 83, 88 | 88, 92 |
| 9V | 63, 71 | 83, 88 | 75, 94 | 83, 96 | 83, 92 |
| 14 | 63, 83 | 75, 83 | 81, 94 | 92, 92 | 88, 92 |
| 18C | 88, 96 | 88, 96 | 56, 69 | 88, 92 | 88, 100 |
| 19F | 79, 96 | 88, 96 | 94, 100 | 96, 100 | 88, 100 |
| 23F | 100, 100 | 100, 100 | 100, 100 | 96, 100 | 92, 96 |
The first value given is the percentage of sera within one dilution of the median OPA titer, and the second value given is the percentage of sera within two dilutions of the median OPA titer.
FIG. 1.
Reported OPA titers (log2) for each quality control serum (a prevaccination serum is followed by its corresponding postvaccination serum) for Pnc serotypes 6B (A), 14 (B), and 23F (C).
Agreement to the median OPA titer.
The exact or true OPA titer for each of the serum samples tested is not known; therefore, levels of agreement were determined based on the median value obtained from all participating laboratories. Serum samples with OPA titers below the level of quantification of the assay (a titer of <8) were expected among the 12 unvaccinated serum samples; however, this does not eliminate the possibility of functional antibodies being induced by natural exposure to the Pnc serotype tested. It is possible that OPA activity is present in some of these sera if tested at a serum dilution of 1:4. Samples at or above the minimum level of quantification (a titer of ≥8), were expected among the 12 postvaccination serum samples. However, as shown in Fig. 1 for serotype 23F, we also found a high proportion of postvaccination sera with OPA titers of 4, especially for serotypes 4 and 23F (42 and 50%, respectively).
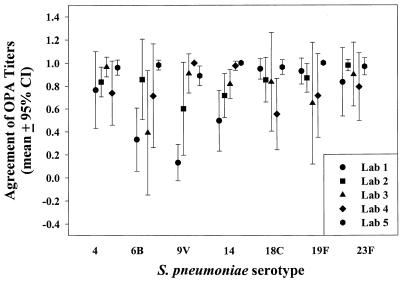
The median OPA titer for each serum sample was compared to the laboratory's reported OPA titer for each serum. The total number of OPA titers in agreement or disagreement at a given cutoff titer was calculated. Laboratories varied in the level of agreement to the median OPA titers (Fig. 2). Laboratory 1 had lower agreements for serotypes 6B (0.33), 9V (0.13), and 14 (0.50). Laboratory 2 had a lower agreement for serotype 14 (0.71). Laboratory 3 had lower agreements for serotypes 6B (0.39) and 19F (0.65). Laboratory 4 had a lower agreement for serotype 18C (0.55). Laboratory 5 reported OPA titers with levels of agreement of ≥0.89 for all the serotypes evaluated. The mean interlaboratory level of agreement for serum samples with OPA titers above the minimum level of quantification was calculated to be 0.8 (99% CI = 0.75 to 0.85).
FIG. 2.
Agreement (mean ± 95% CI) of OPA titers compared to the median OPA titers given in Table 1. The level of agreement to the median was determined in 2 by 2 tables with the formula a/(a + c), where a is the number of sera at or above the given titer by both the test laboratory and the median and c is the number of sera below the given titer by the test laboratory but at or above the given titer by the median.
OPA correlation with ELISA.
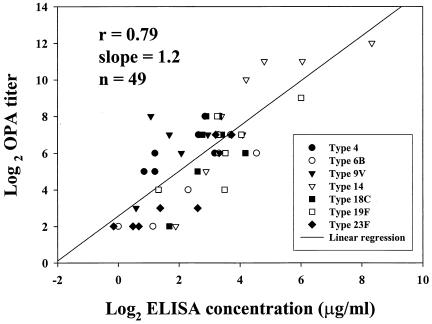
We compared the median OPA titers of 7 sera that were also included in a multilaboratory evaluation of Pnc ELISA IgG methods (18). The ELISA IgG concentrations were obtained from the database reported to CDC by the participating laboratories. This database was used in the analysis that has been published previously by Plikaytis et al. (18) and compared to the median OPA titers obtained in this study. The overall correlation was r = 0.79, and the slope was 1.2. The scatter grams for individual Pnc serotypes are depicted in Fig. 3. All 7 sera were postvaccination sera.
FIG. 3.
Pearson's product moment correlation coefficient (r = 0.79) between log2-transformed data for median OPA titers obtained in this study and the ELISA IgG concentrations (in micrograms/milliliter) assigned to a subset of 7 sera through a multilaboratory evaluation of ELISA methods (18). The reference ELISA concentrations for serum samples 380773 (96-730), 380807 (96-734), 380964 (96-738), 380828 (96-742), 380638 (96-744), 380808 (96-748), and 380860 (96-752) were obtained from the database, as reported to CDC by the participating laboratories. This database was used in the ELISA previously described by Plikaytis et al. (18). The correlation coefficient (r = 0.76, slope = 1.19) between continuous OPA titers (laboratory 5) and ELISA IgG concentrations (n = 49) was similar to the coefficient shown in this figure for median OPA titers (discontinuous data).
DISCUSSION
Measurement of functional antibody activity by opsonophagocytosis has been recognized as an important aspect of Pnc vaccine evaluation. The viability OPA has been successfully implemented by multiple laboratories (12, 16, 17, 23), and it has been recognized as the reference method for the evaluation of other OPA methods. Opsonophagocytosis can be measured by many methods including flow cytometric, chromogenic, and radioisotopic assays (11, 12, 15, 16, 23, 24). We provide the median OPA titers, as determined by this multilaboratory study, for a set of 12-paired sera (Table 1) to facilitate the evaluation of other OPA methods. This type of information will help other investigators in the development and validation of other standardized techniques that may be faster (1-day assays or multiplex assays) in the determination of opsonophagocytosis elicited by Pnc vaccines.
When performing this multilaboratory study for OPA evaluation, the main obstacle encountered by most laboratories was the manipulation of the HL-60 cells. The participating laboratories had the tissue culture capacity; however, ensuring effective differentiation of the HL-60 cells into PMN-like phagocytic cells was difficult for some study participants. HL-60 cells obtained from the American Type Culture Collection under the designation CCL240 were effectively differentiated into PMN-like cells upon the addition of 100 mM DMF. The use of disinfectants which could leave toxic residues in the CO2 incubators, such as formaldehyde, phenol, or sodium hypochlorite, should be avoided. Temperature, CO2 content, and humidity should be constant during cell growth and differentiation. Once the laboratories gained expertise and were familiar with the differentiation protocol with DMF, the study objectives were met in a short period of time and the data were submitted to CDC for analysis. Therefore, we recommend that 100 mM DMF is used for a period of 5 days at 37°C and 5% CO2. Only HL-60 cells that have been actively growing to a density of 4 × 105 to 7 × 105 cells/ml are used for differentiation with DMF. The viability of undifferentiated and differentiated cells is maintained over 90% in RPMI 1640 medium supplemented with 20% fetal calf serum. For this viability OPA, an inoculum of 2 × 105 HL-60 cells/ml should be used for differentiation with a 20% carryover of tissue culture medium from the undifferentiated cell stock. Initially, Romero-Steiner et al. reported a high passage restriction for the differentiation of HL-60 cells (21); however, if the above-mentioned conditions are maintained, there is no longer a passage restriction for the differentiation of this cell line (16). Differentiated cells change from round cells to elongated sickle cell-like cells, and they become phagocytic due to the expression of surface receptors for iC3b, C3b, and Ig G (21). Although monitoring of these receptors is not necessary for this assay, this change in surface markers can be used as an indicator for optimal differentiation. PMN-like HL-60 cells halt the cell division cycle. This is observed in the laboratory as a stable cell count which will eventually start to decline due to cell death. Tissue cultures should be maintained free of mycoplasma contamination, and cultures should be monitored periodically for the presence of mycoplasma.
Other laboratories had difficulty in growing some of the Pnc strains that were sent by CDC (in particular, serotypes 19F and 18C), therefore requiring additional shipments of strains. In general, once all participants had all the materials needed and had gained preliminary experience with the assay conditions, the level of difficulty decreased and the completion of the study became expedited. Most participants found the extended laboratory protocol provided by the CDC useful. This protocol in now available from a website (www.vaccine.uab.edu).
