Abstract
Crohn's disease may be triggered by an infection, and it is plausible to consider that such an infection may be animal borne and ingested with our food. There has been considerable interest in the past in determining whether Mycobacterium avium subsp. paratuberculosis (M. avium) might be the etiologic agent in Crohn's disease since it causes a disease in cattle that is similar to Crohn's disease in humans. We aimed to determine if there was an association between Crohn's disease and infection with M. avium or other zoonotic agents and compared the findings with those for patients with ulcerative colitis, unaffected siblings of Crohn's disease patients, or population-based controls without inflammatory bowel disease. Patients under age 50 years with Crohn's disease or ulcerative colitis, unaffected siblings of patients, or healthy controls drawn from a population-based age- and gender-matched registry were enrolled in a study in which subjects submitted to a questionnaire survey and venipuncture. A nested cohort underwent colonoscopy plus biopsy. Samples were batched and submitted to PCR for the detection of M. avium and other zoonotic agents known to cause predominately intestinal disease in cattle, sheep, or swine. Only one patient with ulcerative colitis, no patients with Crohn's disease, and none of the sibling controls were positive for M. avium, whereas 6 of 19 healthy controls were positive for M. avium. Since the control subjects were significantly older than the case patients, we studied another 11 patients with inflammatory bowel disease who were older than age 50 years, and another single subject with ulcerative colitis was positive for M. avium. One other subject older than age 50 years with ulcerative colitis was positive for circovirus, a swine-borne agent of infection. In conclusion, by performing PCR with mucosal samples from patients with Crohn's disease and controls, no association between Crohn's disease and infection with M. avium or any of the other six zoonotic agents studied could be found.
Recently, there has been heightened interest in pursuing a microbial etiology in Crohn's disease. Mycobacteria are among the potential candidate microbes. In the early part of the 20th century the similarities between segmental human intestinal disease and granulomatous disease in cattle were identified (12). In the latter part of the 20th century, with the advent of a modern understanding of the clinical manifestations of Crohn's disease, parallels were drawn between Crohn's disease and mycobacterial diseases of both humans and animals. Crohn's disease is a segmental granulomatous intestinal disease with a predilection for the terminal ileum and right colon. When Mycobacteria tuberculosis affects the gut it causes a segmental granulomatous disease associated with pain, diarrhea, and wasting, with a predilection for the terminal ileum and right colon. Mycobacterium avium subsp. paratuberculosis (M. avium) causes a disease in cattle known as Johne's disease, also a granulomatous inflammatory enteritis associated with diarrhea, wasting, and a predilection for the ileum (35). The similarities between Johne's disease and Crohn's disease can be summarized as follows: (i) both diseases typically begin early in life; (ii) clinical manifestations of both diseases more typically begin after sexual maturity; (iii) both diseases cluster in families or herds; (iv) the main target site for disease is the ileum; (v) these diseases share similar host responses, as seen by histopathology; and (vi) the clinical symptoms of diarrhea and weight loss are similar (11). Granulomas are universally present in animals with Johne's disease, but they are present in approximately one-third to one-half of subjects with Crohn's disease. This may reflect the potential diverse repertoire of responses to a single pathogen that individuals can mount. Alternatively, this may highlight the fact that Crohn's disease is a variety of diseases. Those patients who mount granulomatous responses may have antigenic triggers different from those who do not. Thus, on consideration of the diverse pathological responses of Crohn's disease and the diverse disease phenotypes, it is reasonable to consider that a single organism may be associated with only some cases of Crohn's disease or that multiple organisms cause the different clinical manifestations of Crohn's disease.
It is rational to consider that an infectious etiology for Crohn's disease might be the ingestion of foodstuffs. Furthermore, the epidemiology of the disease, whose incidence is rising in Western societies, concurrent with the low rates of Crohn's disease in developing nations over the second half of the 20th century and high rates among immigrants to Western societies, is consistent with the possibility that a critical infection may be acquired from cattle or sheep via milk or meat ingestion, staples of Western diets, and cause Crohn's disease in patients with the appropriate genetic predisposition (3).
In the United States approximately 22% of all dairy herds and 8% of beef herds are infected with M. avium (11). A survey of dairy operations with at least 30 milk cows involving 20 states (representing 79.4% of the dairy population) across the United States found evidence of Johne's disease in 22% of the herds. However, over half of the infected herds had only one infected cow (28). This is also a problem in Canadian cattle. A survey of 14,932 cows in 304 randomly selected dairy herds in Ontario, Canada, found that 6.1% of the animals tested were seropositive for M. avium by enzyme-linked immunosorbent assay (24). Of all cows presenting to abattoirs for slaughter, M. avium was isolated from the tissues of 5.5% (24).
Investigators have used molecular biology-based techniques in search of M. avium gene sequences. We believed that it was critical to pursue this question of M. avium infection of mucosal tissue with an appropriate, unbiased, population-based control group. Furthermore, we hypothesized that it may be plausible that other cattle- or sheep-borne infections may be relevant to human disease and worth pursuing in a study of the tissues of Crohn's disease patients and controls.
Bovine viral diarrhea (BVD) virus causes a complex of diseases in cattle, including enteritis, during acute or transient infection, which is usually mild but which is occasionally severe enough to kill even adult cows. Brachyspira hyodysenteriae is the causative agent of swine dysentery, a disease of actively growing pigs, and Brachyspira pilosicoli is associated with intestinal spirochetosis of pigs in the postweaning period, dogs, birds, and humans (usually those that are immunocompromised) (14). Q fever is a zoonotic disease caused by Coxiella burnetii, a species of bacteria that is distributed globally (2). Many human infections are inapparent. Cattle, sheep, and goats are the primary reservoirs of C. burnetii. Infection has been noted in a wide variety of other animals, including other breeds of livestock and domesticated pets. Infection of humans usually occurs by inhalation of these organisms from air that contains airborne barnyard dust contaminated with dried placental material, birth fluids, and excreta of infected herd animals. Humans are often very susceptible to the disease, and very few organisms may be required to cause infection. The pattern of illness in patients with Chlamydia psittaci infections is very similar to that in patients with C. burnetii infections (22).
Circovirus causes an entity known as postweaning multisystemic wasting syndrome (PMWS) in pigs. Pigs show chronic wasting, pallor, and enlarged lymph nodes. They usually develop jaundice and a decreased growth rate. In some less specific cases, there are some respiratory and digestive (diarrhea, gastric ulcers) signs. Porcine circovirus type 2 has been demonstrated in the lesions of animals with PMWS, sometimes in association with other viruses such as porcine reproductive and respiratory syndrome virus and parvovirus. However, porcine circovirus type 2 is also found in herds which do not have problems with PMWS.
In Manitoba, Canada, we have developed a population-based database of inflammatory bowel disease (IBD) and reported Crohn's disease incidence rates (15 cases/105 population/year) that were among the highest in the published literature (4). The agricultural economy of rural Manitoba and the production of locally consumed beef, dairy, and other animal products enhance the relevance of pursuing M. avium and other zoonoses as potential etiologies for Crohn's disease in Manitoba. We developed a cohort of population-based controls and had a cadre of siblings of patients with Crohn's disease who also served as controls. Patients with ulcerative colitis served as relevant disease controls. We enrolled subjects in an endoscopy plus biopsy study which facilitated the acquisition of mucosal tissue.
MATERIALS AND METHODS
Study subjects.
A population based case-control study was undertaken. In developing an administrative definition of IBD for the purposes of creating a population-based database, subjects identified through the administrative database of Manitoba Health, the single provincial health insurer, were mailed questionnaires regarding their histories of IBD and willingness to participate in future studies. Sixty percent of the subjects responded and mailed back the questionnaires (4). Those who returned the questionnaires (and thereby consented to be known to the researchers) were logged in the University of Manitoba Research Registry. At the beginning of the present study there were 2,890 subjects in the Research Registry. Individuals in this Research Registry who agreed to participate in future studies furnished a mailing address and a telephone number to facilitate contact. We accessed the registry to enroll subjects under the age of 50 years and mailed them information sheets and questionnaires for the case-control study that we were initiating.
Control selection.
As part of a larger case-control study, a population-based set of controls was selected from the Manitoba Health population registry. The Manitoba Health population registry contains demographic information on all individuals registered with the Manitoba Health public health insurance system. The registry is regularly updated with vital registrations and information from medical and hospital transactions and closely matches population estimates derived from the Canadian census (Statistics Canada) (31). A random sample of registered persons was selected with stratification for age (5-year intervals) and gender to achieve balance with the case series for those two variables. Using the specified stratification, Manitoba Health's Information Services generated a mailing list of eligible controls and sent an information package prepared by the investigators explaining the study and requesting participation. The investigators did not know the identities of the controls unless they received a mailed response. For a second control group, we asked patients with IBD to refer us to one or more siblings.
All cases and controls completed a questionnaire and submitted to venipuncture. Controls were invited to participate in the colonoscopy plus biopsy study, and those who agree were paid an honorarium. Approximately 10% of the controls who were enrolled in our study in which we collected questionnaire data and blood agreed to participate in the colonoscopy plus biopsy study. Cases were asked to contact the study personnel when they were to undergo their next colonoscopy for clinical reasons. All cases who were to undergo colonoscopies agreed to tissue collection for study purposes.
Colonoscopy plus biopsies.
At colonoscopy we obtained eight biopsy specimens from the cecum and eight biopsy specimens from the rectum, which were studied separately, from the cases and controls. For subjects with a previous cecal resection, biopsy specimens were obtained from the right colon distal to the ileocolonic anastomosis. All biopsy specimens were snap-frozen in liquid nitrogen and stored at −70°C.
PCR.
All laboratory procedures were performed by using published guidelines for quality assurance of PCR in diagnostic laboratory settings (29). Each stage of the PCR process was carried out in one of four separate rooms which were designated for specific use in order to minimize the risk of cross contamination (29). Separate sets of designated gloves, lab coats, micropipettors, and filter barrier tips were used in each room for all steps. Every set of PCRs that were performed included two positive control samples and at least one negative control sample. In the past 2 years our team has performed a total of over 20,000 routine PCR tests. Relevant to this report, several individual tissue specimens were positive by PCR for the organisms evaluated in the study described in this report, including M. avium (n = 80), BVD virus (n = 4,000), B. pilosicoli (n = 190), B. hyodysenteriae (n = 130), circovirus (n = 600), C. psittaci (n = 120), and C. burnetii (n = 50). In the Nayar, Hamel laboratory all specimens positive by PCR tests are subjected to either DNA sequencing or analysis of the patterns observed on electrophoresis gels after restriction enzyme digestion of the PCR products. Samples from among these specimens with positive results served as positive controls in our study.
Nucleic acid purification.
Nucleic acids were extracted from tissue samples essentially as described previously (19), except that tissues were homogenized in only 1 ml of lysis buffer in 10-ml polypropylene snap-cap tubes. The tissue lysis buffer used in this study is a modification (19) of the commonly used Chomczynski lysis buffer (9). Specifically, 0.2 M sodium acetate was used at pH 7.0 instead of pH 4.0.
PCR amplification.
A nested PCR assay for detection of the IS900 sequence of M. avium was performed by using previously described primer sequences (33) and previously described reaction and thermocycling conditions (19). The lysis buffer that our laboratory uses (19) to detect M. avium in Johne's disease cases in cattle is used with the understanding that its sensitivity will be slightly compromised in order to minimize the risk of false-positive results. The sensitivity of our M. avium-specific nested PCR assay is approximately 10 mycobacterial genomes, which is comparable to that described previously (26, 33). The first PCR used primers (5′ to 3′) p90 (GAAGGGTGTTCGGGGCCGTCGCTTAGG) and p91 (GGCGTTGAGGTCGATCGCCCACGTGAC). After the first PCR was performed, 1 μl was tested by PCR with nested primers (5′ to 3′) AV1 (ATGTGGTTGCTGTGTTGGATGG) and AV2 (CCGCCGCAATCAACTCCAG).
The procedure that our laboratory uses is suitable for detection of the vegetative state of M. avium, which is associated with Johne's disease in cattle.
Other organisms.
Reverse transcription-PCR (RT-PCR) for detection of BVD virus was performed as described previously (20). The PCR assay for detection of B. pilosicoli used unpublished primer sequences designed by our laboratory which targeted a 601-bp region of the 16S rRNA gene (GenBank accession number U14928) between nucleotide positions 160 and 760. Primers Spil-1 (AGAGTAGAGGAAAGTTTTTTCGCTTCACGA) and Spil-2 (GTACAACGTTTACGGCTAGGACTACCAGGG) were used. The PCR assay for detection of B. hyodysenteriae used unpublished primer sequences designed by our laboratory which targeted a 586-bp region of a probe sequence (GenBank accession number U16319) between nucleotide positions 248 and 833. Primers Shyo-248 (CTCTTGGGTGGGCGTTATAATCG) and Shyo-833 (GTCCTGTAGGAACTGTTGCAGGCT) were used. The PCR assay for detection of type 2 circovirus used previously described primer sequences (19). The PCR assay for detection of C. psittaci used unpublished primer sequences designed by our laboratory which targeted a 221-bp region of the major outer membrane protein gene (GenBank accession number M36703) between nucleotide positions 55 and 275. Primers momp-1 (TGCTTAAGGCTGTTTTCACTTGCA) and momp-2 (GTAAGGAGAGAGCGGAACCCGT) were used. The PCR assay for detection of C. burnetii used unpublished primer sequences designed by our laboratory which targeted a 442-bp region of the transposase (IS1111a) gene (GenBank accession number M80806) between nucleotide positions 138 and 642. Primers Cox-821-For (TTGTCGGCGTTTATTGGGTTGGTC) and Cox-1262-Rev (GGTTGATGCTTATCGGGCTATCGG) were used.
Gel electrophoresis and photography were performed as described previously (19). The sensitivities of the regular RT-PCR and PCR assays have been estimated to be from 103 to 105 genome copies, and this decreases to 100 copies for the nested PCR. The sensitivities of our assays were comparable to those published previously. We have previously reported that the sensitivity of our BVD RT-PCR test is approximately 0.1 50% tissue culture infectious doses (20). The assays for the other organisms had similar sensitivities. The investigators performing the PCR studies (G.N. and A.H.) were blinded to study subject information.
This study was approved by the University of Manitoba Research Ethics Board and the Manitoba Health Human Information Privacy Committee.
RESULTS
A total of 24 patients with Crohn's disease (12 males, 12 females; mean age, 33.5 ± 1.7 years), 28 patients with ulcerative colitis (16 males, 12 females; mean age, 36.2 ± 1.9 years), and 28 controls (15 males, 13 females; mean age, 41.1 ± 1.1 years) were enrolled in the study. Among the patients with Crohn's disease enrolled in the study, 33% were known to have granulomatous disease, as determined histologically. The controls were significantly older than the patients in the Crohn's disease group (P = 0.003) and the ulcerative colitis group (P = 0.01); however, there were no significant differences between the groups by gender. There was no difference between the three groups in terms of the distribution of urban and rural residents. