Abstract
Objective
To assess the association of HIV infection, HIV disease parameters (including CD4+ T-cell counts, HIV viral load, and AIDS) and antiretroviral medication use with subclinical carotid artery atherosclerosis.
Design
Cross-sectional study nested within a prospective cohort study
Methods
Among participants in the Women's Interagency HIV Study (1,331 HIV-infected women, 534 HIV-uninfected women) and Multicenter AIDS Cohort Study (600 HIV-infected men, 325 HIV-uninfected men), we measured subclinical carotid artery lesions and common carotid artery intima-media thickness (CIMT) using B-mode ultrasound. We estimated adjusted mean CIMT differences and prevalence ratios (PRs) for carotid lesions associated with HIV-related disease and treatments, with multivariate adjustment to control for possible confounding variables.
Results
Among HIV-infected individuals, a low CD4+ T cell count was independently associated with an increased prevalence of carotid lesions. Compared to the reference group of HIV-uninfected individuals, the adjusted PR for lesions among HIV-infected individuals with CD4+ T-cell count <200 cells/mm3 was 2.00 (95% confidence interval 1.22, 3.28) in women and 1.74 (95% confidence interval 1.04, 2.93) in men. No consistent association of antiretroviral medications with carotid atherosclerosis was observed, except for a borderline significant association between protease inhibitor use and carotid lesions in men (with no association among women). History of clinical AIDS and HIV viral load were not significantly associated with carotid atherosclerosis.
Conclusions
Beyond traditional cardiovascular disease risk factors, low CD4+ T-cell count is the most robust risk factor for increased subclinical carotid atherosclerosis in HIV-infected women and men.
Recent studies among HIV-infected populations have linked use of combination antiretroviral therapy with increased risk of cardiovascular disease (CVD) events [1-4] and subclinical atherosclerosis [5-7]. Among HIV-infected individuals, low CD4+ T-cell count has also been identified as a vascular risk factor [5]. However, the data have not been consistent as other studies have not confirmed the reported associations of antiretroviral use [8-11] or low CD4+ T-cell count [2] with clinical or subclinical CVD.
Several potential mechanisms have been described that may link HIV disease and its treatment with increased risk of vascular disease. Individuals with untreated HIV infection have decreased levels of high-density lipoprotein cholesterol (HDL-C) and increased levels of triglycerides [12-14], which are adverse risk factors for vascular disease. On the other hand, persons with untreated HIV infection also have reductions in low-density lipoprotein cholesterol (LDL-C) and total cholesterol, which may be favorable characteristics with regard to atherosclerotic risk [12-14]. Initiation of antiretroviral therapy tends to normalize lipid parameters (total cholesterol, LDL-C and triglycerides, but not HDL-C) [14]. Some therapies, notably protease inhibitors (PI), may have adverse effects on LDL-C and triglyceride levels, blood pressure, and risk of diabetes [15-19]. Use of nucleoside analogs has been associated with body fat changes, insulin resistance and diabetes [20-22]. Circulating markers of inflammation such as C-reactive protein (CRP) may be elevated among individuals with HIV infection, especially during progression to AIDS [23].
The purpose of the present investigation was to assess the association of HIV infection, HIV disease parameters (including CD4+ T-cell counts, HIV viral load, and AIDS) and antiretroviral medication use with subclinical vascular disease. We report the results from two studies, conducted in parallel, within the Women's Interagency HIV Study (WIHS) and the Multicenter AIDS Cohort Study (MACS). WIHS and MACS are population-based US cohort studies that offer several important strengths, including large sample size, inclusion of HIV-uninfected persons who were recruited from the same at-risk populations as the HIV-infected individuals, and extensive longitudinal, protocol-driven data collection on clinical, behavioral, and demographic variables. The present report describes data from the baseline phase of a WIHS-MACS Carotid Ultrasound Substudy, which features standardized image acquisition and measurement of carotid artery intima-media thickness, a quantitative measure of atherosclerosis burden.
Methods
Study participants The WIHS [24] and MACS [25] are ongoing prospective cohort studies of individuals in the United States with HIV infection and HIV-uninfected comparison groups. The WIHS began in 1994 and has enrolled a total of 3,766 women across six sites in San Francisco, Los Angeles, Chicago, Washington, DC, Brooklyn, and Bronx, NY. The WIHS cohort initially enrolled 2,054 HIV-infected women and 569 HIV-uninfected women in 1994−1995 and then was expanded in 2001−2002 with the addition of 737 HIV-infected women and 406 HIV-uninfected women. Both HIV-infected and HIV-uninfected WIHS participants were recruited from venues such as primary care clinics, hospital-based programs, community outreach sites, women's support groups, drug rehabilitation programs, and HIV testing sites.
The MACS was initiated in 1984 as a study of men who have sex with men at four study sites in Baltimore/Washington, DC, Chicago, Los Angeles, and Pittsburgh. A total of 6,973 men were enrolled during 3 time periods: 1,813 HIV-infected and 3,141 HIV-uninfected men in 1984−1985, 425 HIV-infected and 243 HIV-uninfected men in 1987−1990, and 705 HIV-infected and 646 HIV-uninfected men, primarily minority individuals, in 2001−2003. Among the 4,030 men who were seronegative at enrollment, 637 subsequently became HIV-infected.
In April 2004, through coordinated efforts by the WIHS and MACS investigators, a vascular disease substudy was initiated in each cohort which included B-mode ultrasound imaging of the carotid arteries. All WIHS participants were eligible for the vascular substudy, while in MACS, eligibility was restricted to men who reported no history of coronary heart disease (CHD) (including angina, myocardial infarction [MI], or coronary revascularization). For the present analyses, we excluded WIHS women with a history of CHD (100 HIV+, 7.5% and 38 HIV-, 7.1%). The MACS vascular substudy also excluded men who were who under 40 years of age or more than 300 lbs; this was because the MACS vascular substudy also included coronary artery calcium measurements (described elsewhere) [26], which provide little information among younger individuals and for technical reasons are difficult to perform in the morbidly obese. After applying exclusion criteria, carotid ultrasound measurements were available for a total of 1331 HIV-infected and 534 HIV-uninfected WIHS participants and 600 HIV-infected and 325 HIV-uninfected MACS participants.
All individuals provided informed consent and these studies were approved by local Institutional Review Boards.
