Abstract
Maternal smoking doubles the risk of delivering a low birth weight infant. The purpose of this study was to analyze differential gene expression in umbilical cord tissue as a function of maternal smoking, with an emphasis on growth-related genes. We recruited 15 pregnant smokers and 15 women who never smoked during pregnancy to participate. RNA was isolated from umbilical cord tissue collected and snap frozen at the time of delivery. Microarray analysis was performed using the Affymetrix GeneChip Scanner 3000. Six hundred seventy-eight probes corresponding to 545 genes were differentially expressed (i.e., had an intensity ratio > +/−1.3 and a corrected significance value p < 0.005) in tissue obtained from smokers versus nonsmokers. Genes important for fetal growth, angiogenesis, or development of connective tissue matrix were up regulated among smokers. The most highly up-regulated gene was CSH1, a somatomammotropin gene. Two other somatomammotropin genes (CSH2 and CSH-L1) were also up regulated. The most highly down-regulated gene was APOBEC3A; other down-regulated genes included those that may be important in immune and barrier protection. Validation of the three somatomammotropin genes showed a high correlation between qPCR and microarray expression. We conclude that maternal smoking may be associated with altered gene expression in the offspring.
Keywords: prenatal tobacco exposure, pregnancy, microarray, genetics
Although a number of adverse perinatal, neonatal, and childhood health outcomes have been attributed to prenatal tobacco smoke exposure, the most consistent and measurable association is between prenatal tobacco exposure and low birth weight (1). Since low-birth-weight infants have an exponential increase in mortality rate compared to infants of normal birth weight (2), the public health significance of smoking during pregnancy is substantial. Moreover, low birth weight increases the risk for cardiovascular disease, metabolic syndrome, and type 2 diabetes among adults (3). The exact relationship between low birth weight and the onset of disease in adulthood is unknown. However, there has been growing evidence that environmental factors that cause fetal growth restriction may cause “fetal reprogramming,” and increase the risk of disease in adulthood (3).
The aim of the present study was to examine the effects of prenatal tobacco exposure on mRNA expression in umbilical cord tissue. Tobacco smoke contains more than 4,000 chemicals, including carcinogens and mutagens, which either alone or in combination may influence gene expression (4). Since the umbilical cord is exclusively fetal tissue, it may facilitate an examination of the effects of prenatal tobacco exposure on the fetal vascular system (5). Based on the magnitude of up-regulation or down-regulation of messenger RNA expression, we sought to identify the most differentially regulated genes in fetal tissue in relation to maternal smoking. This information may help to understand how smoking causes poor infant outcomes (including low birth weight) and increases the risk of childhood and adult diseases.
MATERIALS AND METHODS
The institutional review board at the University of Connecticut Health Center (UCHC) approved the study. Participants provided written consent before study procedures. Subjects were recruited from the labor and delivery unit at UCHC. Inclusion criteria included gestational age ≥32 weeks and no active maternal infection. We administered a medical history (which elicited information on smoking, other drug use and medication use) before delivery. Umbilical cord (UC) tissue was collected shortly after delivery. Infant outcomes were obtained from chart review.
Laboratory Methods
Tissue collection and RNA isolation
UC tissue midway between infant and placenta was collected within 10 minutes postpartum, cut into 1.5-cm lengths, flash frozen by immersion in liquid nitrogen and stored at −80°C. A random sample of UC tissue was selected and RNA was isolated using a modified guanidinium isothiocyanate/CsCl procedure (6). The frozen tissue was ground to a fine powder using a mortar and pestle cooled with liquid nitrogen, and (~300 mg) transferred into a tube containing 1 ml of GTC lysis buffer (4M guanidinium isothiocyanate in 50 mM sodium citrate, TrisHCl or HEPES, 0.1 % sodium sarcosyl). Samples were then sheared using a polytron tissue disrupter to reduce the molecular weights of extracellular matrix material and genomic DNA.
For estimation of blood contamination, an aliquot was analyzed spectrophotometrically at a wavelength of 418 nm. The homogenate was then layered on top of a 1.5 ml cushion of CsCl buffer (5.7 M CsCl/10 mM sodium citrate pH7.0 and 1 mM EDTA), and centrifuged at 45,000 rpm, at 22°C, in a SW50 rotor for 23 hours. RNA sediment was dissolved in 100 µl H2O (RNAsefree), concentrated by overnight precipitation at −20°C in the presence of 0.3M NaOAc and 75% ethanol (final concentration) and sedimented for 10 min at 15,000 rpm, at 4°C, with the air dried pellets dissolved at a concentration of ~1mg/ml. RNA was quantified by spectrophotometry at 260 nm, and purity examined by 260/280 ratio. RNA quality was further examined by electrophoresis on a 1.1% agarose/2.2M formaldehyde gel.
Microarray hybridization
For each sample, 1.5 µg of RNA was converted into biotinylated cRNA and hybridized to arrays following protocols supplied by the array manufacturer (Affymetrix, Santa Clara, CA). The RNA was used for first and second strand cDNA synthesis and the double-stranded cDNA was transcribed in the presence of biotinylated ribonucleotides to generate biotinylated cRNA, which was then purified by ion exchange column chromatography. The biotinylated cRNA was fragmented, and hybridized to human U133 Version 2.0 Plus GeneChips (Affymetrix, Santa Clara, CA) without technical replication. The chip includes 54,674 probes covering all currently identified transcripts (over 47,000 transcripts from ~39,000 genes). After 16 hours of hybridization at 45°C, the arrays were stained in an Affymetrix Fluidics Station using a two-stage signal amplification protocol based on detection of the biotinylated targets by streptavidin-phycoeritherin (SAPE) according to Affymetrix instructions. The signal was quantified by detection of bound phycoerythericin using an Affymetrix GeneChip Scanner 3000.
qPCR Methodology
Total RNA (100ng) was reverse transcribed using a commercial kit (High Capacity cDNA Archive, Applied Biosystems Inc. ABI, Foster City, CA) in parallel with calibrator total RNA from pooled human tissues (Universal Reference Total RNA PN636690, Clontech Laboratories, Mountain View, CA). Triplicate TaqMan real-time PCR reactions were prepared with cDNA from 2 ng of RNA and amplified in 384-well plates using an ABI 7900HT Sequence Detection System. Average threshold cycle number (Ct) for each sample was converted to the equivalent amount of the calibrator RNA by use of standard curves on each plate of cDNA prepared from pooled human tissue reference RNA. Relative amounts of target RNAs were normalized to the amount of two ubiquitously expressed genes [encoding beta-2 microglobulin (B2M), and glyceraldehyde phosphate dehydrogenase (GAPDH)]. Pre-made and validated TaqMan qPCR probe and primers for each target or reference gene were obtained from Applied Biosystems [Hs01862611_g1 chorionic somatomammotropin hormone 1 (placental lactogen, CSH1); Hs00831897_s1 chorionic somatomammotropin hormone 2 (CSH2); Hs00741469_g1 chorionic somatomammotropin hormone-like 1 (CSHL1); 4326319E-0411004 B2M; 4326317E-0411007 GAPDH]. Controls in which the reverse transcriptase was omitted from the RT reaction produced no amplification during the qPCR TaqMan reaction.
Data Analysis
Microarray analyses
Microarray analysis was conducted using the R/MAANOVA open source software (Version 1.4) as part of the Bioconductor and R language open source software library (version 2.4.0) (8). Robust Multiarray Averaging (RMA) is an analysis option within the R/MAANOVA package and a commonly applied technique for normalization of Affymetrix arrays. Statistical analysis was performed on RMA-normalized data using R/MAANOVA(7) with a fixed effect permutation ANOVA model consisting of the independent variable, smoking status (smoker versus non-smoker), and the covariables gender of the offspring and hemoglobin content of the UC lysates (which controlled the analysis for the degree of UC contamination by blood). Using the magnitude of absorption at 418 nm for each sample as an indicator of its hemoglobin (and by extension hemoglobin mRNA) content, we trichotomized the samples into those with low absorption (OD418 < 1.5), intermediate absorption (OD418 < 2.5), and high blood content (OD418 ≥ 2.5).
To identify differentially regulated genes, we performed the F-tests F1, F2, F3 and Fs that are implemented in the R/MAANOVA software and assessed gene-centric (F1-test) and array centric (F3 test) variance through comparison of the respective lists of regulated genes with those provided by the F2 and Fs-tests, which interpolate between gene centric and array centric variance. R/MAANOVA uses the Benjamini Hochberg test to perform the false discovery rate, which was set to a p value of < 0.005, and pooled across all gene lists to create an inclusive master list. We selected the subset of highly significantly differentially regulated genes that demonstrated a change in their expression level of at least 1.3 fold.
The differentially regulated genes were organized into clusters using the hierarchical clustering module of D-Chip software (revised 2006) (8) by both genes and samples with the clustering parameter set to (a) Euclidian distance and (b) a p-value of 0.05. Differentially expressed genes were annotated using all publicly available databases including the ENTREZ, GO and IHOP databases.
Analysis of clinical variables
Analyses were conducted using SPSS software, version 15 (SPSS, Inc., Chicago, Illinois) and StatXact, version 4 (Cytel Software Corp., Cambridge, Massachusetts). Summary statistics (means, standard deviations, and percentages) were calculated to describe the characteristics of subjects in the study samples. For continuous variables, mean values were compared using the two-sample t-test. For categorical variables, distributions of frequencies between samples were compared using the chi-squared test or the Fisher exact test.
Validation of Microarray Results Using Quantitative PCR
For the CSH1, CSH2, and CSHL1 genes, relationships between gene expression values as measured by qPCR and by microarray analysis were evaluated with correlation coefficients and their corresponding p-values. In these analyses, gene expression values were normalized using expression levels of the B2M gene. The Spearman rank correlation coefficient was applied to account for skewness. Microarray expression values of different probes related to the same gene were averaged (after logarithmic transformation) to determine a single expression value before calculating a correlation with the qPCR value for the same gene.
RESULTS
Demographics of the subject population are shown in Table 1. Smokers were younger and they delivered infants at an earlier gestational age and at a lower birth weight than non-smokers. Smokers were also more likely to report other drug use during pregnancy. Additionally, the proportion of male offspring born to nonsmoking mothers was higher. Other characteristics were similar between groups.
Table 1.
Characteristics of the study population (count, mean ± s.d., or percent, as indicated)
| Non-Smoking Mothers | Smoking Mothers | P-Value | |
|---|---|---|---|
| Number of Subjects | 15 | 15 | |
| Maternal Age (yrs) | 29.8 ± 5.5 | 26.2 ± 6.5 | 0.12 |
| Caucasian/White Race | 46.7% | 60.0% | 0.62 |
| Hispanic Ethnicity | 26.7% | 20.0% | 0.68 |
| Gravidity/Total Pregnancies | 0.26 | ||
| 1 | 26.7% | 20.0% | |
| 2 | 53.3 | 20.0 | |
| 3 | 6.7 | 20.0 | |
| ≥ 4 | 13.3 | 33.3 | |
| Unknown | 0.0 | 6.7 | |
| Parity/Prior Births | 0.06 | ||
| 0 | 33.3% | 40.0% | |
| 1 | 60.0 | 20.0 | |
| ≥ 2 | 6.7 | 33.3 | |
| Unknown | 0.0 | 6.7 | |
| Maternal Body mass index | 30.1 ± 5.4 | 32.4 ± 6.8 | 0.33 |
| Evidence of Alcohol or Drugs at Delivery | 0.0% | 33.3% | 0.04 |
| Infant Sex | 0.07 | ||
| Male | 60.0% | 26.7% | |
| Female | 40.0 | 73.3 | |
| Gestational Age (wks) | 38.9 ± 1.5 | 36.8 ± 2.5 | 0.01 |
| Infant Weight (g) | 3319 ± 425 | 2610 ± 911 | 0.01 |
| Infant Length (in) | 19.9 ± 1.2 | 18.0 ± 1.8 | 0.004 |
| Cigarettes/day | |||
| Before pregnancy | 20.6 ± 10.2 | ||
| In week before study entry | 6.9 ± 8.7 | ||
| During 1st trimester | 14.5 ± 8.6 | ||
| During 3rd trimester | 10.5 ± 7.8 |
Six hundred seventy-eight probes corresponding to 545 genes were differentially expressed (i.e., an intensity ratio that exceeded +/−1.3 and a corrected significance value p < 0.005) in tissue obtained from smokers versus nonsmokers. Of the 545 genes, 371 were up regulated and 174 were down regulated in smokers. Of the 545 differentially regulated genes, 458 genes are known. Only 87 probe identification numbers were not annotated as determined by the absence of a gene symbol.
The 25 genes with the largest increase or the largest decrease in mRNA abundance in the umbilical cord tissue of smokers compared to that of controls are shown in Table 2 and Table 3. If more than one probe for the same gene was differentially expressed the mean value of the various probes is reported.
Table 2.
Up-regulated genes in smokers (Limited to 25)
| Entrez ID | Symbol | mean Ratio | P_IDs | Gene Name | Gene Function | (Potential role in Development |
|---|---|---|---|---|---|---|
| 1442 | CSH1 | 2.48 | 211739_x_at | chorionic somatomammotropin hormone 1 | Fetal Growth | GS(15) |
| 3050 | HBZ | 2.26 | 206647_at | hemoglobin, zeta /// hemoglobin, zeta | O2 binding | HY(40) |
| 4629 | MYH11 | 2.23 | 1568760_at | myosin, heavy polypeptide 11, smooth muscle | hematopoieses | TM(28) |
| 58155 | PTBP2 | 2.17 | 1560271_at | Polypyrimidine tract binding protein 2 | Cell cycle | CG(41) |
| 1443 | CSH2 | 2.16 | 203807_x_at | chorionic somatomammotropin hormone 2 | Collagen synthesis | GS(15) |
| 3371 | TNC | 2.12 | 216005_at | Tenascin C (hexabrachion) | Tissue component | CG(24) |
| 6453 | ITSN1 | 2.07 | 215791_at | Intersectin 1 (SH3 domain protein) | endocytosis | GS(21) |
| 2120 | ETV6 | 2.03 | 1561167_at | Ets variant gene 6 (TEL oncogene) | Fusion protein | AG(42) |
| 4628 | MYH10 | 2.03 | 237491_at | Myosin, heavy polypeptide 10, non-muscle | cytokinesis | TM(27, 43) |
| 23240 | KIAA0922 | 2.03 | 239946_at | KIAA0922 | ||
| 51379 | CRLF3 | 2.01 | 235803_at | Cytokine receptor-like factor 3 | ||
| 81 | ACTN4 | 2 | 241788_x_at | Actinin, alpha 4 | Cell motility | TM(26) |
| 10658 | CUGBP1 | 2 | 242440_at | CUG triplet repeat, RNA binding protein 1 | Translation p21 | TM |
| 6310 | ATXN1 | 1.99 | 232744_x_at | Ataxin 1 | Trinucletide repeats | |
| 653471 | LOC653471 | 1.98 | 217653_x_at | Ribosome biogenesis protein BMS1 homolog | ||
| 643314 | KIAA0754 | 1.97 | 215268_at | hypothetical LOC643314 | ||
| 8847 | DLEAH2 | 1.95 | 1556821_x_at | deleted in lymphocytic leukemia, 2 | ||
| 1444 | CSHL1 | 1.94 | 205958_x_at | chorionic somatomammotropin hormone-like 1 | Fetal Growth | GS(15) |
| 1523 | CUTL1 | 1.94 | 240798_at | Cut-like 1, CCAAT displacement protein | Gene expression | CG(44) |
| 11030 | RBPMS | 1.94 | 241897_at | RNA binding protein with multiple splicing | Cell Growth | CG(45) |
| 11039 | SMA4 | 1.94 | 215599_at | SMA4 /// similar to Beta-glucuronidase precursor | ||
| 8662 | EIF3S9 | 1.93 | 242550_at | eukaryotic translation initiation factor 3, sub 9 | CG(46) | |
| 51232 | CRIM1 | 1.93 | 233073_at | Cysteine rich transmembrane BMP regulator 1 | Capillary formation | AG(22) |
| 23433 | RHOQ | 1.91 | 239258_at | Ras homolog gene family, member Q | Exocyst complex | CG(47) |
| 4919 | ROR1 | 1.89 | 1559394_a_at | Receptor tyrosine kinase-like orphan receptor 1 | Neurite growth | TM |
CG- Cell and organ growth; AG- Angiogenesis, TM- Tissue and Matrix remodeling, GS- Growt-related signals; BI - Barrier and immune function, HY-Hypoxia related responses
Table 3.
Down-regulated genes in smokers (Limited to 25)
| Entrez ID | Symbol | mean Ratio | P_IDs | Gene Name | Gene Function | (Potential role in Development |
|---|---|---|---|---|---|---|
| 200315 | APOBEC3A | −2.78 | 210873_x_at | apolipoprotein B mRNA editing enzyme | Cell regulation | BI(32) |
| 4680 | CEACAM6 | −2.72 | 211657_at | carcinoembryonic antigen-related CAM 6 | Tissue architecture | CG(48) |
| 2568 | GABRP | −2.53 | 205044_at | gamma-aminobutyric acid (GABA) A receptor, pi | ||
| 25984 | KRT23 | −2.48 | 218963_s_at | keratin 23 (histone deacetylase inducible) | Structural protein | BI(49) |
| 114569 | MAL2 | −2.45 | 224650_at | mal, T-cell differentiation protein 2 | transcytosis | BI(34) |
| 3854 | KRT6B | −2.39 | 213680_at | keratin 6B | Structural protein | BI(49) |
| 5275 | SERPINB13 | −2.34 | 211361_s_at | serpin peptidase inhibitor, clade B (ovalbumin) | Inhibit proteinases | AG(23) |
| 220 | ALDH1A3 | −2.31 | 203180_at | aldehyde dehydrogenase 1 family, member A3 | detoxification | |
| 144568 | A2ML1 | −2.29 | 1564307_a_at | alpha-2-macroglobulin-like 1 | Inhibit proteases | BI(35) |
| 647456 | CD24 | −2.29 | 208650_s_at | CD24 molecule | Cell receptor | |
| 5918 | RARRES1 | −2.28 | 221872_at | retinoic acid receptor responder 1 | Retinoic acid related | |
| 8796 | SCEL | −2.28 | 232056_at | sciellin | Cell differentiation | BI(30) |
| 6947 | TCN1 | −2.27 | 205513_at | transcobalamin I (vitamin B12 binding protein) | ||
| 5055 | SERPINB2 | −2.25 | 204614_at | serpin peptidase inhibitor, clade B (ovalbumin), | PAI-2 (proteinase inhibitor) | TM(25) |
| 3934 | LCN2 | −2.18 | 212531_at | lipocalin 2 (oncogene 24p3) | Granulocyte maturation | BI(36) |
| 4118 | MAL | −2.17 | 204777_s_at | mal, T-cell differentiation protein | T-cell differentiation | BI(33) |
| 8710 | SERPINB7 | −2.16 | 206421_s_at | serpin peptidase inhibitor, clade B Megsin | Proteinase inhibitor | AG(38) |
| 3713 | IVL | −2.11 | 214599_at | involucrin | Epidermal differentiation | |
| 26298 | EHF | −2.11 | 225645_at | Ets homologous factor | Transcriptional repressor | BI(37) |
| 9052 | GPRC5A | −2.1 | 203108_at | G protein-coupled receptor, family C, group 5, | Cell growth promotion | |
| 6698 | SPRR1A | −2.01 | 214549_x_at | small proline-rich protein 1A | CG | |
| 3860 | KRT13 | −2 | 207935_s_at | keratin 13 | Structural protein | BI(49) |
| 6699 | SPRR1B | −2 | 205064_at | small proline-rich protein 1B (cornifin) | Disrupts mitosis | CG |
| 6707 | SPRR3 | −2 | 232082_x_at | small proline-rich protein 3 | Neoplastic marker | BI |
| 1824 | DSC2 | −1.97 | 204751_x_at | desmocollin 2 |
CG- Cell and organ growth; AG- Angiogenesis, TM- Tissue and Matrix remodeling, GS- Growth-related signals; BI – Barrier and immune function, HY-Hypoxia related responses
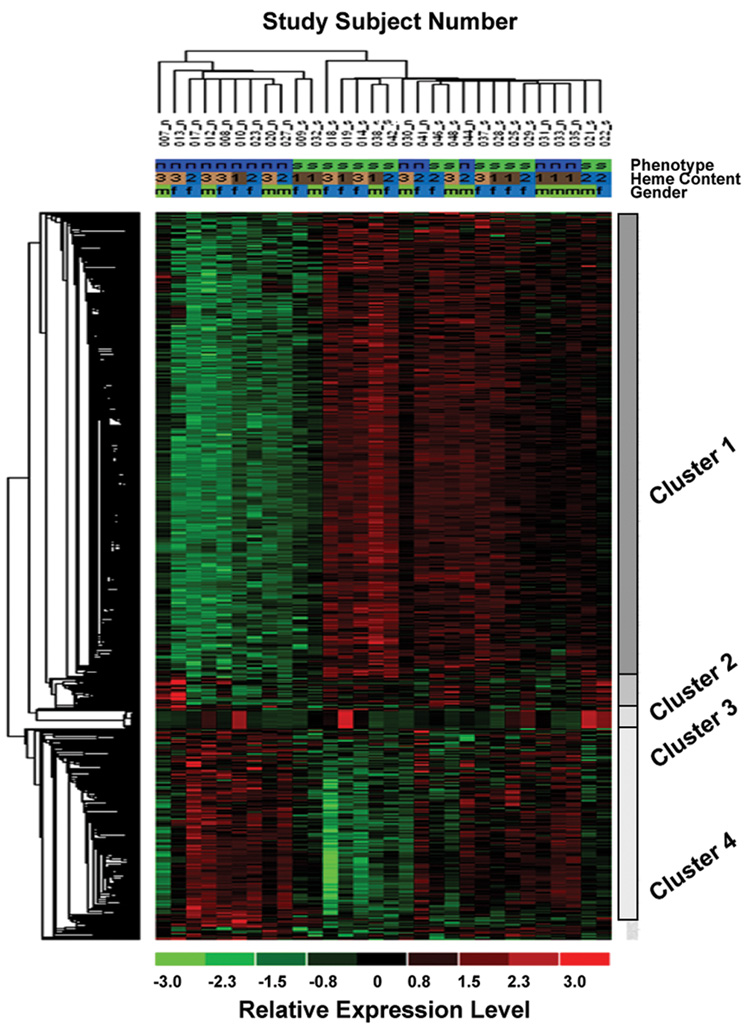
Hierarchical clustering based on similarity in gene expression using all differentially expressed genes (>1.3-fold increased or decreased RNA level) tied cases into two major groups (Figure 1). One group consisted of 9 non-smokers and 2 smokers and the other group consisted of 6 non-smokers and 13 smokers. A predominant cluster of transcripts (cluster 1) consisted of 431 probes that were up regulated in smokers compared to non-smokers. The second largest cluster (cluster 4) consisted of 197 probes that were down-regulation in smokers compared to nonsmokers. Further, small groups of genes (cluster 2 and 3 with 28 and 17 probes, respectively), did not show a consistent pattern of change as a function of smoking status. The genes in each cluster can be found in Supplementary Data Table I (available online at www.pedresearch.com). All genes that were examined in this microarray analysis can be found at GEO (http://www.ncbi.nlm.nih.gov/geo). Gene ontology (GO) analysis was performed with the DAVID ontology program V2.0 (9) using the lists of Affymetrix identification numbers for genes with either increased or decreased gene expression (> 1.3-fold) in UC tissue from smokers. The upregulated and downregulated set of genes were each classified into 5 biologic functional families as shown in Table 4.
Figure 1. Hierarchical Clustergram.
678 probes identified through ANOVA as being significantly regulated among the 30 subjects were clustered using the hierarchical clustering module in the D-Chip software using Euclidian distance and at a cluster significance value of 0.05. Two major subgroups of subjects (Phenotype: s=smokers; n=non-smokers) and four major clusters of probes can be identified (see text). Heme content (1=low, 2=moderate, 3=high) and sex of the baby (m=male, f=female) were used as covariates in ANOVA. The relative expression for each individual at a given probe is reflected by its color intensity (green=down-regulated, red=up-regulated).
Table 4.
Gene ontology classification using DAVID to group genes of similar functional families.
| Group | # genes | Enrichment score | Type of gene |
|---|---|---|---|
| Up regulated* | |||
| 1 | 71 | 6.37 | Transcription regulatory / nuclear |
| 2 | 14 | 5.8 | RNA binding or RNA processing proteins |
| 3 | 23 | 5.1 | Primary metabolism / Protein metabolism |
| 4 | 24 | 4.27 | Protein kinases |
| 5 | 10 | 2.36 | Vesicle associated intracellular transport |
| Down regulated** | |||
| 1 | 18 | 5.62 | Transmembrane protein |
| 2 | 19 | 4.47 | Immunoglobulin domain containing transmembrane protein |
| 3 | 7 | 4.4 | Cytoskeleton / intermediate filament protein |
| 4 | 4 | 3.16 | Membrane protein |
| 5 | 6 | 3.01 | Epidermal morphogenesis |
There were 472 upregulated Affymetrix® IDs with 384 matching DAVID dataset entries. Of these 142 were grouped.
There were 201 downregulated Affymetrix® IDs with 170 matching DAVID dataset entries. Of these 54 were grouped.
The correlation coefficients between qPCR and microarray gene expression values for CSH1, CSH2, and CSHL1 were +0.73, +0.68, and +0.69, respectively (p< 0.0001) as shown in Table 5.
Table 5.
Correlations* between QPCR and microarray expression values for the same gene
| B2M Normalization | GAPDH Normalization | ||||
|---|---|---|---|---|---|
| QPCR | Microarray Probe | Corr. | P-value | Corr. | P-value |
| Gene | |||||
| CSH1 | CSH1-202493_x_at | +0.77 | < 0.0001 | +0.76 | < 0.0001 |
| CSH1-206475_x_at | +0.63 | 0.0002 | +0.62 | 0.0002 | |
| CSH1-208356_x_at | +0.72 | < 0.0001 | +0.74 | < 0.0001 | |
| CSH1-208357_x_at | +0.74 | < 0.0001 | +0.74 | < 0.0001 | |
| CSH1-211739_x_at | +0.69 | < 0.0001 | +0.70 | < 0.0001 | |
| CSH1-208068_x_at | +0.63 | 0.0002 | +0.65 | 0.0001 | |
| CSH2 | CSH2-203807_x_at | +0.63 | 0.0002 | +0.65 | 0.0001 |
| CSH2-207770_x_at | +0.58 | 0.0008 | +0.56 | 0.001 | |
| CSH2-207770_x_at1 | +0.59 | 0.0006 | +0.58 | 0.0007 | |
| CSH2-208341_x_at | +0.72 | < 0.0001 | +0.74 | < 0.0001 | |
| CSH2-208342_x_at | +0.75 | < 0.0001 | +0.77 | < 0.0001 | |
| CSHL1 | CSHL1-205958_x_at | +0.64 | 0.0001 | +0.64 | 0.0001 |
| CSHL1-207285_x_at | +0.63 | 0.0002 | +0.62 | 0.0002 | |
| CSHL1-208293_x_at | +0.61 | 0.0003 | +0.63 | 0.0002 | |
| CSHL1-208294_x_at | +0.67 | < 0.0001 | +0.67 | < 0.0001 | |
| CSHL1-208295_x_at | +0.79 | < 0.0001 | +0.78 | < 0.0001 | |
Spearman rank correlation coefficients
DISCUSSION
To our knowledge, this is the first study to examine the effects of prenatal tobacco exposure on gene expression in the umbilical cord of infants born to smokers. As expected, we found that cigarette smoking was associated with reduced birth weight (1, 10). Using microarray analysis of UC tissue, we identified 545 genes that were differentially expressed as a function of smoking status. Differentially expressed genes appear to cluster into a number of categories, including those involved in growth factor signaling or direct growth promotion, cellular growth and differentiation, angiogenesis, extracellular matrix remodeling and connective tissue growth, and barrier and immune function. Correlation of the expression levels of three somatomammotropin genes with the qPCR results supported the validity of the microarray results.
Tobacco smoke is a complex mixture of toxicants, a number of which (e.g., carbon monoxide, hydrogen cyanide, nicotine, carcinogens) have been implicated in reducing fetal growth (4). Consistent with the theory that carcinogens may play a role in reduced birth weight, Wang et al. (11) found that pregnant smokers with the inducible CYP1A1 genotype were more likely to deliver a low birth weight baby than smokers with the wild type variant. Further, one recent study examining the effects of tobacco exposure on gene expression in placental tissue (a composite of maternal and fetal tissue) found that phase 1 drug metabolism genes (particularly CYP1A1) were up-regulated in smokers (12). Our study differs from that study in that we examined the effect of tobacco exposure on UC tissue (which is exclusively fetal tissue). The results that we obtained may reflect effects of prenatal tobacco exposure on fetal vascular gene expression. The difference in results from these two studies of gene expression may be explained by the fact that different areas in the placenta have differing patterns of gene expression (9), while the UC is not typically involved in drug metabolism.
Although the exact mechanisms are unknown (13), changes in the pattern of gene expression in the UC tissue of smokers can be understood in the context of fetal adaptation to a nutrient-poor, or growth-limiting, environment. For example, studies of the effects of fetal malnutrition and anemia suggest that with fetal adaptation there are increases in growth-related genes, especially those encoding growth hormone (GH1, GH2) and chorionic somatomammotropin (CSH-1, CSH-2 and CSH-L genes). These genes are localized within a 66.5 kb cluster on human chromosome 17q23 (14). The proteins encoded by the genes in this cluster have a complex mechanism of action and regulation in both the pregnant mother and the fetus (15). Of note, fetal growth is mainly affected by human somatomammotropins via their effects on the GH-receptor or the prolactin receptor. In addition to direct effects on fetal growth, these hormones have indirect effects that are mediated by insulin-like growth factors (IGFs) (15, 16). Along with other growth factors, human somatomammotropin regulates IGF production and modulates intermediary metabolism, including the availability of glucose and amino acids to the fetus (15, 16). Sheep adapt to poor fetal growth by increasing somatomammotropin activity in the fetal circulation (17). Our finding that the three CSH genes are the most highly up regulated among smokers may reflect a similar adaptive response. In addition to its association with lower birth weight, maternal smoking increases the risk of childhood obesity (18). In view of this, and the findings reported here, it is interesting that the growth hormone-lactogen gene cluster has been implicated in the association between low birth weight and the risk of metabolic syndrome later in life (19).
A number of growth factor signaling/ direct growth promotion genes also showed differential expression in UC tissue obtained from smokers. Similarly, down-regulation of the CEACAM6 gene could lead to a delay in the termination of insulin’s action, with growth promoting effects (20). The increased expression of the ITSN1 (intersectin) gene may serve to increase epidermal growth factor-receptor turnover, another growth-enhancing adaptation (21). Increased expression of CRIM1, whose protein product promotes capillary tube formation (22) and decreased expression of SERPINB13 [which encodes an inhibitor of angiogenesis (23)] may promote angiogenesis and fetal growth. Finally, up-regulation of another gene, the tenascin gene [TSN; which promotes tissue healing and regeneration (24)], could contribute to the development of extracellular matrix (i.e., fibroblasts and smooth muscle cells) in the offspring of smokers.
Another potential interaction of genes that were differentially expressed involves the down-regulation of SERPINB2 [which encodes a plasminogen activator inhibitor (PAI-2)], which may decrease tissue remodeling by inhibiting the plasminogen activators (25). The effects of decreased expression of SERPINB2 may be augmented by increased expression of ACTN4, which encodes actinin alpha 4, a protein that interacts with SERPINB2 and modulates its effects (26).
Two genes involved in the myosin chain showed increased expression in smokers. One of them, MYH10, is important in hypoxia-related myocardial adaptation (27) and the other, MYH11, is associated with arterial smooth muscle stiffening (28). It is noteworthy that stiffening of arteries is seen in infants with intra-uterine growth retardation (IUGR) (29). Further, one of the down regulated genes, SCEL (which encodes sciellin), is involved in stress-bearing blood vessel remodeling (30).
Some of the genes down-regulated in cord tissue of smokers may provide immune and barrier protection (31). The most highly down-regulated gene, APOBEC3A, is a proto-oncogene with presumed protective immune function through its action as a cytidine deaminase (32). Decreased expression of MAL (33), MAL2 (34), and KRT6B may result in impaired epithelial barrier protection. Other down-regulated genes, A2ML1 (35), LCN2 (36), and EHF (37), are associated with impaired immune cell function. Reduced expression of SerpinB7, and a lower concentration of its protein product megsin (which plays a role in megakaryocyte differentiation) may contribute to the development of thrombocytopenia that is seen in IUGR infants (38).
These findings provide insight into potential processes by which the fetus adapts to the adverse effects of maternal smoking. Strengths of this study include the well-defined study population, use of clinical variables to adjust for potential confounds in the analysis of the microarray results, and the validation of key findings using qPCR technology. One limitation of the study is that smoking could not be validated biochemically due to the inadequacy of infant hair samples for cotinine analysis. However, subjects were informed that their smoking status would be validated, which by itself can reduce reporting bias, particularly in relation to smoking. Further, the most common reporting bias in pregnant smokers is non-disclosure of smoking (39), which would have reduced the differences in gene expression seen between groups. Finally, although we adjusted for clinical variables that differed as a function of smoking status, it is possible that other variables that were not adjusted for will have influenced the results, including the clustering of groups based on smoking status.
In summary, we found that 545 genes were differentially expressed in the fetal tissue of offspring born to women who smoke. These findings require replication and subsequent studies should examine the potential mechanisms (e.g., epigenetic modification) by which maternal smoking exerts adverse effects on the fetus. Larger-scale studies are also needed to correlate gene expression results with longer-term clinical outcomes.
Supplementary Material
Acknowledgments
The technical assistance of Christine Abreu, Pamela Fall, John Glynn, Anupinder Kaur, Shama Praveen, Bruce Morris and Denise Ortiz in this work is greatly appreciated. We also thank the other members of our tobacco research group (Marc Lalande, Henry Furneaux, Melinda Saunders, Anna Dongari-Batzoglou, John Greene, and Roxanne Stepnowski) who participated in valuable discussions during the conduct of the study and the analysis of study findings
Financial Support: This research was supported by the State of Connecticut Department of Public Health Tobacco Research Funds, The Patrick and Catherine Weldon Donaghue Medical Research Foundation, and NIH grants RR06192 (University of Connecticut General Clinical Research Center), AA13736, and DA15167
Abbreviations
- cRNA
copy ribonucleic acid
- qPCR
quantitative polymerase chain reaction
- UC
umbilical cord
Footnotes
The manuscript contains supplementary material available online at www.pedresearch.com
Publisher's Disclaimer: Pediatric Research Articles Ahead of Print contains articles in unedited manuscript form that have been peer-reviewed and accepted for publication. As a service to our readers, we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting and review of the resulting proof before it is published in its final definitive form. Please note that during the production process errors may be discovered, which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Walsh RA. Effects of maternal smoking on adverse pregnancy outcomes: examination of the criteria of causation. Hum Biol. 1994;66:1059–1092. [PubMed] [Google Scholar]
- 2.Kristensen S, Salihu HM, Keith LG, Kirby RS, Fowler KB, Pass MA. SGA subtypes and mortality risk among singleton births. Early Hum Dev. 2007;83:99–105. doi: 10.1016/j.earlhumdev.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- 4.Stratton KR, Shetty P, Wallace R, Bondurant S. Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction. Washington DC: National Academy Press; 2001. pp. 148–158. [PubMed] [Google Scholar]
- 5.Ulm MR, Plockinger B, Pirich C, Gryglewski RJ, Sinzinger HF. Umbilical arteries of babies born to cigarette smokers generate less prostacyclin and contain less arginine and citrulline compared with those of babies born to control subjects. Am J Obstet Gynecol. 1995;172:1485–1487. doi: 10.1016/0002-9378(95)90482-4. [DOI] [PubMed] [Google Scholar]
- 6.Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 7.Kerr MK, Martin M, Churchill GA. Analysis of variance for gene expression microarray data. J Comput Biol. 2000;7:819–837. doi: 10.1089/10665270050514954. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Wong L. Emerging patterns and gene expression data. Genome Inform. 2001;12:3–13. [PubMed] [Google Scholar]
- 9.Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- 10.DiFranza JR, Lew RA. Effect of maternal cigarette smoking on pregnancy complications and sudden infant death syndrome. J Fam Pract. 1995;40:385–394. [PubMed] [Google Scholar]
- 11.Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, Niu T, Wise PH, Bauchner H, Xu X. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. 2002;287:195–202. doi: 10.1001/jama.287.2.195. [DOI] [PubMed] [Google Scholar]
- 12.Huuskonen P, Storvik M, Reinisalo M, Honkakoski P, Rysa J, Hakkola J, Pasanen M. Clin Pharmacol Ther. 2007. Microarray Analysis of the Global Alterations in the Gene Expression in the Placentas From Cigarette-smoking Mothers. in press. [DOI] [PubMed] [Google Scholar]
- 13.Angiolini E, Fowden A, Coan P, Sandovici I, Smith P, Dean W, Burton G, Tycko B, Reik W, Sibley C, Constancia M. Regulation of placental efficiency for nutrient transport by imprinted genes. Placenta. 2006;27:S98–S102. doi: 10.1016/j.placenta.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Oliver MH, Hawkins P, Harding JE. Periconceptional undernutrition alters growth trajectory and metabolic and endocrine responses to fasting in late-gestation fetal sheep. Pediatr Res. 2005;57:591–598. doi: 10.1203/01.PDR.0000155942.18096.9C. [DOI] [PubMed] [Google Scholar]
- 15.Osafo J, Wei Y, Kenth G, Goodyer CG. Growth hormone during development. Rev Endocr Metab Disord. 2005;6:173–182. doi: 10.1007/s11154-005-3048-6. [DOI] [PubMed] [Google Scholar]
- 16.Handwerger S, Freemark M. The roles of placental growth hormone and placental lactogen in the regulation of human fetal growth and development. J Pediatr Endocrinol Metab. 2000;13:343–356. doi: 10.1515/jpem.2000.13.4.343. [DOI] [PubMed] [Google Scholar]
- 17.Holland MD, Hossner KL, Williams SE, Wallace CR, Niswender GD, Odde KG. Serum concentrations of insulin-like growth factors and placental lactogen during gestation in cattle. I. Fetal profiles. Domest Anim Endocrinol. 1997;14:231–239. doi: 10.1016/s0739-7240(97)00023-4. [DOI] [PubMed] [Google Scholar]
- 18.Wideroe M, Vik T, Jacobsen G, Bakketeig LS. Does maternal smoking during pregnancy cause childhood overweight? Paediatr Perinat Epidemiol. 2003;17:171–179. doi: 10.1046/j.1365-3016.2003.00481.x. [DOI] [PubMed] [Google Scholar]
- 19.Day IN, Chen XH, Gaunt TR, King TH, Voropanov A, Ye S, Rodriguez S, Syddall HE, Sayer AA, Dennison EM, Tabassum F, Barker DJ, Cooper C, Phillips DI. Late life metabolic syndrome, early growth, and common polymorphism in the growth hormone and placental lactogen gene cluster. J Clin Endocrinol Metab. 2004;89:5569–5576. doi: 10.1210/jc.2004-0152. [DOI] [PubMed] [Google Scholar]
- 20.Najjar SM. Regulation of insulin action by CEACAM1. Trends Endocrinol Metab. 2002;13:240–245. doi: 10.1016/s1043-2760(02)00608-2. [DOI] [PubMed] [Google Scholar]
- 21.Martin NP, Mohney RP, Dunn S, Das M, Scappini E, O'Bryan JP. Intersectin regulates epidermal growth factor receptor endocytosis, ubiquitylation, and signaling. Mol Pharmacol. 2006;70:1643–1653. doi: 10.1124/mol.106.028274. [DOI] [PubMed] [Google Scholar]
- 22.Glienke J, Sturz A, Menrad A, Thierauch KH. CRIM1 is involved in endothelial cell capillary formation in vitro and is expressed in blood vessels in vivo. Mech Dev. 2002;119:165–175. doi: 10.1016/s0925-4773(02)00355-6. [DOI] [PubMed] [Google Scholar]
- 23.Shellenberger TD, Mazumdar A, Henderson Y, Briggs K, Wang M, Chattopadhyay C, Jayakumar A, Frederick M, Clayman GL. Headpin: a serpin with endogenous and exogenous suppression of angiogenesis. Cancer Res. 2005;65:11501–11509. doi: 10.1158/0008-5472.CAN-05-2262. [DOI] [PubMed] [Google Scholar]
- 24.Chiquet-Ehrismann R, Chiquet M. Tenascins: regulation and putative functions during pathological stress. J Pathol. 2003;200:488–499. doi: 10.1002/path.1415. [DOI] [PubMed] [Google Scholar]
- 25.Hu ZY, Liu YX, Liu K, Byrne S, Ny T, Feng Q, Ockleford CD. Expression of tissue type and urokinase type plasminogen activators as well as plasminogen activator inhibitor type-1 and type-2 in human and rhesus monkey placenta. J Anat. 1999;194:183–195. doi: 10.1046/j.1469-7580.1999.19420183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magdolen U, Schroeck F, Creutzburg S, Schmitt M, Magdolen V. Non-muscle alpha-actinin- 4 interacts with plasminogen activator inhibitor type-1 (PAI-1) Biol Chem. 2004;385:801–808. doi: 10.1515/BC.2004.105. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto T, Yamasaki S, Taguchi S. Alterations in the expression of myosin heavy chain isoforms in hypoxia-induced hypertrophied ventricles in rats. Comp Biochem Physiol B Biochem Mol Biol. 2003;136:139–145. doi: 10.1016/s1096-4959(03)00182-9. [DOI] [PubMed] [Google Scholar]
- 28.Zhu L, Vranckx R, Khau Van Kien P, Lalande A, Boisset N, Mathieu F, Wegman M, Glancy L, Gasc JM, Brunotte F, Bruneval P, Wolf JE, Michel JB, Jeunemaitre X. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet. 2006;38:343–349. doi: 10.1038/ng1721. [DOI] [PubMed] [Google Scholar]
- 29.Mori A, Uchida N, Inomo A, Izumi S. Stiffness of systemic arteries in appropriate- and small-for-gestational-age newborn infants. Pediatrics. 2006;118:1035–1041. doi: 10.1542/peds.2006-0386. [DOI] [PubMed] [Google Scholar]
- 30.Young PP, Modur V, Teleron AA, Ladenson JH. Enrichment of genes in the aortic intima that are associated with stratified epithelium: implications of underlying biomechanical and barrier properties of the arterial intima. Circulation. 2005;111:2382–2390. doi: 10.1161/01.CIR.0000164235.26339.78. [DOI] [PubMed] [Google Scholar]
- 31.Simchen MJ, Beiner ME, Strauss-Liviathan N, Dulitzky M, Kuint J, Mashiach S, Schiff E. Neonatal outcome in growth-restricted versus appropriately grown preterm infants. Am J Perinatol. 2000;17:187–192. doi: 10.1055/s-2000-9423. [DOI] [PubMed] [Google Scholar]
- 32.Navaratnam N, Sarwar R. An overview of cytidine eaminases. Int J Hematol. 2006;83:195–200. doi: 10.1532/IJH97.06032. [DOI] [PubMed] [Google Scholar]
- 33.Marazuela M, Acevedo A, Adrados M, Garcia-Lopez MA, Alonso MA. Expression of MAL, an integral protein component of the machinery for raft-mediated pical transport, in human epithelia. J Histochem Cytochem. 2003;51:665–674. doi: 10.1177/002215540305100512. [DOI] [PubMed] [Google Scholar]
- 34.Marazuela M, Acevedo A, Garcia-Lopez MA, Adrados M, de Marco MC, Alonso MA. Expression of MAL2, an integral protein component of the machinery for basolateral-to-apical transcytosis, in human epithelia. J Histochem Cytochem. 2004;52:243–252. doi: 10.1177/002215540405200212. [DOI] [PubMed] [Google Scholar]
- 35.Galliano MF, Toulza E, Gallinaro H, Jonca N, Ishida-Yamamoto A, Serre G, Guerrin M. A novel protease inhibitor of the alpha2-macroglobulin family expressed in the human epidermis. J Biol Chem. 2006;281:5780–5789. doi: 10.1074/jbc.M508017200. [DOI] [PubMed] [Google Scholar]
- 36.Bjorkqvist M, Kallman J, Fjaertoft G, Xu S, Venge P, Schollin J. Human neutrophil lipocalin: normal levels and use as a marker for invasive infection in the newborn. Acta Paediatr. 2004;93:534–539. [PubMed] [Google Scholar]
- 37.van Dijk TB, Baltus B, Caldenhoven E, Handa H, Raaijmakers JA, Lammers JW, Koenderman L, de Groot RP. Cloning and characterization of the human interleukin-3 (IL-3)/IL-5/ granulocyte-macrophage colony-stimulating factor receptor betac gene: regulation by Ets family members. Blood. 1998;92:3636–3646. [PubMed] [Google Scholar]
- 38.Silverman GA, Bird PI, Carrell RW, Church FC, Coughlin PB, Gettins PG, Irving JA, Lomas DA, Luke CJ, Moyer RW, Pemberton PA, Remold-O'Donnell E, Salvesen GS, Travis J, Whisstock JC. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem. 2001;276:33293–33296. doi: 10.1074/jbc.R100016200. [DOI] [PubMed] [Google Scholar]
- 39.Pichini S, Basagana XB, Pacifici R, Garcia O, Puig C, Vall O, Harris J, Zuccaro P, Segura J, Sunyer J. Cord serum cotinine as a biomarker of fetal exposure to cigarette smoke at the end of pregnancy. Environ Health Perspect. 2000;108:1079–1083. doi: 10.1289/ehp.001081079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hofmann O, Mould R, Brittain T. Allosteric modulation of oxygen binding to the three human embryonic haemoglobins. Biochem J. 1995;306:367–370. doi: 10.1042/bj3060367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rahman L, Bliskovski V, Kaye FJ, Zajac-Kaye M. Evolutionary conservation of a 2-kb intronic sequence flanking a tissue-specific alternative exon in the PTBP2 gene. Genomics. 2004;83:76–84. doi: 10.1016/s0888-7543(03)00207-6. [DOI] [PubMed] [Google Scholar]
- 42.Wang LC, Swat W, Fujiwara Y, Davidson L, Visvader J, Kuo F, Alt FW, Gilliland DG, Golub TR, Orkin SH. The TEL/ETV6 gene is required specifically for hematopoiesis in the bone marrow. Genes Dev. 1998;12:2392–2402. doi: 10.1101/gad.12.15.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Leon H, Scott NA, Martin F, Simonet L, Bernstein KE, Wilcox JN. Expression of nonmuscle myosin heavy chain-B isoform in the vessel wall of porcine coronary arteries after balloon angioplasty. Circ Res. 1997;80:514–519. doi: 10.1161/01.res.80.4.514. [DOI] [PubMed] [Google Scholar]
- 44.Michl P, Knobel B, Downward J. CUTL1 is phosphorylated by protein kinase A, modulating its effects on cell proliferation and motility. J Biol Chem. 2006;281:15138–15144. doi: 10.1074/jbc.M600908200. [DOI] [PubMed] [Google Scholar]
- 45.Sun Y, Ding L, Zhang H, Han J, Yang X, Yan J, Zhu Y, Li J, Song H, Ye Q. Potentiation of Smad-mediated transcriptional activation by the RNA-binding protein RBPMS. Nucleic Acids Res. 2006;34:6314–6326. doi: 10.1093/nar/gkl914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong Z, Zhang JT. Initiation factor eIF3 and regulation of mRNA translation, cell growth, and cancer. Crit Rev Oncol Hematol. 2006;59:169–180. doi: 10.1016/j.critrevonc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays. 2007;29:356–370. doi: 10.1002/bies.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scholzel S, Zimmermann W, Schwarzkopf G, Grunert F, Rogaczewski B, Thompson J. Carcinoembryonic antigen family members CEACAM6 and CEACAM7 are differentially expressed in normal tissues and oppositely deregulated in hyperplastic colorectal polyps and early adenomas. Am J Pathol. 2000;156:595–605. doi: 10.1016/S0002-9440(10)64764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schweizer J, Bowden PE, Coulombe PA, Langbein L, Lane EB, Magin TM, Maltais L, Omary MB, Parry DA, Rogers MA, Wright MW. New consensus nomenclature for mammalian keratins. J Cell Biol. 2006;174:169–174. doi: 10.1083/jcb.200603161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.