Abstract
Objective
Magnetic resonance (MR) imaging is used widely for assessment of patients with cognitive impairment, but the pathological correlates are unclear, especially when multiple pathologies are present.
Methods
This report includes 93 subjects from a longitudinally followed cohort recruited for the study of Alzheimer’s disease (AD) and subcortical cerebrovascular disease (CVD). MR images were analyzed to quantify cortical gray matter volume, hippocampal volume, white matter hyperintensities, and lacunes. Neuropathological examination quantified CVD parenchymal pathology, AD pathology (defined as Consortium to Establish a Registry for Alzheimer’s Disease scores and Braak and Braak stage), and hippocampal sclerosis. Subjects were pathologically classified as 12 healthy control subjects, 46 AD, 14 CVD, 9 mixed AD/CVD, and 12 cognitively impaired patients without significant AD/CVD pathology. Multivariate models tested associations between magnetic resonance and pathological findings across the entire sample.
Results
Pathological correlates of cortical gray matter volume were AD, subcortical vascular pathology, and arteriosclerosis. Hippocampal volume was related to AD pathology and hippocampal sclerosis, and the effects of hippocampal sclerosis were greater for subjects with low levels of AD pathology. White matter hyperintensities were related to age and to white matter pathology. Number of MRI lacunes was related to subcortical vascular pathology.
Interpretation
In this clinical setting, the presence of lacunes and white matter changes provide a good signal for vascular disease. The neuropathological basis of MR defined cerebral cortical and hippocampal atrophy in aging and dementia is complex, with several pathological processes converging on similar brain structures that mediate cognitive decline.
Alterations in brain structure are a profound concomitant of aging and dementia. From a clinical perspective, structural magnetic resonance imaging (MRI) has become a standard component of the dementia evaluation,1 helping to rule out infarcts and other nonneurodegenerative causes of dementia. Research studies have linked brain atrophy, especially in the hippocampus and medial temporal lobe structures, to clinically diagnosed Alzheimer’s disease (AD),2–4 and prediction of AD in patients with mild cognitive impairment,5,6 whereas epidemiological studies show convincing associations between white matter hyperintensities (WMH) and a host of vascular factors.7,8 There are, however, few studies that examine relations between MRI and neuropathology, which is a serious limitation because the extent of AD pathology cannot be ascertained without neuropathological examination.
Knowing the pathological phenomena that underlie changes in MR measures of brain structure may help to clarify the significance of neuroimaging in complex clinical situations and elucidate underlying mechanisms of disease. Because of the early and severe involvement of the hippocampus by neurofibrillary pathological change in AD,9 it is not surprising that hippocampal atrophy seen on MRI is frequently associated with the accumulation of neurofibrillary tangles at postmortem examination.10 However, hippocampal atrophy may be associated with other pathological processes, including hippocampal sclerosis (HS) and frontotemporal dementia.10 Although WMH are associated with vascular risk factors, they may also be associated with AD-associated amyloid angiopathy.11,12 Conversely, evidence linking cerebrovascular risk factors with cerebral atrophy13,14 suggests that brain atrophy may reflect the effects of vascular pathology as well as AD.
Thus, although some data support relatively specific relations between postmortem findings and MRI, the reality of these associations is complex. This is a particularly important issue when multiple pathological processes occur simultaneously, as is frequently the case in older individuals who often have coexistent pathologically confirmed AD and cerebrovascular disease (CVD).15 We have enrolled and followed a cohort of subjects with a wide range of cognitive ability and a high prevalence of both CVD and AD in a longitudinal study. Previous reports from this project have shown that measures of cerebral cortical and hippocampal atrophy are major determinants of cognition16 and cognitive decline,17 with relatively small contributions from WMH and lacunes. We now report the associations between neuropathology and MR findings among cohort cases who have been autopsied. The goal of this report is to define how AD and CVD together produce changes in brain structure that are related to cognitive decline and dementia. Our approach involved the prospective evaluation of antemortem quantitative MR followed up with neuropathological evaluation, relating imaging and pathological variables to one another together with continuous dimensions of severity because that is how these processes occur in the “real world.” The analysis of this cohort is useful both for defining the pathological basis of MR findings observed during life and for understanding how different pathological processes might lead to cognitive decline through specific and overlapping pathways.
Subjects and Methods
Subjects
The sample was drawn from the first 127 sequentially autopsied cases in the ischemic vascular dementia (IVD) program project, a prospective, longitudinal study of CVD, AD, and normal aging. Among these cases coming to autopsy, 15 were excluded because of incomplete neuropathological data, and 7 were excluded because they had other definitive causes of dementia (5 cases with Lewy body dementia absent AD, and 2 cases with frontotemporal lobar degeneration). Quantitative MRI data were not available for 12 subjects, leaving the 93 cases reported in this study. The autopsy cases were drawn from a total sample of 704 subjects included in the November 2006 database, 189 of whom were deceased, giving an autopsy rate of 67.2%. This article focuses on MR pathological correlations; clinicopathological and neuropsychological-pathological correlations are reported elsewhere.18–20 We use the terms CVD and AD to refer to the pathological diagnoses of CVD and AD, whereas IVD, subcortical IVD, and probable or possible AD are used to denote the clinical situations in which these processes are present.
Subjects were recruited from three university-affiliated dementia centers as described in previous publications.19,21 Control subjects were recruited from the community. Exclusion criteria at the time of enrollment included age younger than 55 years, non–English-speaking, history of cortical strokes, history of dementia that was believed to be related to processes other than AD or CVD, and history of severe illnesses or medications likely to affect cognition.
All subjects underwent a clinical evaluation that included a history and physical examination, laboratory tests, neuropsychological testing with a standardized battery and a standardized research MRI protocol followed by a consensus conference to arrive at a diagnosis as previously described.19 Dementia was diagnosed by Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria,22 probable or possible AD was established according to National Institute of Neurological and Communication Disorders-Alzheimer’s Disease and Related Disorders Association criteria,23 diagnosis of IVD used the California Alzheimer’s Disease Diagnostic and Treatment Centers criteria,24 and mixed dementia was diagnosed if the contributions of both AD and CVD were considered approximately equal. Subjects with cognitive impairments not severe enough to meet dementia criteria, or those who evinced no functional impairment, were diagnosed as having cognitive impairment, no dementia. Diagnostic case conferences utilized all available data including clinical MRI scans, but they did not use quantitative MRI data, which were derived separately in a standardized manner. Neuropsychological testing was repeated annually and MRI every 2 years for subjects with cognitive impairment or dementia; these examinations were every 2 years and every 4 years for cognitively intact persons. At each reevaluation, clinical diagnosis was reviewed and changed by consensus if warranted by intervening data. For this analysis, we chose the last MR examination before autopsy, together with the clinical data closest in time to this examination.
Magnetic Resonance Imaging Acquisition and Analysis
MRI acquisition and analysis has been reported previously,21,25 and full details can be found in supplementary online data. In brief, MR double spin-echo (DSE) and volumetric T1 (magnetization-prepared rapid gradient echo or spoiled gradient echo recalled) sequences were obtained on 1.5-Tesla MR systems (either Siemens Vision, Erlangen, Germany, or General Electric Signa, Milwaukee, WI) with a quadrature head coil. Tissues were segmented using an automated algorithm with manual editing to define gray matter, white matter, cerebrospinal fluid, and WMH, and hippocampal volume (HV) was measured on the coronal T1 data sets using a semiautomated algorithm. Final variables for this study include HV, cortical gray matter (CGM), and WMH, each normalized to the total intracranial volume. Lacunes were manually defined as discrete lesions more than 2mm in diameter that were hyperintense relative to cerebrospinal fluid on proton density images; they were slightly smaller than, but otherwise similar to, the lesions identified by the Cardiovascular Health Study criteria.26
Neuropathological Examination
Neuropathological examination was described previously and utilized a CVD pathology score.19 The quantitative data rated for each case included measures of Alzheimer’s pathology, HS, cerebrovascular brain injury, and blood vessel abnormalities. Alzheimer’s pathology utilized Braak and Braak staging of neurofibrillary pathology9 and Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuritic plaque scores.27 HS was defined as well-demarcated regions of segmental hippocampal neuronal loss and gliosis, generally involving CA1, prosubiculum, or both, and was graded on a 0 to 3 scale for each hemisphere: 0 indicated absence of HS, 1 indicated involvement of a single CA region (usually CA1), 2 indicated extension of segmental neuronal loss and gliosis to the prosubiculum, and 3 indicated involvement of the entire pyramidal cell layer over multiple coronal sections.
The severity of cerebrovascular ischemic brain injury was rated using the CVD pathology scoring system developed within this project.19 The overall summary score was the cerebrovascular disease parenchymal score (CVDPS), ranging from 0 to 300, that was composed of scores for cystic infarcts (CYST INF), lacunar infarcts (LAC INF), and microinfarcts (MICRO INF), each ranging from 0 to 100. In addition, infarcts were categorized by location as cortical (CORTICAL INF) and subcortical (SUBCORTICAL INF), also standardized to a scale of 0 to 100. Complete and incomplete white matter infarct and demyelination scores were combined to generate a white matter pathology (WMP) score standardized to a scale of 0 to 100.
The blood vessel abnormalities were graded by neuropathologists after reviewing all tissue blocks and brain photographs. Atherosclerosis was graded based on a review of the circle of Willis and meningeal blood vessels, whereas arteriosclerosis was rated in the basal ganglia and thalamus. Severity, grade, or extent of atherosclerosis and arteriolosclerosis were each rated on a 4-point scale (0–3 points). Cerebral amyloid angiopathy (CAA) was rated in Brodmann area 17 using Vonsattel and colleagues’28 0 to 4 scale; CAA-associated microangiopathies, which we considered Vonsattel stage 4, were noted as being present or absent in a given brain specimen.29
For categorical analyses, cutoff scores were selected for Braak and Braak stage and CVDPS score to operationally define pathological subgroups as described in a previous publication.19 We considered a Braak stage ≥ IV, where AD is considered to be moderately likely by National Institute on Aging-Reagan criteria,30 to indicate AD. We used a CVDPS score ≥ 20, which divides the sample approximately into tertiles, as a cutoff score for CVD (CVDPS = 0, n = 37; 0 < CVDPS < 20, n = 33; CVDPS ≥ 20, n = 23). Cases with Braak and Braak stage < IV and CVDPS < 20 were classified as having no significant pathological abnormality. We further subdivided the no significant pathological abnormality group into 12 normal control cases (NCs: cognitively normal and no significant pathology) and 12 OTHER cases (cognitively impaired without significant pathology).
Data Analysis
Because pathological variables were continuous and diagnostic classification therefore relied on arbitrary cutoff scores, our primary analyses used linear and ordinal logistic regression models to investigate the associations between the neuropathological and imaging measures. Dependent variables in these models were the imaging variables, either volume of CGM, HV, log WMH, or number of lacunes. The independent variables were age at death and three measures of neuropathology (AD, HS, and CVD), as well as blood vessel abnormalities. We used this approach, rather than defining pathology as the dependent measure, because the goal of the study was to define factors that explained MR findings, rather than factors that predicted pathology.
Because the distribution of WMH volume and number of lacunes were right skewed, a log transformation was applied to the WMH variable, and number of lacunes was analyzed using ordinal logistic rather than linear regression (no lacunes, 1–2 lacunes, or 3–10 lacunes). As colinearity was observed for Braak and Braak stage and CERAD plaque score (Spearman’s r = 0.85; p < 0.0001) and for the atherosclerosis and arteriolosclerosis rating (Spearman’s r = 0.71; p < 0.0001), combined scores were generated: AD-PATH (defined as [2 × CERAD score + Braak score]/2) and ARTERIO (defined as atherosclerosis + arteriolosclerosis scores).