Data collection High resolution B-mode carotid artery ultrasound was used to image the far wall of the right common carotid artery (CCA), internal carotid artery (ICA), and carotid bulb according to the procedure of Hodis and colleagues [27]. Sonographers at each of the WIHS and MACS sites were uniformly trained at the University of Southern California Atherosclerosis Research Unit Core Imaging and Reading Center (CIRC). Subclinical atherosclerosis was measured by: 1) right distal CCA intima-media thickness (CIMT); and 2) presence of lesions defined as a focal intima-media thickness (IMT) >1.5 mm in any of the imaged segments. Intima-media thickness was centrally measured from standardized ultrasound images of the carotid artery by automated computerized edge detection (Prowin, Patent, 2005, 2006) [28]. The coefficient of variation of repeated measures of CIMT, with repeat scans guided by the initial images, was 1.8% (ICC = 0.98) at WIHS sites (n = 113 WIHS women) and 1.0% (ICC = 0.99) at MACS sites (n = 38 healthy volunteers). Data from the vascular measurements was combined with demographic, clinical, and laboratory variables collected in standardized fashion at baseline and semi-annual WIHS and MACS core visits.
Study variables The definition of highly-active antiretroviral therapy (HAART) was based on the US Department of Health and Human Services (DHHS) treatment guidelines (www.aidsinfo.gov) as use of: (a) 2+ nucleoside reverse transciptase inhibitors (NRTIs) in combination with at least one PI or one non-nucleoside reverse transcriptase inhibitor (NNRTI); (b) one NRTI in combination with at least one PI and at least one NNRTI; or (c) an abacavir or tenofovir containing regimen of 3+ NRTIs in the absence of both PIs and NNRTIs, except for the three-NRTI regimens consisting of: abacavir + tenofovir + lamivudine, or didanosine + tenofovir + lamivudine. Cumulative duration of exposure to PIs, NNRTIs, and NRTIs was defined based on data that was reported semi-annually on the use of individual antiretroviral medications. History of AIDS defining illness according to the 1993 Centers for Disease Control and Prevention case definition was determined by self-report in WIHS, and by medical records confirmation in MACS. For subjects who were already treated with HAART at the time of study enrollment, we obtained prior medication history and the CD4+ T-cell count prior to HAART initiation by medical record review. Additional CD4+ T-cell counts and HIV-1 RNA quantification levels were determined from specimens collected at the time of the semi-annual study visits. Beginning in 1997, participants were instructed to fast for at least 8 hours prior to blood draws. Laboratory values including glucose, total cholesterol, HDL-C, LDL-C, and triglycerides were measured at central laboratories.
Statistical Analysis Our analysis proceeded in three steps. First, we compared average CIMT levels and the prevalence of carotid lesions among HIV-infected individuals versus those who were HIV-uninfected. Second, we investigated the association of CIMT and carotid lesions with measures of HIV disease progression, by conducting separate analyses with variables for recent and nadir CD4+ T-cell counts, recent and peak HIV viral loads, and history of AIDS defining illness (each variable was considered in a separate model). Third, we examined CIMT and carotid lesions in relation to duration of specific antiretroviral therapies among HIV-infected participants, with separate models for the duration of PI use, NNRTI use, and NRTI use (per 2 years of use).
For these models, we utilized multivariate linear regression to estimate adjusted mean differences in CIMT associated with HIV-related and treatment-related variables. Multivariate log-binomial/Poisson regression was used to assess the adjusted prevalence ratios (PR) relating HIV-related and treatment-related variables with the presence of lesions in any segment of the right CCA, ICA or carotid bulb. All multivariate models were adjusted for age, race/ethnicity, education, income, family history of MI, smoking, alcohol, opiate and injection drug use, and study site. In order to assess whether associations between HIV-related variables and CIMT or lesions might be mediated by metabolic CVD risk factors, we also performed further adjustment for diabetes (based on measured glucose levels or history of diabetes treatment), body mass index (BMI), systolic blood pressure, LDL-C, HDL-C, and use of lipid-lowering medication. Data was complete for all analytical covariates for 1100 HIV-infected (89%) and 433 HIV-uninfected (87%) WIHS participants and 515 HIV-infected (86%) and 300 HIV-uninfected (92%) MACS participants. We utilized (single-chain) Markov-chain Monte Carlo multiple-imputation methods for missing data, using separate imputations for each constructed model [29]. Analyses were conducted using SAS (Version 9.1, Cary, NC) with appropriate multiple-imputation inference. The authors had full access to the data, take responsibility for its integrity, and have read and agree to the manuscript as written.
Results
Participant characteristics Table 1 presents characteristics of WIHS and MACS participants stratified by HIV and HAART use. Statistically significant differences were present by HIV infection and HAART use in age, race/ethnicity, education (among men only), income, smoking, alcohol and injection drug use, family history of MI (among men only), diabetes (among men only), lipids and use of lipid-lowering medication, BMI, and systolic blood pressure (among women only) (Table 1). Older age was associated with higher mean CIMT and higher prevalence of carotid lesions (Figure).
Table 1.
Characteristics of HIV-infected and HIV-uninfected women and men participating in the Women's Interagency HIV Study (WIHS) and Multicenter AIDS Cohort Study (MACS)
| Women (WIHS) | Men (MACS) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HIV+/HAART | HIV+/HAART | ||||||||
| HIV-N=477 | Never N=170 | Ever N=1021 | p-value | HIV-N=309 | Never N=61 | Ever N=489 | p-value | ||
| Age (years) | |||||||||
| <35 | 42% | 24% | 24% | <0.0001 | -- | -- | -- | <0.0001 | |
| 35−39 | 15% | 24% | 21% | -- | -- | -- | |||
| 40−44 | 20% | 19% | 22% | 15% | 21% | 26% | |||
| 45−49 | 12% | 14% | 19% | 22% | 28% | 31% | |||
| 50−54 | 7% | 14% | 10% | 22% | 28% | 24% | |||
| ≥ 55 | 4% | 6% | 5% | 41% | 23% | 20% | |||
| Race/ethnicity | |||||||||
| African-American | 62% | 69% | 56% | 0.005 | 24% | 43% | 27% | 0.04 | |
| Hispanic | 28% | 21% | 31% | 7% | 8% | 8% | |||
| Caucasian/Other | 10% | 11% | 14% | 69% | 49% | 65% | |||
| Education > high school | 35% | 31% | 31% | 0.26 | 83% | 69% | 81% | 0.04 | |
| Annual household income | |||||||||
| <$12K (W) / < $10K (M) | 42% | 51% | 53% | 0.0001 | 16% | 33% | 20% | 0.003 | |
| $12−36K (W)/$10−40K (M) | 43% | 30% | 34% | 27% | 36% | 34% | |||
| ≥ $36K (W) / ≥ $40K(M) | 12% | 18% | 11% | 49% | 26% | 41% | |||
| Not reported | 3% | 1% | 2% | 8% | 5% | 5% | |||
| Current smokers | 50% | 50% | 43% | 0.01 | 27% | 46% | 30% | 0.01 | |
| Alcohol consumption | |||||||||
| None | 36% | 49% | 55% | <0.0001 | 12% | 20% | 20% | <0.0001 | |
| 1−2 drinks/week | 45% | 36% | 35% | 51% | 43% | 58% | |||
| >2 drinks/week | 19% | 14% | 10% | 37% | 38% | 22% | |||
| History of Injection drug use | 20% | 32% | 25% | 0.004 | 7% | 20% | 15% | 0.001 | |
| Family history of MI | 11% | 11% | 14% | 0.20 | 19% | 21% | 26% | 0.04 | |
| METABOLIC RISK FACTORS* | |||||||||
| Diabetes | 11% | 10% | 12% | 0.87 | 15% | 7% | 21% | 0.008 | |
| Used lipid-lowering medication | 1% | 5% | 6% | 0.0003 | 20% | 12% | 30% | 0.0008 | |
| Median BMI (IQR) | 29.3 | 28.2 | 26.6 | <0.0001 | 26.1 | 25.0 | 25.0 | <0.0001 | |
| (24.5−34.4) | (24.3−31.8) | (23.2−31.5) | (23.9−29.3) | (22.5−27.6) | (22.8−27.6) | ||||
| Median systolic BP (IQR) | 116 | 119 | 115 | 0.03 | 124 | 124 | 123 | 0.87 | |
| (108−126) | (109−130) | (107−127) | (117−132) | (117−131) | (116−132) | ||||
| Median LDL-C (IQR) | 97 | 98 | 98 | 0.98 | 116 | 107 | 105 | <0.0001 | |
| (75−120) | (78−120) | (75−123) | (96−142) | (79−123) | (83−128) | ||||
| Median HDL-C (IQR) | 53 | 42 | 46 | <0.0001 | 48 | 42 | 42 | <0.0001 | |
| (43−65) | (30−53) | (37−58) | (40−55) | (34−55) | (36−51) | ||||
| HIV CHARACTERISTICS† | |||||||||
| History of clinical AIDS | -- | 22% | 39% | <0.0001 | -- | 2% | 16% | 0.004 | |
| Median CD4+ T-cells/mm3 (IQR) | |||||||||
| Nadir | -- | 404 | 227 | <0.0001 | -- | 381 | 238 | <0.0001 | |
| (291−538) | (107−349) | (273−565) | (130−344) | ||||||
| Current | -- | 479 | 425 | 0.003 | -- | 484 | 510 | 0.75 | |
| (345−668) | (256−626) | (319−685) | (356−705) | ||||||
| HIV-1 RNA (copies/ml) | |||||||||
| <80 | -- | 20% | 48% | <0.0001 | -- | 12% | 70% | <0.0001 | |
| 80−1000 | -- | 23% | 14% | -- | 15% | 9% | |||
| 1000−10,000 | -- | 26% | 17% | -- | 22% | 9% | |||
| >10,000 | -- | 31% | 21% | -- | 51% | 12% | |||
| Antiretroviral therapy | |||||||||
| Current PI HAART | -- | -- | 46% | -- | -- | 43% | |||
| Current non-PI HAART | -- | -- | 33% | -- | -- | 42% | |||
| Former HAART users | -- | -- | 21% | -- | -- | 15% | |||
Metabolic risk factors observed on N=433 HIV- women, N=153 HAART-naïve HIV+ women, N=947 HAART-experienced HIV+ women, N=300 HIV- men, N=57 HAART-naïve HIV+ men, and N=458 HAART-experienced HIV+ men.
HIV characteristics observed on N=167 HAART-naïve women, N=1006 HAART-experienced women, N=59 HAART-naïve men, and N=482 HAART-experienced men.
MI, myocardial infarction; BMI, body mass index; BP, blood pressure; LDL-C, low density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol
Figure. Mean CIMT (A) and prevalence of carotid lesions (B), with 95% confidence intervals, by age, HIV status, and sex.
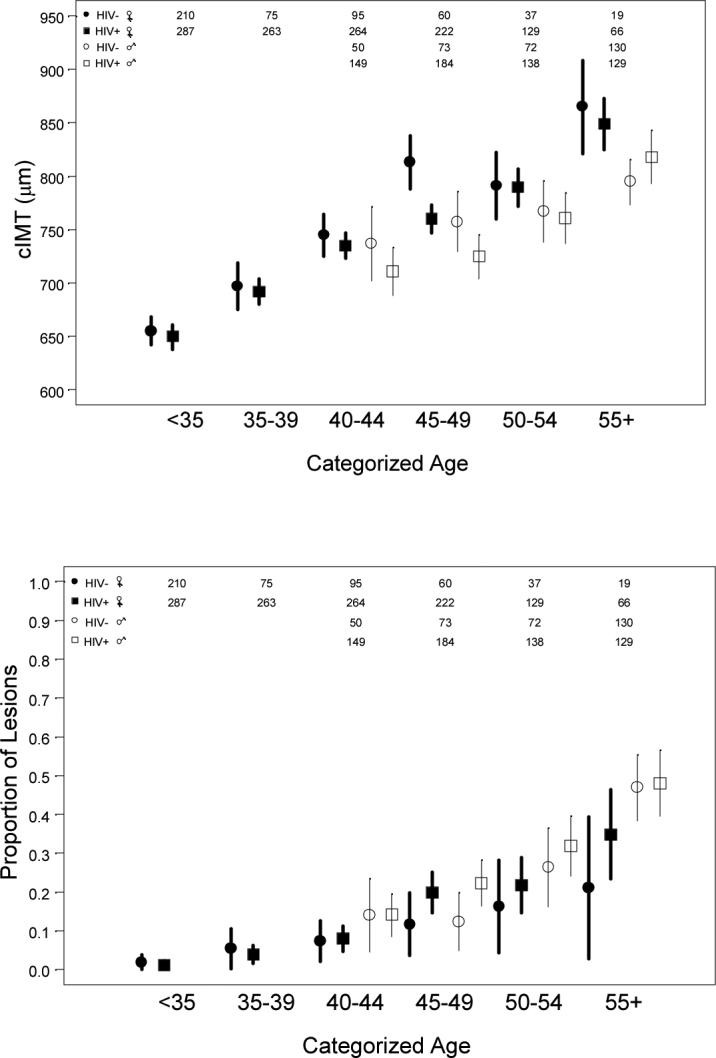
CIMT, carotid intima-media thickness
Sample sizes for each age/sex/HIV group shown at the top.
(A) Unadjusted mean CIMT was 722 μm in HIV-infected women, 716 μm in HIV-uninfected women, 750 μm in HIV infected men, and 771 μm in HIV-uninfected men. Y axis: CIMT in μm
(B) Carotid lesions defined as focal intima-media thickness >1.5 mm in any segment of the right common carotid artery, internal carotid artery, or carotid bulb. Unadjusted prevalence of lesions was 10% in HIV-infected women, 6% in HIV-uninfected women, 28% in HIV-infected men, and 30% in HIV-uninfected men. Y axis: prevalence of lesions
HIV-infected versus HIV-uninfected In multivariate analyses, HIV-infected women had significantly lower CIMT than HIV-uninfected women (adjusted mean difference in CIMT = −12 μm, 95% confidence interval −23, −2 μm, p = 0.02), after adjustment for age, race, education, income level, family history of MI, current smoking, alcohol consumption, opiate use, injection drug use, and study site (Table 2). This association was attenuated and not statistically significant after additional adjustment for metabolic variables including history of diabetes, lipid levels and hyperlipidemic therapy, BMI, and blood pressure. In men, CIMT did not differ significantly between HIV-infected and HIV-uninfected groups, although the point estimates suggested that as in women, HIV-infected men appeared to have lower CIMT. Carotid lesions were not significantly associated with HIV infection in either sex.
Table 2.
Comparison of mean CIMT and prevalence of carotid lesions among HIV-infected and -uninfected participants in women (Women's Interagency HIV Study) and men (Multicenter AIDS Cohort Study)
| |
|
|
Without Metabolic Risk Factor Adjustment(1) |
With Metabolic Risk Factor Adjustment(2) |
||
|---|---|---|---|---|---|---|
| Carotid intima-media thickness (C IMT) | ||||||
| N | Mean C IMT (μm) | Adjusted Mean Difference in C IMT (95% CI) | P-value | Adjusted Mean Difference in C IMT (95% CI) | P-value | |
| Women | ||||||
| HIV-uninfected | 496 | 716 | R E F | R E F | ||
| HIV-infected | 1,231 | 722 | −12 (−23, −2) | 0.02 | −7 (−18, 3) | 0.17 |
| Men | ||||||
| HIV-uninfected | 325 | 771 | R E F | R E F | ||
| HIV-infected | 600 | 750 | −11 (−30, 9) | 0.27 | −7 (−27, 12) | 0.47 |
| Carotid lesions | ||||||
|---|---|---|---|---|---|---|
| N | Prevalence of Lesions (%) | Adjusted Prevalence Ratio (95% CI) | P-value | Adjusted Prevalence Ratio (95% CI) | P-value | |
| Women | ||||||
| HIV-uninfected | 496 | 6% | R E F | R E F | ||
| HIV-infected | 1,231 | 10% | 1.24 (0.83, 1.85) | 0.29 | 1.13 (0.74, 1.72) | 0.57 |
| Men | ||||||
| HIV-uninfected | 325 | 30% | R E F | |||
| HIV-infected | 600 | 28% | 1.23 (0.94, 1.62) | 0.13 | 1.21 (0.91, 1.60) | 0.20 |
Adjusted for age, race, education, income level, family history of myocardial infarction, current smoking, alcohol consumption, opiate use, history of injection drug use, and study site
In addition to factors listed for (1), also adjusted for history of diabetes, use of cholesterol medications, BMI, systolic blood pressure, LDL and HDL cholesterol
CD4+ T-cell count, AIDS, and HIV viral load No association was observed between the most recent CD4+ T-cell count and mean CIMT (data not shown). Multivariate-adjusted analyses indicated a significant 70% to 100% increase in the prevalence of carotid lesions among HIV-infected individuals with CD4+ T-cell count below 200 cells/mm3, as compared to HIV-uninfected individuals (Table 3). Results were similar after additional adjustment for metabolic CVD risk factors (Table 3), and after excluding from the analysis HIV-infected individuals who had never been treated with antiretroviral therapies (data not shown). In additional analyses, which incorporated historical data on CD4+ T-cell count and AIDS, neither the lowest measured CD4+ T-cell count, nor prior AIDS defining illness, were associated with CIMT or lesions. The most recent HIV viral load measurement was not associated with CIMT or lesions.
Table 3.
Association between CD4+ T-cell count and prevalence of carotid lesions among participants in women (Women's Interagency HIV Study) and men (Multicenter AIDS Cohort Study)
| |
|
Without Metabolic Risk Factor Adjustment (1) |
With Metabolic Risk Factor Adjustment (2) |
||
|---|---|---|---|---|---|
| Women | |||||
| N | Adjusted Prevalence Ratio (95% CI) | P-value | Adjusted Prevalence Ratio (95% CI) | P-value | |
| HIV-uninfected | 496 | REF | REF | ||
| HIV-infected | |||||
| 500 < CD4 | 487 | 0.91 (0.56, 1.48) | 0.71 | 0.88 (0.53, 1.45) | 0.61 |
| 350 < CD4 < 500 | 269 | 1.21 (0.71, 2.05) | 0.49 | 1.17 (0.68, 2.01) | 0.57 |
| 200 < CD4 < 350 | 288 | 1.26 (0.76, 2.08) | 0.37 | 1.10 (0.64, 1.86) | 0.74 |
| CD4 < 200 | 187 | 2.00 (1.22, 3.28) | 0.006 | 1.70 (1.00, 2.89) | 0.05 |
| Men | |||||
|---|---|---|---|---|---|
| N | Adjusted Prevalence Ratio (95% CI) | P-value | Adjusted Prevalence Ratio (95% CI) | P-value | |
| HIV-uninfected | 325 | REF | REF | ||
| HIV-infected | |||||
| 500 < CD4 | 303 | 1.29 (0.95, 1.76) | 0.10 | 1.24 (0.90, 1.70) | 0.18 |
| 350 < CD4 < 500 | 147 | 1.03 (0.69, 1.54) | 0.88 | 1.01 (0.67, 1.52) | 0.97 |
| 200 < CD4 < 350 | 100 | 1.10 (0.69, 1.75) | 0.69 | 1.13 (0.70, 1.83) | 0.61 |
| CD4 < 200 | 50 | 1.74 (1.04, 2.93) | 0.04 | 1.93 (1.12, 3.32) | 0.02 |
Adjusted for age, race, education, income level, family history of myocardial infarction, current smoking, alcohol consumption, opiate use, history of injection drug use, and study site
In addition to factors listed for (1), also adjusted for history of diabetes, use of cholesterol medications, BMI, systolic blood pressure, LDL and HDL cholesterol
Duration of antiretroviral medication use Compared with women, men had longer median cumulative duration of antiretroviral drug exposure (including PIs, NNRTIs, and NRTIs) (Table 4). Among men, we observed an association between longer duration of PI exposure and increased PR for carotid lesions (adjusted PR per 2 years of PI use = 1.11, 95% confidence interval 1.00, 1.24, p = 0.05), although this association was not significant after further adjustment for metabolic risk factors (p = 0.08) (Table 4). In contrast to the results in men, no significant association between duration of PI use and carotid lesions was found in women. Further, NRTI and NNRTI use were not significantly associated with carotid lesions among women or men, and no associations were observed between duration of antiretroviral therapy and mean CIMT (data not shown).