The level of agreement with the median OPA titers varied depending on the participating laboratory. Laboratory 1 had lower levels of agreement (<0.75) with the median OPA titers for 3 of 7 serotypes evaluated. In contrast, laboratory 5 had very high levels of agreement (>0.89) with the median OPA titers for all serotypes tested. It was observed that most laboratories were capable of obtaining OPA titers closer to the median titer if the serum sample was below the level of quantification of the assay. However, as the median OPA titer increased, the reported OPA values diverged more from the median and the level of agreement dropped in some cases. Laboratory 3 had difficulties with the agreement of OPA titers for Pnc serotype 18C. However, participation in this study improved the level of agreement of laboratory 3 for this serotype after completion of the study. Although no clear technical explanation exists for the differences observed (Table 1) with serotypes 6B and 9V, it is possible that these differences may be due to the quantity of capsular Ps during the growth of the target strains (14). The use of the same Pnc reference strains by different testing laboratories is highly recommended to increase assay reproducibility.
This study provides a guide for laboratories that are implementing OPA testing for Pnc vaccine evaluation. The viability OPA can be performed in multiple laboratories with a high degree of interlaboratory reproducibility (75 and 88% of the sera tested were within one or two dilutions of the median titer, respectively). When this viability method for determining OPA titers has been compared to other OPAs, it has been found to be highly sensitive for measuring OPA activity in sera with low antibody concentrations from both infants and adults (12, 23). This OPA highly correlates with the current ELISA IgG method (18). The minimum level of quantification (a titer of 8) of this OPA correlates with the minimum level of ELISA IgG (0.2 μg/ml) conferring protection in infants vaccinated with Ps conjugate vaccine, as recently reported (13). OPA should be used for the evaluation of new Pnc Ps-based vaccines.
Acknowledgments
S.R.-S. was funded in part by a postdoctoral fellowship program from the American Society for Microbiology and the National Center for Infectious Diseases, CDC.
We thank Richard R. Facklam, Division of Bacterial and Mycotic Diseases, CDC, for providing the Pnc strains used in this multilaboratory evaluation.
REFERENCES
- 1.Austrian, R., R. M. Douglas, G. Schiffman, A. M. Coetzee, H. J. Koorhof, S. Hayden-Smith, and R. D. Reid. 1976. Prevention of pneumococcal pneumonia by vaccination. Trans. Assoc. Am. Physicians 89:184-189. [PubMed] [Google Scholar]
- 2.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. Hansen, L. Elvin, K. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, K. Edwards, and the Northern California Kaiser Permanente Vaccine Study Center Group. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 3.Butler, J. C., E. D. Shapiro, and G. M. Carlone. 1999. Pneumococcal vaccines: history, current status and future directions. Am. J. Med. 107:69S-76S. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1997. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. 46(RR-8):1-24. [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2000. Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. 49(RR-09):1-38. [PubMed] [Google Scholar]
- 6.Concepcion, N. F., and C. E. Frasch. 2001. Pneumococcal type 22F polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 8:266-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coughlin, R. T., A. C. White, C. A. Anderson, G. M. Carlone, D. L. Klein, and J. Treanor. 1998. Characterization of pneumococcal specific antibodies in healthy unvaccinated adults. Vaccine 16:1761-1767. [DOI] [PubMed] [Google Scholar]
- 8.Eskola, J., T. Kilpi, A. Palmu, et al. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344:403-409. [DOI] [PubMed] [Google Scholar]
- 9.Hausdorff, W. P., J. Bryant, P. R. Paradiso, G. R. Siber. 2000. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin. Infect. Dis. 30:100-121. [DOI] [PubMed] [Google Scholar]
- 10.Hausdorff, W. P., J. Bryant, C. Kloek, P. R. Paradiso, G. R. Siber. 2000. Contribution of specific pneumococcal serogroups to different disease manifestations: implications for conjugate vaccine formulation and use, part II. Clin. Infect. Dis. 30:122-140. [DOI] [PubMed] [Google Scholar]
- 11.Jansen, W. T. M., J. Gootjes, M. Zelle, D. V. Madore, J. Verhoef, H. Snippe, and A. F. M. Verheul. 1998. Use of highly encapsulated Streptococcus pneumoniae strains in a flow-cytometric assay for assessment of the phagocytic capacity of serotype-specific antibodies. Clin Diag. Lab. Immunol. 5:703-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansen, W. T. M., M. Väkeväinen-Anttila, H. Käyhty, M. Nahm, N. Bakker, J. Verhoef, H. Snippe, and A. F. Verheul. 2001. Comparison of a classical phagocytosis assay and a flow cytometry assay for assessment of phagocytic capacity of sera from adults vaccinated with a pneumococcal conjugate vaccine. Clin. Diag. Lab. Immunol. 8:245-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jódar, L., J. Butler, G. Carlone, R. Dagan, C. Frasch, D. Goldblatt, H. Käyhty, K. Klugman, B. Plikaytis, G. Siber, R. Kohberger, I. Chang, and T. Cherian. 2003. Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine 21:3265-3272. [DOI] [PubMed] [Google Scholar]
- 14.Kim, J. O., S. Romero-Steiner, U. B. S. Sørensen, J. Blom, M. Carvalho, S. Barnard, G. Carlone, and J. N. Weiser. 1999. Relationship between cell surface carbohydrates and intrastrain variation on opsonophagocytosis of Streptococcus pneumoniae. Infect. Immun. 67:2327-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin, J. S., M. K. Park, and M. H. Nahm. 2001. Chromogenic assay measuring opsonophagocytic killing capacities of antipneumococcal antisera. Clin. Diagn. Lab. Immunol. 8:528-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez, J. E., S. Romero-Steiner, T. Pilishvili, S. Barnard, J. Schinsky, D. Goldblatt, and G. M. Carlone. 1999. A flow cytometric opsonophagocytic assay for the measurement of functional antibodies elicited after vaccination with the 23-valent pneumococcal polysaccharide vaccine. Clin. Diagn. Lab. Immunol. 6:581-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nahm, M. H., D. E. Briles, and X. Yu. 2000. Development of a multi-specificity opsonophagocytic killing assay. Vaccine 18:2768-2771. [DOI] [PubMed] [Google Scholar]
- 18.Plikaytis, B. D., D. Goldblatt, C. E. Frasch, C. Blondeau, M. J. Bybel, G. S. Giebink, I. Jonsdottir, H. Käyhty, H. B. Konradsen, D. V. Madore, M. H. Nahm, C. A. Schulman, P. F. Holder, T. Lezhava, C. M. Elie, and G. M. Carlone. 2000. An analytical model applied to a multicenter pneumococcal enzyme-linked immunosorbent assay study. J. Clin. Microbiol. 38:2043-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quartaert, S., D. Martin, P. Anderson, G. S. Giebink, J. Hendrichsen, M. Leinonen, D. M. Granoff, H. Russell, G. Siber, H. Faden, and D. V. Madore. 2001. A multi-laboratory evaluation of an enzyme-linked immunoassay quantitating human antibodies to Streptococcus pneumoniae polysaccharides. Immunol. Investig. 30:191-207. [DOI] [PubMed] [Google Scholar]
- 20.Quartaert, S. A., C. S. Kirch, L. J. Quackenbush Weidl, D. C. Phipps, S. Strohmeyer, C. O. Cimino, J. Skuse, and D. M. Madore. 1995. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin. Diagn. Lab. Immunol. 2:590-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romero-Steiner, S., D. Libutti, L B. Pais, J. Dykes, P. Anderson, J. C. Whitin, H. L. Keyserling, and G. M. Carlone. 1997. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin. Diagn. Lab. Immunol. 4:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapiro, E. D., A. T. Berg, R Austrian, D. Schroeder, V. Parcells, A. Margolis, R. K. Adair, and J. D. Clemens. 1991. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N. Engl. J. Med. 325:1453-1460. [DOI] [PubMed] [Google Scholar]
- 23.Väkeväinen, M., W. Jansen, E. Saeland, I. Jonsdottir, H. Snippe, A. Verheul, and H. Käyhty. 2001. Are opsonophagocytic activities of antibodies in infant sera measured by different pneumococcal phagocytosis assays comparable? Clin. Diagn. Lab. Immunol. 8:363-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vidarsson, G., I. Jonsdottir, S. Jonsson, and H. Valdimarsson. 1994. Opsonization and antibodies to capsular and cell wall polysaccharides of Streptococcus pneumoniae. J. Infect. Dis. 170:592-599. [DOI] [PubMed] [Google Scholar]
- 25.Wilkestein, J. A., M. R. Smith, and H. S. Shin. 1975. The role of C3 as an opsonin in the early stages of infection. Proc. Soc. Exp. Biol. Med. 149:397-401. [DOI] [PubMed] [Google Scholar]
- 26.Yu, X., Y. Sun, C. Frasch, N. Conception, and M. H. Nahm. 1999. Pneumococcal capsular polysaccharide preparations may contain non-C-polysaccharide contaminants that are immunogenic. Clin. Diagn. Lab. Immunol. 6:519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]