Abstract
We report an outbreak of Saccharomyces cerevisiae subtype boulardii fungemia among three intensive care unit roommates of patients receiving lyophilized preparations of this fungus. The fungemia was probably due to central venous catheter contamination and resolved after fluconazole treatment. The need for stringent application of proper hygiene when using a probiotic preparation of this organism is emphasized.
CASE REPORTS
Outbreak cases. (i) Case 1.
Case 1 involved a 34-year-old man hospitalized for hypoxia after head and thoracic trauma. He was placed on enteral nutrition, with insertion of a central venous catheter (CVC), and broad-spectrum antibiotic therapy was administered. On day 42 after admission (5 November 2000), he developed a fever, which was unsuccessfully treated with teicoplanin and imipenem. Multiple blood cultures yielded Saccharomyces cerevisiae. The fever and fungemia subsided under treatment with fluconazole at 400 mg/day. The CVC was removed 3 weeks after initiation of fluconazole treatment. The infectious episode resolved, but no catheter culture was performed.
(ii) Case 2.
Case 2 involved a 48-year-old man hospitalized for rupture of a cerebral aneurysm and fever. He was given enteral nutrition, and a CVC was inserted. Teicoplanin alone and then teicoplanin and meropenem were administered. On day 14 (10 November 2000), one blood culture yielded S. cerevisiae. On day 19, the CVC was removed and fluconazole therapy (400 mg/day) was immediately started. No catheter culture was performed. The fever subsided within 48 h of the initiation of fluconazole treatment.
(iii) Case 3.
Case 3 involved a 75-year-old woman admitted for acute myocardial infarction. She was given enteral nutrition, and a CVC was inserted. She was treated with various antibiotic regimens for several febrile episodes. On day 56 (10 April 2001), a blood culture yielded S. cerevisiae. The CVC was removed, leading to immediate defervescence. The CVC tip was positive for S. cerevisiae. Fluconazole therapy (400 mg/day) was started 2 days later and administered for 2 weeks.
None of the three patients described above received any probiotic treatment.
Case 4 (incomplete report).
A 35-year-old woman with multiple traumas who had been hospitalized in the intensive care unit (ICU) at the same time as patients 1 and 2 had blood cultures positive for S. cerevisiae. Unfortunately, her medical record was sequestered for forensic purposes and whether she had received probiotic treatment or not could not be determined. This patient improved and was transferred to the orthopedic division, from which she was discharged 2 months later.
The outbreak setting was an eight-bed ICU in a 400-bed secondary-care hospital in Rome, Italy. During the year preceding the outbreak, about 20% of the ICU patients were hospitalized for emergency surgery, 12% were hospitalized for elective surgery, 18% were hospitalized for trauma, and 50% were hospitalized for medical diseases. The mean (± standard deviation) age of patients was 66 ± 16 years, the mean ICU hospitalization time was 17 ± 18 days, and the mortality rate was 25%.
The ICU consists of a large room with the beds arranged in a semicircle, two small single-patient rooms, and the nurse's room, where drugs, medical records, and computer equipment are kept. There are four sinks in the patient rooms (two in the large one and one each in the small ones). All of these rooms have direct, independent access to the corridor.
A probiotic preparation labeled Codex (Zambon Farmaceutici, Vicenza, Italy) and declared to contain 250 mg of lyophilized Saccharomyces boulardii (see below for correct taxonomy) had been in use at the ICU since 1999. It was usually administered by a nurse wearing standard latex gloves, who opened the package containing the probiotic powder and dissolve it close to a sink located approximately 3 m from the nearest bed in the six-patient room. For administration of the probiotic preparation, the full content of the package was directly poured into the cylinder of a 50-ml syringe to which 30 ml of saline solution was added. The probiotic suspension was finally administered via the enteral nutrition tube.
In October 2000 (just before the outbreak), the probiotic preparation had been administered to four patients but not to the three patients involved in the outbreak (cases 1 to 3) reported in this study. After the first two cases of fungemia occurred, the probiotic preparation was not used further for 3 months. Importantly, the third case of fungemia was diagnosed about 1 month after reintroduction of the probiotic preparation into prophylactic regimens. Consequently, the probiotic preparation was no longer used.
The microorganism was initially identified by growth on Sabouraud chloramphenicol agar and by an automated biochemical identification panel (API Italia). A karyotype obtained by pulsed-field gel electrophoresis, which was performed in accordance with the protocol of Petersen et al. (11), provided a comparison between the two available isolates from the patients and the yeast contained in a package of the probiotic preparation that had been stored in the ICU's pharmacy. All of the yeast isolates were identical (Fig. 1), thus confirming both the exact identification as S. cerevisiae and the clonal relationship of the clinical isolates with the probiotic agent.
FIG. 1.
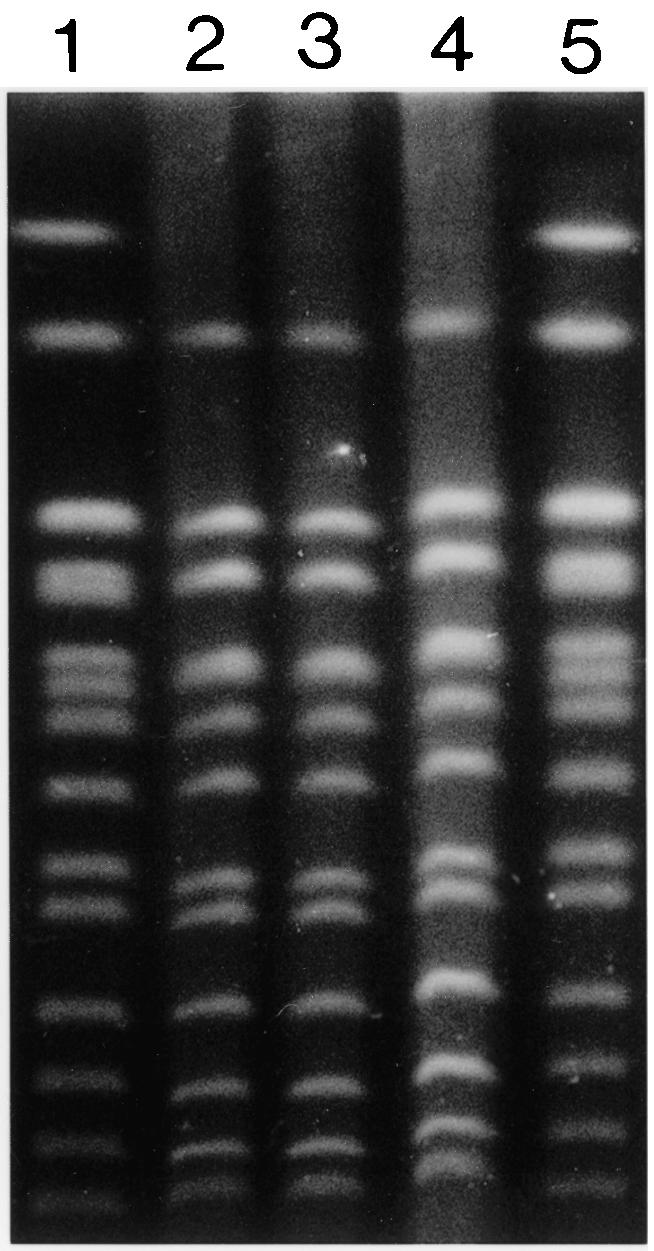
Pulsed-field gel electrophoresis of chromosomal DNA from a packet of an S. cerevisiae subtype boulardii biotherapeutic preparation (Codex) and S. cerevisiae subtype boulardii isolated from the CVC of patient 3 and a blood culture of patient 4. Lanes: 1, S. cerevisiae Bio-Rad (marker); 2, S. cerevisiae subtype boulardii (Codex); 3, S. cerevisiae subtype boulardii from patient 3; 4, S. cerevisiae subtype boulardii from patient 4; 5, S. cerevisiae Bio-Rad (marker).
In recent years, invasive fungal infections due to new or unusual agents have been frequently reported worldwide. This is currently considered to be a consequence of an increase in the population at risk for chronic or debilitating diseases, increased use of immunosuppressive drugs or broad spectrum antibiotics, as well as parenteral nutrition, and use of CVCs. Freeze-dried preparations labeled S. boulardii are used in several countries as a biotherapeutic agent to prevent antibiotic-induced diarrhea in ICU patients (2). This treatment has also been proposed for Crohn's disease and other inflammatory bowel conditions (5). Overall, it is considered to be safe and well tolerated (8). Although S. boulardii is, according to official taxonomic definitions, simply a subtype of the species S. cerevisiae, we will consider here only the fully described cases of fungemia that have been attributed to this subtype and not all cases of S. cerevisiae fungemia. Fungemia caused by S. cerevisiae subtype boulardii should not be considered rare. To our knowledge, 24 well-described clinical cases have been reported in the literature (1, 3, 4, 6, 7, 9, 10, 13-16) (Table 1). In a further report, 12 (93%) of 13 S. cerevisiae blood isolates collected in five hospitals over a 2-year period were retrospectively subtyped as S. cerevisiae subtype boulardii, and their etiologic role in fungemias was demonstrated by a genotypic comparison with commercial preparations labeled S. boulardii (12). Moreover, single cases, especially in patients receiving oral therapy, are unlikely to be published.
TABLE 1.
Characteristics and outcomes of cases of S. boulardii fungemia described in the literaturea
| Case no. (reference) | Age/sexe | Immunocompetence | Presence of CVC | Oral S. boulardii therapy | Intestinal disease | CVC removal | Medical treatmentb | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 (16) | 33 yr/M | Unknown | Yes | Yes | Yes | Unknown | Flu-AmB | Cure |
| 2 (15) | 14 yr/M | Unknown | Yes | Yes | No | Yes | Flu-AmB | Cure |
| 3 (13) | 1 yr/F | No | Yes | Yes | Yes | Unknown | Flu | Cure |
| 4 (4) | 49 yr/M | Unknown | Yes | Yes | No | Unknown | Flu | Cure |
| 5 (1) | 51 yr/F | Unknown | Yes | Yes | Yes | Unknown | AmB | Cure |
| 6 (9) | 78 yr/F | Unknown | Yes | Yes | No | Yes | Flu | Cure |
| 7 (9) | 31 yr/F | No | Yes | Yes | Unknown | Yes | AmB | Cure |
| 8 (9) | 36 yr/F | No | Yes | Yes | Unknown | Yes | AmB | Cure |
| 9 (9) | 2 yr/M | Unknown | Yes | Yes | Yes | Unknown | Unknown | Unknown |
| 10 (6) | 2 yr/M | Unknown | Yes | Yes | Yes | Yes | AmB | Cure |
| 11 (6) | 36 yr/M | No | Yes | Yes | Unknown | Unknown | Flu | Cure |
| 12 (6) | 47 yr/M | Unknown | Yes | Yes | Yes | Yes | Flu | Cure |
| 13 (6) | 78 yr/F | Unknown | Yes | Yes | Yes | No | None | Cure |
| 14 (10) | 3 mo/M | Unknown | Yes | Yes | Yes | Yes | AmB | Cure |
| 15 (10) | 1 mo/F | Unknown | Yes | No | Yes | Yes | AmB | Cure |
| 16 (7) | 50 yr/M | Yes | Yes | Yes | No | Unknown | None | Deathd |
| 17 (7) | 51 yr/F | No | Yes | Yes | Yes | Unknown | Flu | Deathd |
| 18 (7) | 50 yr/M | Yes | Yes | No | Yes | Yes | Flu | Cure |
| 19 (7) | 82 yr/F | Unknown | Yes | Yes | No | Unknown | None | Cure |
| 20 (7) | 75 yr/M | Unknown | Yes | Yes | No | Unknown | None | Cure |
| 21 (7) | 77 yr/M | No | Yes | Yes | Yes | Unknown | AmB | Deathd |
| 22 (7) | 71 yr/F | No | Yes | Yes | No | Unknown | None | Cure |
| 23 (3) | 8 mo/M | No | Yes | Yes | Unknown | Yes | AmBc | Cure |
| 24 (14) | 74 yr/M | Yes | No | Yes | Yes | Unknown | Flu | Deathd |
| 25 (this study) | 34 yr/M | Yes | Yes | No | No | No | Flu | Cure |
| 26 (this study) | 48 yr/M | Yes | Yes | No | No | Yes | Flu | Cure |
| 27 (this study) | 75 yr/F | Yes | Yes | No | No | Yes | Flu | Cure |
All had positive blood cultures.
Flu, fluconazole; AmB, amphotericin B; Flu-AmB, both fluconazole and amphotericin B.
Positive blood culture under antifungal prophylactic treatment.
Patient's death was probably not related to the fungemia.
M, male; F, female.
Importantly, fungemia by S. cerevisiae subtype boulardii can occur in patients who are not treated with a probiotic preparation of this organism when they share a room with treated patients (Table 1 and Fig. 2), as a result of airborne or interpersonal colonization. It has been demonstrated that after a package of freeze-dried yeast is opened, viable cells persist on room surfaces after 2 h at a 1-m distance and may persist on the bare hands of operators even after vigorous hand washing (6). Two studies have so far reported a case of fungemia in a patient not receiving S. cerevisiae subtype boulardii therapy (7, 10). However, our study is the first one describing multiple contemporary cases of S. cerevisiae subtype boulardii fungemia in patients in the same ward who did not receive a probiotic preparation of this organism as biotherapeutic treatment.
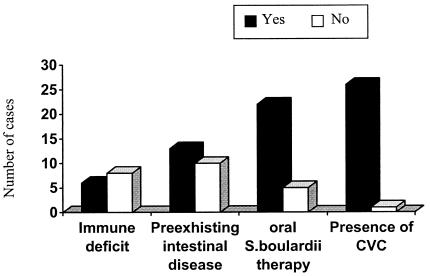
FIG. 2.
Overall occurrence of selected risk factors for S. cerevisiae subtype boulardii fungemia in the cases reported here.
Contamination of a CVC insertion site is likely to be one of the main mechanisms of S. cerevisiae subtype boulardii fungemia, as suggested by Hennequin et al. (6). In our study, the role of CVCs in the pathogenesis of the infection has been demonstrated by CVC tip culture (in one of our patients), and it is also probable in the other two cases since neither patient was orally administered the probiotic preparation. Fungemias are not limited to immunocompromised patients (Fig. 2), and for this reason, S. cerevisiae subtype boulardii should be considered a potentially dangerous microorganism. Although no case of death clearly attributable to fungemia due to this yeast has been reported, two studies documented septic shock in the presence of positive blood cultures that yielded S. cerevisiae subtype boulardii as a single isolate (6, 10). In one case, fungemia developed despite prophylactic antifungal treatment (3). In several additional cases, this microorganism was the only cause of fever in debilitated patients in which the metabolic stress caused by fever could have contributed to a downward prognostic shift unless prompt therapy was instituted. Four patients (three of whom were treated with antifungal agents) died, but death was not attributed to fungemia (7, 14) (Table 1). All of the other cases described in the literature and the three patients described here as well had a rapid response, even if they were immunocompromised, after suspension of treatment with the probiotic preparation, CVC removal, and/or antifungal therapy. These observations, taken together, underscore the prognostic importance of the rapid diagnosis of fungemia.
Decreased susceptibility to fluconazole was reported in some strains of S. cerevisiae subtype boulardii (6), although this did not lead to therapeutic failure. However, none of the patients reported in the literature had any intravascular devices, such as prosthetic valves or aorto-bifemoral grafts, that could be contaminated during fungemia and could be potential factors of increased risk of fungemia and therapeutic failure.
In conclusion, we describe the largest outbreak of S. cerevisiae subtype boulardii fungemia involving patients not receiving biotherapy with the fungus. A review of the literature showed that (i) fungemias can occur in immunocompetent patients and may contribute to morbidity and mortality in immunocompromised patients, (ii) enteral translocation of ingested microorganisms and CVC insertion site contamination are the main portals of entry into the bloodstream, (iii) prevention of CVC-related fungemias can be achieved by simple prophylactic measures, and (iv) fluconazole and amphotericin B are effective therapeutic options; CVC removal alone was also effective in some cases.
Finally, our study emphasizes the risk of infection if the package containing the lyophilized fungal preparation is opened in a patient's room and without proper infection control precautions, such as changing gloves before administration of the probiotic preparation and careful hand washing.