Simple univariate regression models between each MRI measure and three measures of neuropathology (AD-PATH, CVDPS, and HS) were examined first in the initial steps of model building to explore the variables that were most appropriate for inclusion in final multivariate models. We also fit regression models for CGM and HV using CVDPS or HS as independent variables, stratified by high and low AD-PATH scores. In this way, we examined the relation between non-AD pathology (HS or CVD) and MR outcomes apart from AD, as well as interactions between non-AD and AD pathology on MR outcome.
We developed multivariate models that included the significant terms from the simple regression models. In primary multivariate analyses, AD-PATH, HS, and one of several measures of parenchymal cerebrovascular brain injury were modeled as independent variables: (1) AD-PATH, HS, and CVDPS; (2) AD-PATH, HS, CYST INF, LAC INF, and MICRO INF; (3) AD-PATH, HS, CORTICAL INF, and SUBCORTICAL INF; and (4) AD-PATH, HS, and WMP. Secondary multivariate analyses further investigated the association of ARTERIO and CAA with the imaging variables with adjustment for the other neuropathology measures, as well as the demographic variables of age, sex, and ethnicity. In the final multivariate models, we also included the lag between MRI and death; these models did not differ substantially from models that did not contain this variable. The final models included all significant components from previous models and are reported as the total r2 for all variables in the model. All analyses were conducted using Statistical Analysis System version 9.0 (SAS Institute, Cary, NC). A p value less than 0.05 was considered statistically significant.
Results
Table 1 shows the subject characteristics, including mean MR variables, grouped by last clinical diagnosis before death. At the time of last clinical evaluation, the majority of subjects (n = 63) had dementia, although there was a substantial proportion of cognitively normal (n = 15) and cognitive impairment, no dementia (n = 15) subjects. For all subjects, mean age at death was 83.5 ± 7.2 years. Mean interval between last neuropsychological testing and death was 1.9 ± 1.7 years, and between the last MRI and death was 2.7 ± 2.0 years.
Table 1.
Demographic Data by Clinical Diagnosis Closest to Death (N = 93)
| Cognitively Normal (n = 15) | CIND (n = 15) | SIVD (n = 11) | pAD (n = 32) | Mixed AD/SIVD (n = 20) | Pa | |
|---|---|---|---|---|---|---|
| Mean age at death, yr (SD) | 84.2 (6.5)b | 82.3 (5.6) | 84.9 (8.4) | 82 (8.3) | 85.6 (6.1) | 0.41 |
|
| ||||||
| Mean education, yr (SD) | 13.3 (2.5) | 15.1 (3) | 13.3 (2.8) | 14.9 (3.6) | 12.3 (2.7) | 0.02 |
| Cortical gray matter, % (SD) | 38.5 (2.2) | 35.4 (3.6) | 34.2 (2.8) | 35 (2.4) | 34.3 (4.3) | 0.001 |
|
| ||||||
| Hippocampal volume, % (SD) | 0.3 (0.1) | 0.3 (0) | 0.2 (0) | 0.2 (0) | 0.2 (0.1) | <0.0001 |
|
| ||||||
| White matter hyperintensities, % (SD) | 1.1 (1) | 2.2 (1.9) | 3.5 (2.9) | 0.9 (1) | 2.7 (2.3) | 0.0002 |
| Number of lacunes (SD) | 0.5 (0.7) | 3.1 (3) | 2.9 (2.9) | 0.9 (1.5) | 1.8 (2.5) | 0.003 |
| Mean time from last MRI to death, yr (range) | 3.3 (0.8–8.3) | 1.5 (0.2–3.1) | 2.7 (0.7–6.6) | 3.3 (0.5–7.8) | 2.2 (0.3–8.7) | 0.02 |
|
| ||||||
| Mean global cognitive score (SD) | 94.4 (16.2) | 91.1 (17.9) | 49.9 (17.9) | 44.9 (19) | 51.6 (18.2) | <0.0001 |
|
| ||||||
| Mean last MMSE score (SD) | 28.4 (1.4) | 28.1 (2.1) | 17.6 (6.1) | 11.9 (8.4) | 12.8 (6.8) | <0.0001 |
| Mean time from last neuropsychological testing to death, yr (SD) | 1.7 (2) | 0.9 (0.8) | 2.1 (2) | 2.2 (1.6) | 2.1 (1.9) | 0.19 |
|
| ||||||
| Sex, n (%)
| ||||||
| Male | 6 (40) | 9 (60) | 5 (45) | 20 (63) | 12 (60) | 0.61 |
| Female | 9 (60) | 6 (40) | 6 (55) | 12 (38) | 8 (40) | |
|
| ||||||
| Race, n (%) | ||||||
| White | 14 (93) | 14 (93) | 8 (73) | 31 (97) | 15 (75) | 0.09 |
|
| ||||||
| Hispanic | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 1 (5) | |
|
| ||||||
| Black | 0 (0) | 1 (7) | 1 (9) | 0 (0) | 2 (10) | |
| Asian | 1 (7) | 0 (0) | 2 (18) | 0 (0) | 2 (10) | |
p value from analysis of variance for continuous variables, from Fisher’s exact test for categoric variables.
CIND = cognitive impairment, no dementia; SIVD = subcortical ischemic vascular dementia; pAD = probable or possible Alzheimer’s disease; AD = Alzheimer’s disease; SD = standard deviation; MRI = magnetic resonance imaging; MMSE = Mini-Mental State Examination.
Associations between Magnetic Resonance Variables and Pathological Diagnoses
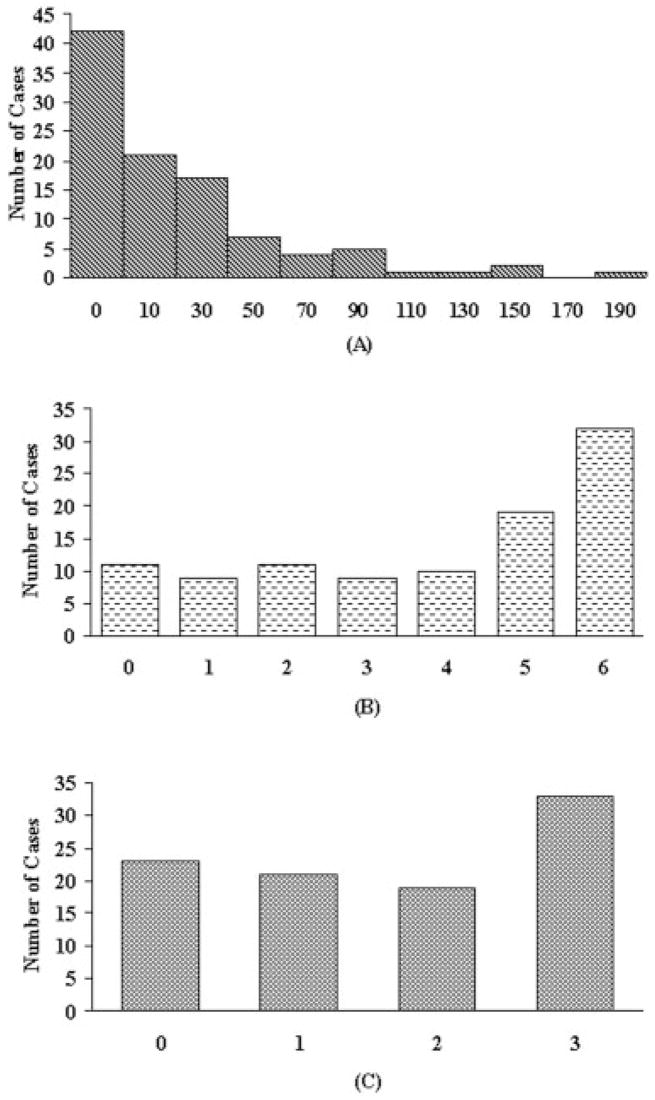
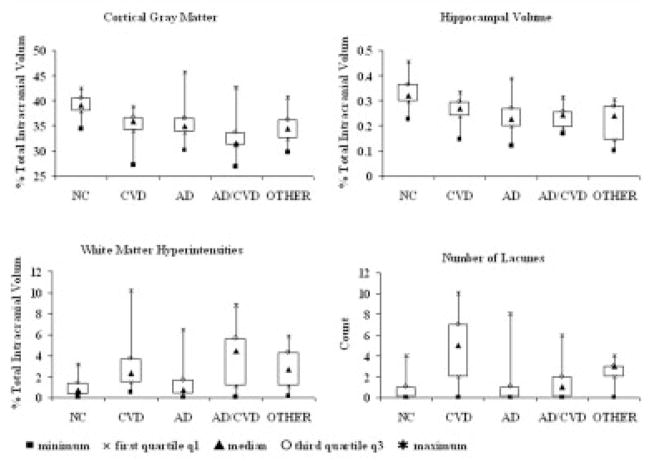
A wide range of CVDPS, Braak and Braak, and CERAD neuritic plaque scores were found in the sample (Fig 1). Using the cutoff scores for Braak and Braak stage and CVDPS to dichotomize continuous processes as described earlier, we determined that 46 cases had predominantly AD pathology, 14 had predominantly CVD pathology, 9 had mixed AD/CVD pathology, and 24 did not meet the threshold for significant CVD and AD pathology (no significant pathological abnormality), of which 12 were noncognitively impaired and considered NCs and 12 were cognitively impaired (OTHER). Figure 2 shows box plots of the MR variables grouped by these pathological diagnoses. CGM was lowest in the mixed AD/CVD group (33.0 ± 4.3) and decreased to a similar extent in the CVD (35.0 ± 3.1), AD (35.3 ± 2.9), and OTHER groups (34.6 ± 3.2) compared with the NC group (38.9 ± 2.2) (p values of <0.0001, 0.001, 0.0004, and 0.0008, respectively). HV was lowest in the AD (0.23 ± 0.06), mixed AD/CVD (0.23 ± 0.05), and OTHER groups (0.21 ± 0.07), and decreased in the CVD group (0.26 ± 0.05) compared with the NC group (0.34 ± 0.06) (p values of <0.0001, <0.0001, <0.0001, and 0.001, respectively). WMH volume was increased in the CVD (2.7 ± 2.5), mixed AD/CVD (3.9 ± 3.0), and OTHER groups (2.7 ± 1.8) compared with the NC group (1.0 ± 0.9) (p = 0.01, 0.0003, and 0.01, respectively). Number of lacunes was increased in CVD (4.5 ± 3.1) and OTHER (2.2 ± 1.5) groups compared with the NC group (0.3 ± 0.5) (p values of <0.0001 and 0.02, respectively).
Fig 1.
Frequency histograms indicating the distribution of neuropathological abnormalities in the 93 autopsied cases. (A) Cerebrovascular disease parenchymal score, (B) Braak stage, and (C) Consortium to Establish a Registry for Alzheimer’s Disease plaque score.
Fig 2.
Box plots showing the distribution of quantitative magnetic resonance imaging volumes by pathological group. Solid squares denote minimum; multiply signs denote first quartile; solid triangles denote median; open circles denote third quartile; asterisks denote maximum. AD = Alzheimer’s disease; CVD = cerebrovascular disease; NC = cognitively normal and no significant pathology; OTHER = cognitively impaired without significant pathology.
Associations between Magnetic Resonance Cortical Gray Matter and Pathology
Univariate analyses showed AD-PATH and CVDPS to be significant inverse correlates of CGM, whereas HS was not a significant pathology predictor of CGM. Among the CVD components, LAC INF, MICRO INF, SUBCORTICAL INF, and WMP, rather than CYST INF or CORTICAL INF, were significant correlates of CGM. Among blood vessel pathology variables, ARTERIO rather than CAA was inversely associated with CGM. In the final multivariate model (Table 2), the three variables AD-PATH, ARTERIO, and SUBCORTICAL INF explained 25% of the variance in CGM. Age at death, ethnicity, and sex were not significantly associated with CGM. We further examined the relation between SUBCORTICAL INF score and CGM and ARTERIO and CGM in cases with low versus high AD (based on a median split in AD-PATH). No interactions were found between SUBCORTICAL and AD-PATH or between ARTERIO and AD-PATH.
Table 2.
Final Multivariate Linear Regression Models Evaluating the Association between Neuropathological Variables and Magnetic Resonance Imaging Measures at Visit Closest to Death (N = 93)
| Dependent Variable | Independent Variable | β | SE | p | r2 |
|---|---|---|---|---|---|
| Cortical gray matter volume | Intercept | 38.72 | 0.92 | <0.0001 | 0.25 |
| AD-PATH | −0.47 | 0.17 | 0.006 | ||
|
| |||||
| ARTERIO | −0.6 | 0.24 | 0.02 | ||
| SUBCORTICAL INF | −0.05 | 0.03 | 0.045 | ||
|
| |||||
| Hippocampal volumea | Intercept | 0.27 | 0.01 | <0.0001 | 0.33 |
|
| |||||
| AD-PATH | −0.01 | 0.003 | <0.0001 | ||
| HS | −0.02 | 0.006 | 0.009 | ||
|
| |||||
| White matter hyperintensitiesb | Intercept | −0.01 | 0.09 | 0.89 | 0.32 |
| WMP | 0.02 | 0.003 | <0.0001 | ||
| Age at death | 0.02 | 0.007 | 0.01 | ||
All models were adjusted for time from last magnetic resonance imaging (MRI) scan to death. Units of all MRI variables are percentage intracranial volume.
Adjusted for sex in the final model.
White matter hyperintensities were log transformed.
SE = standard error; AD-PATH = Alzheimer’s disease pathology (0–6); ARTERIO = sum of atherosclerosis and arteriolosclerosis scores (0–6); SUBCORTICAL INF = subcortical infarct scores (0–100); HS = hippocampal sclerosis (0–4); WMP = white matter pathology score (0–100).
Associations between Magnetic Resonance Hippocampal Volume and Pathology
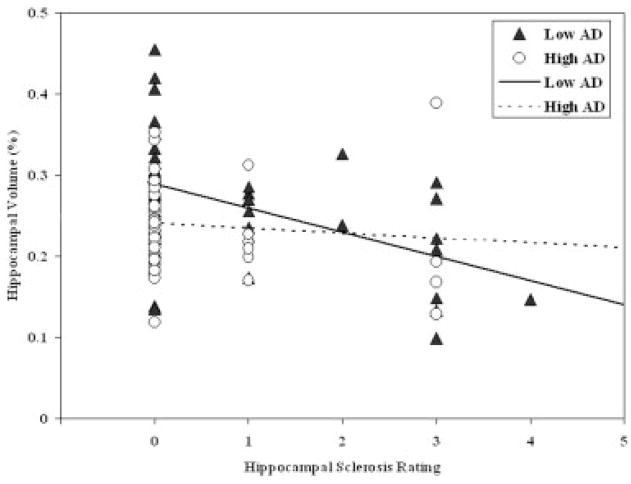
For HV, univariate analyses demonstrated AD-PATH and HS, but not CVDPS, to be important inverse correlates. Secondary analyses showed significant inverse associations of HV with CAA, but not ARTERIO. HV was not associated with age at death (p = 0.20), but was lower in male subjects. After adjusting for sex, in the final multivariate model, AD-PATH and HS jointly accounted for 33% of the HV variance (see Table 2). Of note, CAA was negatively associated with HV at a marginally significant level (β = −0.01; standard error, 0.006; p = 0.066) after adjustment for AD-PATH, HS, and sex. HS scores and Braak stage were not correlated with one another (r = −0.01; p = 0.90), and there was no association between a Braak and Braak stage ≥ IV and the presence of HS (χ2 = 0.90; p = 0.34). Figure 3 illustrates the relation between HS and HV according to the extent of AD pathology: HS has a stronger and significant effect in the group with low AD pathology (Spearman’s r = −0.44; p = 0.002), but a weak and nonsignificant effect in the group with high AD pathology (Spearman’s r = −0.1; p = 0.52) (interaction p = 0.075).
Fig 3.
Relation between pathologically measured hippocampal sclerosis and magnetic resonance measured hippocampal volume for two groups of subjects defined by the extent of Alzheimer’s disease (AD) pathology using a median split. Triangles denote low AD; circles denote high AD; solid line denotes low AD; dotted line denotes high AD.
Associations between Magnetic Resonance White Matter Hyperintensities and Pathology
In univariate analyses against log WMH, positive correlations were noted with AD-PATH, CVDPS, and HS. All combinations of CVD variables (CYST INF, LAC INF, MICRO INF, CORT INF, SUBCORT INF, and WMP) were positively correlated with log WMH. Secondary analysis also showed ARTERIO to be positively associated with WMH (β = 0.13; standard error, 0.03; p = 0.0002). No significant relation between CAA and log WMH was found in the sample as a whole, although there was a weak trend in the high AD-PATH group (r = 0.16; p = 0.30, no significant interaction). In the final multivariate model for log WMH (see Table 2), age at death and WMP explained 32% of the variance in log WMH. Of note, ARTERIO was positively associated with log WMH at a marginally significant level (β = 0.07; standard error, 0.04; p = 0.059) after adjustment for age at death and WMP.
Associations between Magnetic Resonance Lacunes and Pathology
The relation between pathology and number of MR-visualized lacunes was assessed using ordinal logistic regression. There were 46 cases with no lacunes, 22 with 1 to 2 lacunes, and 22 cases with 3 to 10 lacunes. In univariate analyses, CVDPS was marginally associated with increased number of lacunes (p = 0.09), whereas higher AD-PATH was associated with decreased number of lacunes (p = 0.02); no significant relation was noted between HS and lacunes. For type of blood vessel pathology, ARTERIO, not CAA, was positively associated with lacunes. The final ordinal logistic regression for number of MR lacunes included WMP (odds ratio, 1.03; 95% confidence interval, 1.02–1.06; p = 0.02) and AD-PATH (odds ratio, 0.76; 95% confidence interval, 0.61–0.94; p = 0.01).
Discussion
Results of this study show that four quantitative MR measures of anatomy (CGM, HV, WMH, and lacunes) have complex and overlapping associations with three major neuropathological substrates (AD, HS, and CVD) in a large sample with a wide range of vascular and degenerative pathology. Strong evidence from multivariate models indicates that CGM is related to both Alzheimer’s and vascular pathology. HV is related to AD pathology, and also to HS, and these two pathological processes appear to be independent of one another, explaining HV in different subjects. Both WMH and lacunes are strongly reflective of CVD.
These results suggest that the neuropathological basis of MR findings in aging and dementia are complex, with multiple pathological processes converging on similar brain structures that mediate cognitive decline. Although it is known that hippocampal atrophy does not necessarily reflect AD pathology,10 these results show that even CGM atrophy reflects a vascular and AD component. In addition, although WMH are associated with vascular pathology, Figure 2 demonstrates that AD and CVD pathology often coexist and produce substantial reductions in CGM volume.
From the perspective of understanding the pathway to cognitive decline and dementia, these findings expand on the previous clinical-imaging correlations reported from this cohort. As noted, CGM and HV are the strongest predictors of neuropsychological performance16 and cognitive decline,31 whereas WMH and lacunes contribute only modestly to cognitive function in multivariate models. Our current findings shed light on these associations and suggest that vascular disease can affect cognition not only through effects on sub-cortical structures and white matter, but also by exacerbating cortical atrophy. Indeed, the effects of AD and vascular pathology on cerebral cortex and perhaps hippocampus provide a compelling link in which disparate pathological processes converge on structures crucial to cognition.
Our data agree with imaging-autopsy correlation studies that show associations between hippocampal atrophy seen on MR10,32–35 and AD pathology, but the data also highlight the importance of HS as an independent cause, particularly in cases with comparatively little AD pathological change. HS often goes unrecognized as a cause of amnesia and progressive dementia in late life, is often diagnosed as AD,36,37 and has been reported in 12% of older adults with dementia drawn from a community-based autopsy series.38 The pathogenesis of HS remains controversial; associations with both vascular and degenerative processes have been observed or suggested.19,37–40 Our data indicate that in elderly adults with progressive amnesia and hippocampal atrophy, HS should be considered as a possible underlying causative factor, although diagnostic tests for HS are not available.
Epidemiological data have established associations between age and vascular risk factors and WMH using semiquantitative scales.7,41,42 Cross-sectional and longitudinal data link WMH to slowed processing speed, 43,44 dysexecutive syndromes, 45 and incident dementia.11 Nevertheless, pathological studies have shown associations with lacunar infarction, demyelination and pallor, gliosis, dilated perivascular spaces, and CAA,12,46–50 leaving a persisting impression that WMH is nonspecific. In this study, WMH was measured volumetrically, which offers an advantage over the commonly used semiquantitative rating scales that show a ceiling effect.51 We confirm positive associations between log WMH with age and CVD-related variables, and no associations with AD or CAA. We cannot state with certainty whether the pathological “incomplete infarction” (pallor, demyelination, and axonal loss) that we found to be associated with WMH reflects CVD and not Wallerian degeneration. However, the latter appears less likely in view of the lack of association between WMH and AD pathology. In the setting of progressive cognitive impairment in late life, this study suggests that WMH is associated with vascular disease and complete and incomplete infarction, and is thus a more specific finding than previously supposed.
There is a paucity of studies that focus on the pathology of MRI-defined lacunes. Discrete hyperintensities on T2-weighted MRI may represent incomplete or complete infarcts or dilated perivascular spaces.52
This study confirms expected positive associations between MR-defined lacunes with infarcts and arteriosclerosis. Because perivascular spaces were not included as a pathological variable, there is a remaining need to correlate MRI-lacunes and pathology on a lesion-by-lesion basis. Nonetheless, the results support the value of MR-defined lacunes as a marker for vascular rather than AD-related pathology.
This study has several limitations. Patients with a wide range of dementing disease were excluded, as were those with large cortical infarcts, limiting our inferences about these conditions. The ratings of atherosclerosis and arteriolosclerosis were semiquantitative and were based on an overall impression. Although the Braak and Braak staging and CERAD neuritic plaque score measures of AD pathology are widely recognized, the CVD-PATH scoring system reported here is novel. The CVD subscores were standardized to avoid weighting effects and were suitable for multiple regression or ordinal logistic regression analyses, but the β values or odds ratio do not translate directly to pathology findings. The analyses in this study were based on cross-sectional data obtained between MRI closest to autopsy, an interval of several years; changes in pathology during this period would weaken MR-pathology correlations. Although the delay between MR and postmortem examination was long in some cases, our analytic approach accounted for this problem. In fact, estimates of association were essentially unaltered with adjustment for years since MRI examination. Finally, although our multivariate models showed significant associations between pathology and MR variables, a large proportion of variance in each of these models remains unexplained. This may reflect imprecision in the techniques, as well as the likelihood that some pathological findings, particularly AD and microin-farcts, are difficult to detect with MRI.
Qualitative MRI is now a mainstay of clinical diagnosis of cognitive decline, and volumetric measures are rapidly becoming the standard for research. Historically, investigative studies begin by contrasting AD and normal aging. When the circle is widened to include mixed pathologies, autopsy findings are essential. This study provides an opportunity to relate quantitative MRI with pathology in a mixed sample of AD, CVD, and normal aging. In this clinical setting, the presence of lacunes and white matter changes provide a good signal for underlying vascular disease. Although cerebral cortical and hippocampal atrophy help to differentiate disease states from normal aging, different pathological processes share the final common pathway leading from structural atrophy to cognitive decline. Indeed, it is likely that this convergence on structures critical to cognition, hippocampus and cortex, are mechanistically important in determining cognitive decline in AD and vascular disease. However, more specific molecular and microstructural markers are needed to differentiate the causative agents underlying these structural changes, and thus suggest best approaches to treatment.
Acknowledgments
This work was supported by the NIH (National Institute on Aging): AG12435 (H.C.C.), AG10129 (CD.), AG16570 (H.V.V.), AG05142 (H.C.C.).
Footnotes
This article includes supplementary materials available via the Internet at http://www.interscience.wiley.com/jpages/0364-5134/suppmat
References
- 1.Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1143–1153. doi: 10.1212/wnl.56.9.1143. [DOI] [PubMed] [Google Scholar]
- 2.Scahill RI, Schott JM, Stevens JM, et al. Mapping the evolution of regional atrophy in Alzheimer’s disease: unbiased analysis of fluid-registered serial MRI. Proc Natl Acad Sci U S A. 2002;99:4703–4707. doi: 10.1073/pnas.052587399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scab JP, Jagust WJ, Wong STS, et al. Quantitative NMR measurements of hippocampal atrophy in Alzheimer’s disease. Magn Reson Med. 1988;8:200–208. doi: 10.1002/mrm.1910080210. [DOI] [PubMed] [Google Scholar]
- 4.Jack CR, Petersen RC, O’Brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer’ disease. Neurology. 1992;42:183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- 5.Jack CR, Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Killiany RJ, Gomez-Isla T, Moss M, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer’s disease. Ann Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- 7.Breteler MMB, van Swieten JC, Bots ML, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam study. Neurology. 1994;44:1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- 8.DeCarli C, Miller BL, Swan GE, et al. Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the National Heart, Lung, and Blood Institute Twin Study. Arch Neurol. 2001;58:643–647. doi: 10.1001/archneur.58.4.643. [DOI] [PubMed] [Google Scholar]
- 9.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 10.Jack CR, Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58:750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Dijk EJ, Prins ND, Vermeer SE, et al. Plasma amyloid beta, apolipoprotein E, lacunar infarcts, and white matter lesions. Ann Neurol. 2004;55:570–575. doi: 10.1002/ana.20050. [DOI] [PubMed] [Google Scholar]
- 12.Gurol ME, Irizarry MC, Smith EE, et al. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology. 2006;66:23–29. doi: 10.1212/01.wnl.0000191403.95453.6a. [DOI] [PubMed] [Google Scholar]
- 13.DeCarli C, Miller BL, Swan GE, et al. Predictors of brain morphology for the men of the NHLBI twin study. Stroke. 1999;30:529–536. doi: 10.1161/01.str.30.3.529. [DOI] [PubMed] [Google Scholar]
- 14.Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behav Neurosci. 2003;117:1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- 15.Neuropathology Research Group, Medical Research Council Cognitive Function and Aging Study. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Aging Study. Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 16.Mungas D, Jagust WJ, Reed BR, et al. MRI predictors of cognition in subcortical ischemic vascular disease and Alzheimer’s disease. Neurology. 2001;57:2229–2235. doi: 10.1212/wnl.57.12.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mungas D, Harvey D, Reed BR, et al. Longitudinal volumetric MRI change and rate of cognitive decline. Neurology. 2005;65:565–571. doi: 10.1212/01.wnl.0000172913.88973.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vinters HV, Ellis WG, Zarow C, et al. Neuropathologic substrates of ischemic vascular dementia. J Neuropathol Exp Neurol. 2000;59:931–945. doi: 10.1093/jnen/59.11.931. [DOI] [PubMed] [Google Scholar]
- 19.Chui HC, Zarow C, Mack WJ, et al. Cognitive impact of sub-cortical vascular and Alzheimer disease pathology. Ann Neurol. 2006;60:677–687. doi: 10.1002/ana.21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reed BR, Mungas DM, Kramer JH, et al. Profiles of neuropsychological impairment in autopsy-defined Alzheimer’s disease and cerebrovascular disease. Brain. 2007;130:731–739. doi: 10.1093/brain/awl385. [DOI] [PubMed] [Google Scholar]
- 21.Fein G, Di Sclafani V, Tanabe J, et al. Hippocampal and cortical atrophy predict dementia in subcortical ischemic vascular disease. Neurology. 2000;55:1626–1635. doi: 10.1212/wnl.55.11.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 23.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 24.Chui HC, Victoroff JI, Margolin DI, et al. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer disease diagnostic and treatment centers. Neurology. 1992;42:473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- 25.Du AT, Schuff N, Chao LL, et al. White matter lesions are associated with cortical atrophy more than entorhinal and hippocampal atrophy. Neurobiol Aging. 2005;26:553–559. doi: 10.1016/j.neurobiolaging.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Bryan RN, Wells SW, Miller TJ, et al. Infarctlike lesions in the brain: prevalence and anatomic characteristics at MR imaging of the elderly—data from the Cardiovascular Health Study. Radiology. 1997;202:47–54. doi: 10.1148/radiology.202.1.8988191. [DOI] [PubMed] [Google Scholar]
- 27.Mirra SS, Heyman A, McKeel D, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD): Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 28.Vonsattel JP, Myers RH, Hedley-Whyte ET, et al. Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol. 1991;30:637–649. doi: 10.1002/ana.410300503. [DOI] [PubMed] [Google Scholar]
- 29.Vinters HV, Vonsattel JP. Neuropathologic features and grading of Alzheimer-related and sporadic CAA. In: Verbeek MM, de Wall RMW, Vinters HV, editors. Cerebral amyloid angiopathy in Alzheimer’s disease and related disorders. Dordrecht, the Netherlands; Kluwer Academic: 2000. pp. 137–155. [Google Scholar]
- 30.NIA and Reagan Institute working group on diagnostic criteria for the neuropathological assessment of Alzheimer’s disease. Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 31.Mungas D, Reed BR, Jagust WJ, et al. Volumetric MRI predicts rate of cognitive decline related to AD and cerebrovascular disease. Neurology. 2002;59:867–873. doi: 10.1212/wnl.59.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gosche KM, Mortimer JA, Smith CD, et al. Hippocampal volume as an index of Alzheimer neuropathology: findings from the Nun Study. Neurology. 2002;58:1476–1482. doi: 10.1212/wnl.58.10.1476. [DOI] [PubMed] [Google Scholar]
- 33.Silbert LC, Quinn JF, Moore MM, et al. Changes in premor-bid brain volume predict Alzheimer’s disease pathology. Neurology. 2003;61:487–492. doi: 10.1212/01.wnl.0000079053.77227.14. [DOI] [PubMed] [Google Scholar]
- 34.Nagy Z, Hindley NJ, Braak H, et al. The progression of Alzheimer’s disease from limbic regions to the neocortex: clinical, radiological and pathological relationships. Dement Geriatr Cogn Disord. 1999;10:115–120. doi: 10.1159/000017111. [DOI] [PubMed] [Google Scholar]
- 35.Bobinski M, Wegiel J, Wisniewski HM, et al. Neurofibrillary pathology—correlation with hippocampal formation atrophy in Alzheimer disease. Neurobiol Aging. 1996;17:909–919. doi: 10.1016/s0197-4580(97)85095-6. [DOI] [PubMed] [Google Scholar]
- 36.Ala TA, Beh GO, Frey WH., 2nd Pure hippocampal sclerosis: a rare cause of dementia mimicking Alzheimer’s disease. Neurology. 2000;54:843–848. doi: 10.1212/wnl.54.4.843. [DOI] [PubMed] [Google Scholar]
- 37.Corey-Bloom J, Sabbagh MN, Bondi MW, et al. Hippocampal sclerosis contributes to dementia in the elderly. Neurology. 1997;48:154–160. doi: 10.1212/wnl.48.1.154. [DOI] [PubMed] [Google Scholar]
- 38.Leverenz JB, Agustin CM, Tsuang D, et al. Clinical and neuropathological characteristics of hippocampal sclerosis: a community-based study. Arch Neurol. 2002;59:1099–1106. doi: 10.1001/archneur.59.7.1099. [DOI] [PubMed] [Google Scholar]
- 39.Dickson DW, Davies P, Bevona C, et al. Hippocampal sclerosis: a common pathological feature of dementia in very old (> or = 80 years of age) humans. Acta Neuropathol (Berl) 1994;88:212–221. doi: 10.1007/BF00293396. [DOI] [PubMed] [Google Scholar]
- 40.Hatanpaa KJ, Blass DM, Pletnikova O, et al. Most cases of dementia with hippocampal sclerosis may represent frontotem-poral dementia. Neurology. 2004;63:538–542. doi: 10.1212/01.wnl.0000129543.46734.c0. [DOI] [PubMed] [Google Scholar]
- 41.Longstreth WT, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 42.Jeerakathil T, Wolf PA, Beiser A, et al. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke. 2004;35:1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 43.Junqué C, Pujol J, Vendrell P, et al. Leuko-araiosis on magnetic resonance imaging and speed of mental processing. Arch Neurol. 1990;47:151–156. doi: 10.1001/archneur.1990.00530020047013. [DOI] [PubMed] [Google Scholar]
- 44.de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000;47:145–151. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- 45.Tullberg M, Fletcher E, DeCarli C, et al. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;63:246–253. doi: 10.1212/01.wnl.0000130530.55104.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Swieten JC, van Den Hout JHW, van Ketel BA, et al. Periventricular lesions in the white matter on magnetic resonance imaging in the elderly. A morphometric correlation with arteriolosclerosis and dilated perivascular spaces. Brain. 1991;114:761–774. doi: 10.1093/brain/114.2.761. [DOI] [PubMed] [Google Scholar]
- 47.Munoz DG, Hastak SM, Harper B, et al. Pathologic correlates of increased signals of the centum ovale on magnetic resonance imaging. Arch Neurol. 1993;50:492–497. doi: 10.1001/archneur.1993.00540050044013. [DOI] [PubMed] [Google Scholar]
- 48.Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 49.Scheltens P, Barkhof F, Leys D, et al. Histopathologic correlates of white matter changes on MRI in Alzheimer’s disease and normal aging. Neurology. 1995;45:883–888. doi: 10.1212/wnl.45.5.883. [DOI] [PubMed] [Google Scholar]
- 50.Bronge L, Bogdanovic N, Wahlund LO. Postmortem MRI and histopathology of white matter changes in Alzheimer brains. Dement Geriatr Cogn Disord. 2002;13:205–212. doi: 10.1159/000057698. [DOI] [PubMed] [Google Scholar]
- 51.van Straaten EC, Fazekas F, Rostrup E, et al. Impact of white matter hyperintensities scoring method on correlations with clinical data: the LADIS study. Stroke. 2006;37:836–840. doi: 10.1161/01.STR.0000202585.26325.74. [DOI] [PubMed] [Google Scholar]
- 52.Pullicino PM, Miller LL, Alexandrov AV, Ostrow PT. Infrapu-taminal ‘lacunes’ Clinical and pathological correlations. Stroke. 1995;26:1598–1602. doi: 10.1161/01.str.26.9.1598. [DOI] [PubMed] [Google Scholar]