Table 4.
Association between cumulative use of antiretroviral therapy and prevalence of carotid lesions among HIV-infected participants in women (Women's Interagency HIV Study) and men (Multicenter AIDS Cohort Study)
| |
|
Without Metabolic Risk Factor Adjustment (1) |
With Metabolic Risk Factor Adjustment (2) |
||
|---|---|---|---|---|---|
| Median Duration (Years) | Adjusted Prevalence Ratio per 2 years of drug use (95% CI) | P-value | Adjusted Prevalence Ratio per 2 years of drug use (95% CI) | P-value | |
| Protease inhibitor use | |||||
| Women | 1.50 | 1.03 (0.89, 1.19) | 0.71 | 1.03 (0.89, 1.20) | 0.66 |
| Men | 3.00 | 1.11 (1.00, 1.24) | 0.05 | 1.11 (0.99, 1.24) | 0.08 |
| Non-nucleoside reverse transcriptase inhibitor use | |||||
| Women | 1.00 | 1.08 (0.90, 1.28) | 0.42 | 1.11 (0.92, 1.33) | 0.27 |
| Men | 2.00 | 1.07 (0.94, 1.22) | 0.32 | 1.05 (0.92, 1.20) | 0.47 |
| Nucleoside reverse transcriptase inhibitor use | |||||
| Women | 4.00 | 1.05 (0.92, 1.20) | 0.44 | 1.05 (0.92, 1.20) | 0.48 |
| Men | 6.50 | 1.03 (0.95, 1.11) | 0.50 | 1.02 (0.94, 1.11) | 0.62 |
Adjusted for age, race, education, income level, family history of myocardial infarction, current smoking, alcohol consumption, opiate use, history of injection drug use, current, nadir, and first C D4+ T-cell counts and study site
In addition to factors listed for (1), also adjusted for history of diabetes, use of cholesterol medications, BMI, systolic blood pressure, LDL and HDL cholesterol
Separate models were conducted for PI use, NNRTI use, and NRTI use
CIMT and carotid lesions in relation to traditional CVD risk factors Analyses conducted among all study participants showed significant associations between subclinical carotid atherosclerosis and age, African-American race/ethnicity, smoking, diabetes, BMI, blood pressure, and lipids (Table 5). The presence of carotid lesions was associated with increased CIMT among both HIV-infected women (adjusted Δ CIMT = 34 μm, 95% confidence interval 16, 56, p<0.001) and HIV-infected men (adjusted Δ CIMT = 46 μm, 95% confidence interval 22, 73, p<0.001).
Table 5.
Relationship between standard cardiovascular risk factors, CIMT, and carotid lesions
| Adjusted mean difference in CIMT | Adjusted prevalence ratio (PR) for carotid lesions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Women |
Men |
Women |
Men |
||||||||
| ΔCIMT | 95% CI | P-value | ΔCIMT | 95% CI | P-value | PR | 95% CI | P-value | PR | 95% CI | P-value | |
| Age, years | ||||||||||||
| <35 | −73 | (−86, −59) | <0.01 | N/A | N/A | N/A | 0.23 | (0.10, 0.54) | <0.01 | N/A | N/A | N/A |
| 35−39 | −40 | (−53, −26) | <0.01 | N/A | N/A | N/A | 0.63 | (0.33, 1.20) | 0.16 | N/A | N/A | N/A |
| 40−44 | Ref | Ref | Ref | Ref | ||||||||
| 45−49 | 29 | (15, 44) | <0.01 | 24 | (−1, 49) | 0.06 | 1.85 | (1.15, 2.99) | 0.01 | 1.38 | (0.86, 2.21) | 0.18 |
| 50−54 | 45 | (27, 62) | <0.01 | 60 | (32, 87) | <0.01 | 1.97 | (1.16, 3.33) | 0.01 | 2.13 | (1.34, 3.41) | <0.01 |
| ≥55 | 105 | (83, 128) | <0.01 | 91 | (64, 118) | <0.01 | 3.22 | (1.82, 5.68) | <0.01 | 3.54 | (2.26, 5.54) | <0.01 |
| Race/Ethnicity (vs. African-American) | ||||||||||||
| Hispanic | −29 | (−40, −18) | <0.01 | −28 | (−65, 10) | 0.15 | 0.87 | (0.56, 1.33) | 0.52 | 0.73 | (0.35, 1.53) | 0.40 |
| Caucasian | −17 | (−32, −3) | 0.02 | −54 | (−78, −29) | <0.01 | 1.51 | (0.97, 2.36) | 0.07 | 1.19 | (0.83, 1.71) | 0.35 |
| Current smoker | 23 | (13, 32) | <0.01 | −21 | (−43, 1) | 0.06 | 1.51 | (1.05, 2.18) | 0.03 | 1.59 | (1.18, 2.15) | <0.01 |
| Alcohol use (vs. none) | ||||||||||||
| 1−2 drinks/week | −5 | (−15, 5) | 0.35 | 23 | (−2, 48) | 0.08 | 0.99 | (0.69, 1.43) | 0.97 | 1.12 | (0.77, 1.64) | 0.55 |
| >2 drinks/week | −6 | (−21, 8) | 0.40 | 24 | (−5, 54) | 0.10 | 0.94 | (0.57, 1.55) | 0.81 | 1.12 | (0.73, 1.71) | 0.61 |
| Diabetes | 22 | (8, 37) | <0.01 | 1 | (−22, 25) | 0.90 | 1.40 | (0.93, 2.11) | 0.11 | 0.90 | (0.64, 1.26) | 0.54 |
| Used lipid-lowering medication | 4 | (−17, 25) | 0.71 | 22 | (−3, 48) | 0.10 | 0.60 | (0.30, 1.21) | 0.15 | 1.37 | (1.02, 1.83) | 0.04 |
| BMI (per 5 kg/m2) | 4 | (1, 8) | 0.01 | 27 | (16, 38) | <0.01 | 0.81 | (0.70, 0.93) | <0.01 | 0.98 | (0.85, 1.14) | 0.83 |
| SBP (per 10 mm Hg) | 11 | (8, 14) | <0.01 | 10 | (3, 17) | 0.01 | 1.10 | (1.02, 1.18) | 0.02 | 1.06 | (0.97, 1.17) | 0.20 |
| LDL-C (per 20 mg/dL) | 3 | (1, 6) | 0.02 | 6 | (1, 11) | 0.03 | 1.09 | (1.00, 1.19) | 0.05 | 1.07 | (1.00, 1.15) | 0.04 |
| HDL-C (per 5 mg/dL) | 0 | (−2, 1) | 0.55 | −5 | (−8, −1) | 0.01 | 0.97 | (0.92, 1.01) | 0.17 | 0.98 | (0.93, 1.03) | 0.40 |
All models adjusted for HIV infection, education, income level, gamily history of myocardial infarction, opiate use, injection drug use, and study site in addition to all factors shown.
Discussion
This study did not confirm the presence of increased mean CIMT among HIV-infected as compared with HIV-uninfected individuals, as has been previously reported [5, 6]. However, we did find that the frequency of carotid lesions (IMT > 1.5mm) was significantly increased among HIV-infected men and women with evidence of more advanced HIV disease. Compared with HIV-uninfected persons, the adjusted prevalence of carotid lesions was 70% to 100% higher in HIV-infected persons who had a recent CD4+ T-cell count measurement below 200 cells/mm3. These findings are consistent with prior evidence that low CD4+ T-cell count may increase risk of CVD in HIV-infected populations [5, 30]. Prior studies have also suggested increased risks of incident CVD events with each additional year of combination antiretroviral therapy, particularly for regimens that include PIs [1, 2]. Our data, based upon a surrogate marker for CVD, provide little support for the conclusion that increased atherosclerotic vascular disease is associated with longer duration of PI therapy. There was no evidence of such an association among women, and among men a possible association between PI use and carotid lesions was weak and not significant in multivariate models. This study among two large population-based HIV cohorts has several strengths, including extensive data on an array of potential confounders and metabolic risk mediators, and the inclusion of HIV-uninfected comparison groups who were recruited using comparable methods and from similar venues as the HIV-infected individuals and who also had HIV risk behaviors (eg, injection drug use, receipt of blood products, high-risk sexual practices).
Our finding that low CD4+ T-cell count is a vascular risk factor among HIV-infected individuals is supported by previous observational data [5] and by secondary analyses from the FIRST/ CPCRA 058 clinical trial [30]. In the Strategies for Management of Antiretroviral Therapy (SMART) trial, HIV-infected individuals randomized to episodic antiretroviral therapy (eg, “drug conservation” arm) guided by the CD4+ T-cell count had reduced CD4+ T-cell count and increased risk of major CVD events as compared with those randomized to continuous therapy [31]. The main results from SMART appear to be consistent with our finding that immunosuppression was associated with increased atherosclerosis, although subsequent analyses from SMART found that high (not low) CD4+ T-cell count was a predictor of increased CVD risk in the drug-conservation arm [32]. The association between low CD4+ T-cell count may plausibly be explained by chronic inflammation [23] or specific pathogen exposures [33] in immunosuppressed HIV-infected individuals.
Prior data have suggested that use of antiretroviral therapy may increase CVD risk among HIV-infected adults [1, 2], although this is not entirely consistent with other studies, some of which were based on subclinical rather than clinical CVD [8-10]. The Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group [1], a large collaborative study of HIV-infected cohorts, reported an adjusted 16% increase in the relative hazard of incident MI with each additional year of PI-based combination antiretroviral treatment. The present study did not find consistent evidence for an association between antiretroviral therapies and atherosclerosis. There was no association whatsoever between use of PIs or other antiretroviral therapies and atherosclerosis in women. Among men, a possible association between longer duration of PI-based therapy and increased risk of carotid artery lesions was observed, but this finding was non-significant in adjusted models. The possible reasons why the association between antiretroviral therapies and atherosclerosis would differ by sex are unclear. This may reflect random subgroup differences; poorer drug adherence or less aggressive antiretroviral therapy among women as compared with men; the younger age of women as compared with men in the study; or lower susceptibility to atherosclerosis among women than among men. On the other hand, the lack of clear and consistent findings in this study is evidence against the hypothesis that antiretroviral medications are an important cause of vascular disease.
While we found evidence that men and women with low CD4+ T-cell count have increased occurrence of atherosclerotic lesions, the present study also suggested that overall carotid wall thickness (i.e., mean CIMT) was significantly lower among HIV-infected women than among HIV-uninfected women (with a similar trend in men). A possible explanation may be that HIV infection is associated with favorable levels of some vascular risk factors including total cholesterol, LDL-C, blood pressure, and BMI [14, 19, 34, 35]. Consistent with this proposed mechanism, we found that the association between HIV infection and reduced CIMT was attenuated through adjustment for metabolic variables. We note that our observation of both more frequent carotid lesions, and lower mean CIMT, in the HIV-infected population is strikingly similar to the findings from carotid ultrasound studies of other inflammatory and immune-related conditions. For example, women with systemic lupus erythematosus [36] and rheumatoid arthritis [37] have increased prevalence of carotid lesions, but reduced mean CIMT, compared with healthy controls. Histologic studies of carotid plaques in HIV-infected individuals show extensive inflammatory infiltration of the vascular wall, more similar to arteritis than to classical atheroma [38], which is further evidence of an atypical vascular disease phenotype in the HIV-infected population.
The cross-sectional nature of the present study is an important limitation, because use of antiretroviral medications, CD4+ T-cell cell counts, HIV viral loads, and CVD risk mediators such as lipid levels are dynamic variables that change over time. Although vascular measurements were only performed at a single timepoint, we did make use of extensive longitudinal data on medication use and other HIV-related variables collected as part of the WIHS and MACS core protocols. This is an observational study, and it is possible that associations of HIV infection, medications, and other HIV-related variables with subclinical carotid atherosclerosis may not accurately represent causal relationships. Because this study was conducted after the initial reports liking specific antiretroviral therapies with metabolic disorders and CVD, channeling bias may have affected our results. This would have lead to an underestimation of the risks associated with these therapies, if they tended to be avoided in patients known to be at high vascular risk. As noted, the HIV-uninfected comparison groups had similar characteristics and were recruited using similar methods as the HIV-infected groups, albeit it is possible that relevant behaviors, comorbidities, social factors, health care access, and other health-related practices may not have been balanced between the groups. For technical reasons relating to a concurrent study of coronary calcium [26], men younger than 40 years were excluded from the study, which is a limitation as this group represents a large fraction of the HIV-infected population. Likewise, our study excluded individuals with preexisting CVD, who may be susceptible to adverse effects of HIV infection or therapies. Finally, this was a study of subclinical atherosclerosis measures rather than hard CVD events. While the carotid ultrasound protocol is a well-validated measure of atherosclerosis and predictor of clinical cardiovascular events, other mechanisms such as increased coagulation or impaired endothelial function may also lead to vascular events in HIV-infected populations [39].
In conclusion, we found that HIV-infected women and men with a low CD4+ T-cell count had a significantly increased prevalence of subclinical carotid artery lesions. Use of antiretroviral therapies had no consistent association with atherosclerosis in this study. These findings emphasize that the association of antiretroviral therapy use and other HIV-related variables with atherosclerosis is in need of further study.
Acknowledgments and funding
Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange). MACS centers (Principal Investigators) at The Johns Hopkins University Bloomberg School of Public Health (Joseph B. Margolick, Lisa Jacobson), Howard Brown Health Center and Northwestern University Medical School (John Phair), University of California, Los Angeles (Roger Detels), and University of Pittsburgh (Charles Rinaldo). The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the National Institute of Child Health and Human Development (UO1-HD-32632). The study is co- funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (MO1-RR-00071, MO1-RR-00079, MO1-RR-00083). The MACS is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute and the National Heart, Lung and Blood Institute. UO1-AI-35042, 5-MO1-RR-00722 (GCRC), UO1-AI-35043, UO1-AI-37984, UO1-AI-35039, UO1-AI-35040, UO1-AI-37613, UO1-AI-35041. Also supported by NIH/NHLBI grant 1R01HL083760-01 (to R.C.K.) and grant M01RR-023942-01 from the National Center for Research Resources (NIH/NCRR).
References
- 1.Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte A, El-Sadr W, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 2.Friis-Moller N, Sabin CA, Weber R, d'Arminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 3.Holmberg SD, Moorman AC, Williamson JM, Tong TC, Ward DJ, Wood KC, et al. Protease inhibitors and cardiovascular outcomes in patients with HIV-1. Lancet. 2002;360:1747–1748. doi: 10.1016/S0140-6736(02)11672-2. [DOI] [PubMed] [Google Scholar]
- 4.Mary-Krause M, Cotte L, Simon A, Partisani M, Costagliola D. Increased risk of myocardial infarction with duration of protease inhibitor therapy in HIV-infected men. Aids. 2003;17:2479–2486. doi: 10.1097/00002030-200311210-00010. [DOI] [PubMed] [Google Scholar]
- 5.Hsue PY, Lo JC, Franklin A, Bolger AF, Martin JN, Deeks SG, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109:1603–1608. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 6.Johnsen S, Dolan SE, Fitch KV, Kanter JR, Hemphill LC, Connelly JM, et al. Carotid intimal medial thickness in human immunodeficiency virus-infected women: effects of protease inhibitor use, cardiac risk factors, and the metabolic syndrome. J Clin Endocrinol Metab. 2006;91:4916–4924. doi: 10.1210/jc.2006-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jerico C, Knobel H, Calvo N, Sorli ML, Guelar A, Gimeno-Bayon JL, et al. Subclinical carotid atherosclerosis in HIV-infected patients: role of combination antiretroviral therapy. Stroke. 2006;37:812–817. doi: 10.1161/01.STR.0000204037.26797.7f. [DOI] [PubMed] [Google Scholar]
- 8.Currier JS, Kendall MA, Zackin R, Henry WK, Alston-Smith B, Torriani FJ, et al. Carotid artery intima-media thickness and HIV infection: traditional risk factors overshadow impact of protease inhibitor exposure. Aids. 2005;19:927–933. doi: 10.1097/01.aids.0000171406.53737.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bozzette SA, Ake CF, Tam HK, Chang SW, Louis TA. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med. 2003;348:702–710. doi: 10.1056/NEJMoa022048. [DOI] [PubMed] [Google Scholar]
- 10.Mangili A, Gerrior J, Tang AM, O'Leary DH, Polak JK, Schaefer EJ, et al. Risk of cardiovascular disease in a cohort of HIV-infected adults: a study using carotid intima-media thickness and coronary artery calcium score. Clin Infect Dis. 2006;43:1482–1489. doi: 10.1086/509575. [DOI] [PubMed] [Google Scholar]
- 11.Currier JS, Kendall MA, Henry WK, Alston-Smith B, Torriani FJ, Tebas P, et al. Progression of carotid artery intima-media thickening in HIV-infected and uninfected adults. Aids. 2007;21:1137–1145. doi: 10.1097/QAD.0b013e32811ebf79. [DOI] [PubMed] [Google Scholar]
- 12.Grunfeld C, Kotler DP, Hamadeh R, Tierney A, Wang J, Pierson RN. Hypertriglyceridemia in the acquired immunodeficiency syndrome. Am J Med. 1989;86:27–31. doi: 10.1016/0002-9343(89)90225-8. [DOI] [PubMed] [Google Scholar]
- 13.Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992;74:1045–1052. doi: 10.1210/jcem.74.5.1373735. [DOI] [PubMed] [Google Scholar]
- 14.Riddler SA, Smit E, Cole SR, Li R, Chmiel JS, Dobs A, et al. Impact of HIV infection and HAART on serum lipids in men. Jama. 2003;289:2978–2982. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 15.Lee GA, Seneviratne T, Noor MA, Lo JC, Schwarz JM, Aweeka FT, et al. The metabolic effects of lopinavir/ritonavir in HIV-negative men. Aids. 2004;18:641–649. doi: 10.1097/00002030-200403050-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noor MA, Seneviratne T, Aweeka FT, Lo JC, Schwarz JM, Mulligan K, et al. Indinavir acutely inhibits insulin-stimulated glucose disposal in humans: a randomized, placebo-controlled study. Aids. 2002;16:F1–8. doi: 10.1097/00002030-200203290-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saag MS, Powderly WG, Schambelan M, Benson CA, Carr A, Currier JS, et al. Switching Antiretroviral Drugs for Treatment of Metabolic Complications in HIV-1 Infection: Summary of Selected Trials. Top HIV Med. 2002;10:47–51. [Google Scholar]
- 18.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165:1179–1184. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 19.Seaberg EC, Munoz A, Lu M, Detels R, Margolick JB, Riddler SA, et al. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. Aids. 2005;19:953–960. doi: 10.1097/01.aids.0000171410.76607.f8. [DOI] [PubMed] [Google Scholar]
- 20.Tien PC, Schneider MF, Cole SR, Levine AM, Cohen M, DeHovitz J, et al. Antiretroviral therapy exposure and incidence of diabetes mellitus in the Women's Interagency HIV Study. Aids. 2007;21:1739–1745. doi: 10.1097/QAD.0b013e32827038d0. [DOI] [PubMed] [Google Scholar]
- 21.Brown TT, Li X, Cole SR, Kingsley LA, Palella FJ, Riddler SA, et al. Cumulative exposure to nucleoside analogue reverse transcriptase inhibitors is associated with insulin resistance markers in the Multicenter AIDS Cohort Study. Aids. 2005;19:1375–1383. doi: 10.1097/01.aids.0000181011.62385.91. [DOI] [PubMed] [Google Scholar]
- 22.Tien PC, Schneider MF, Cole SR, Justman JE, French AL, Young M, et al. Relation of Stavudine Discontinuation to Anthropometric Changes Among HIV-Infected Women. J Acquir Immune Defic Syndr. 2007;44:43–48. doi: 10.1097/01.qai.0000248353.56125.43. [DOI] [PubMed] [Google Scholar]
- 23.Lau B, Sharrett AR, Kingsley LA, Post W, Palella FJ, Visscher B, et al. C-reactive protein is a marker for human immunodeficiency virus disease progression. Arch Intern Med. 2006;166:64–70. doi: 10.1001/archinte.166.1.64. [DOI] [PubMed] [Google Scholar]
- 24.Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, et al. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12:1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 26.Kingsley L, Cuervo J, Munoz A, Palella F, Budoff M, Post W, et al. Subclinical coronary atherosclerosis, HIV-infection and antiretroviral therapy: results from the Multicenter AIDS Cohort Study. Abstracts of the International AIDS Society. 2007 [Google Scholar]
- 27.Hodis HN, Mack WJ, Lobo RA, Shoupe D, Sevanian A, Mahrer PR, et al. Estrogen in the prevention of atherosclerosis. A randomized, double- blind, placebo-controlled trial. Ann Intern Med. 2001;135:939–953. doi: 10.7326/0003-4819-135-11-200112040-00005. [DOI] [PubMed] [Google Scholar]
- 28.Selzer RH, Mack WJ, Lee PL, Kwong-Fu H, Hodis HN. Improved common carotid elasticity and intima-media thickness measurements from computer analysis of sequential ultrasound frames. Atherosclerosis. 2001;154:185–193. doi: 10.1016/s0021-9150(00)00461-5. [DOI] [PubMed] [Google Scholar]
- 29.Schafer JL. Analysis of Incomplete Multivariate Data. Chapman and Hall; New York: 1997. [Google Scholar]
- 30.Baker J, Peng G, Rapkin J, Abrams D, Silverberg M, Cavert W, et al. HIV-related Immune Suppression after ART Predicts Risk of Non-opportunistic Diseases: Results from the FIRST Study.. Conference on Retroviruses and Opportunistic Infections, Boston, 2007; 2007; [22 May 2007]. http://www.retroconference.org/2007/Abstracts/28667.htm. [Google Scholar]
- 31.El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 32.Phillips A, Carr A, Neuhaus J, Visnegarwala F, Prineas R, Burman W, et al. Interruption of ART and Risk of Cardiovascular Disease: Findings from SMART.. Abstracts of the 14th Conference on Retroviruses and Opportunistic Infections; 2007. [Google Scholar]
- 33.Hsue PY, Hunt PW, Sinclair E, Bredt B, Franklin A, Killian M, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. Aids. 2006;20:2275–2283. doi: 10.1097/QAD.0b013e3280108704. [DOI] [PubMed] [Google Scholar]
- 34.Bacchetti P, Gripshover B, Grunfeld C, Heymsfield S, McCreath H, Osmond D, et al. Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr. 2005;40:121–131. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mulligan K, Anastos K, Justman J, Freeman R, Wichienkuer P, Robison E, et al. Fat distribution in HIV-infected women in the United States: DEXA substudy in the Women's Interagency HIV Study. J Acquir Immune Defic Syndr. 2005;38:18–22. doi: 10.1097/00126334-200501010-00004. [DOI] [PubMed] [Google Scholar]
- 36.Roman MJ, Shanker BA, Davis A, Lockshin MD, Sammaritano L, Simantov R, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2399–2406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- 37.Roman MJ, Moeller E, Davis A, Paget SA, Crow MK, Lockshin MD, et al. Preclinical carotid atherosclerosis in patients with rheumatoid arthritis. Ann Intern Med. 2006;144:249–256. doi: 10.7326/0003-4819-144-4-200602210-00006. [DOI] [PubMed] [Google Scholar]
- 38.Maggi P, Maserati R, Antonelli G. Atherosclerosis in HIV patients: a new face for an old disease? AIDS Rev. 2006;8:204–209. [PubMed] [Google Scholar]
- 39.Psaty BM, Weiss NS, Furberg CD, Koepsell TD, Siscovick DS, Rosendaal FR, et al. Surrogate End Points, Health Outcomes, and the Drug-Approval Process for the Treatment of Risk Factors for Cardiovascular Disease. JAMA. 1999;282:786–790. doi: 10.1001/jama.282.8.786. [DOI] [PubMed] [Google Scholar]