REFERENCES
- 1.Bassetti, S., S. Frei, and W. Zimmerli. 1998. Fungemia with Saccharomyces cerevisiae after treatment with Saccharomyces boulardii. Am. J. Med. 105:71-72. [DOI] [PubMed] [Google Scholar]
- 2.Bleichner, G., H. Blèhaut, H. Mente, and D. Moyse. 1997. Saccharomyces boulardii prevents diarrhea in critically ill tube-fed patients. Intensive Care Med. 23:517-523. [DOI] [PubMed] [Google Scholar]
- 3.Cesaro, S., P. Chinello, L. Rossi, and L. Zanesco. 2000. Saccharomyces cerevisiae fungemia in a neutropenic patient treated with Saccharomyces boulardii. Support Care Cancer 8:504-505. [DOI] [PubMed] [Google Scholar]
- 4.Fredenucci, I., M. Chomarat, C. Boucaud, and J. Flandrois. 1998. Saccharomyces boulardii fungemia in a patient receiving Ultra-Levure therapy. Clin. Infect. Dis. 27:222-223. [DOI] [PubMed] [Google Scholar]
- 5.Guslandi M., G. Mezzi, M. Sorghi, and P. A. Testoni. 2000. Saccharomyces boulardii in maintenance treatment of Crohn's disease. Dig. Dis. Sci. 68:5998-6004. [DOI] [PubMed] [Google Scholar]
- 6.Hennequin, C., C. Kauffmann-Lacroix, A. Jobert, J. P. Viard, C. Ricour, J. L. Jacquemin, and P. Berche. 2000. Possible role of catheters in Saccharomyces boulardii fungemia. Eur. J. Clin. Microbiol. Infect. Dis. 19:16-20. [DOI] [PubMed] [Google Scholar]
- 7.Lherm, T., C. Monet, B. Nougière, M. Soulier, D. Larbi, C. Le Gall, D. Caen, and C. Malbrunot. 2002. Seven cases of fungemia with Saccharomyces boulardii in critically ill patients. Intensive Care Med. 28:797-801. [DOI] [PubMed] [Google Scholar]
- 8.McCullough, M. J., K. V. Clemons, J. H. McCusker, and D. A. Stevens. 1998. Species identification and virulence attributes of Saccharomyces boulardii (nom. inval.). J. Clin. Microbiol. 36:2613-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niault, M., F. Thomas, J. Prost, F. Hojjat Ansari, and P. Kalfon. 1999. Fungemia due to Saccharomyces species in a patient treated with enteral Saccharomyces boulardii. Clin. Infect. Dis. 28:930. [DOI] [PubMed] [Google Scholar]
- 10.Perapoch, J., A. M. Planes, A. Querol, V. Lopez, I. Martinez-Bendayan, R. Tormo, F. Fernandez, G. Peguero, and S. Salcedo. 2000. Fungemia with Saccharomyces cerevisiae in two newborns, only one of whom had been treated with Ultra-Levura. Eur. J. Clin. Microbiol. Infect. Dis. 19:468-470. [DOI] [PubMed] [Google Scholar]
- 11.Petersen, R. F., T. Nilsson-Tillgren, and J. Piskur. 1999. Karyotypes of Saccharomyces sensu latu species. Int. J. Syst. Bacteriol. 49:1925-1931. [DOI] [PubMed] [Google Scholar]
- 12.Piarroux, R., L. Millon, K. Bardonnet, O. Vagner, and H. Koenig. 1999. Are live Saccharomyces yeasts harmful to patients? Lancet 353:1851-1852. [DOI] [PubMed] [Google Scholar]
- 13.Pletincx, M., J. Legein, and Y. Vandenplas. 1995. Fungemia with Saccharomyces boulardii in a 1-year-old girl with protracted diarrhea. J. Pediatr. Gastroenterol. Nutrition 21:113-115. [DOI] [PubMed] [Google Scholar]
- 14.Rijnders, B. J. A., E. Van Vijngaerden, C. Verwaest, and W. E. Peetermans. 2000. Saccharomyces fungemia complicating Saccharomyces boulardii treatment in a non-immunocompromised host. Intensive Care Med. 26:825. [DOI] [PubMed] [Google Scholar]
- 15.Viggiano, M., C. Badetti, V. Bernini, M. Garabedian, and J. Manelli. 1995. Fongemie à Saccharomyces boulardii chez un brulè grave. Ann. Fr. Anesth. Reanim. 14:356-358. [DOI] [PubMed] [Google Scholar]
- 16.Zunic, P., J. Lacotte, M. Pegoix, G. Buteux, G. Leroy, B. Mosquet, and M. Moulin. 1991. Fongèmia à Saccharomyces boulardii: a propos d'un cas. Therapie 46:498-499. [PubMed] [Google Scholar]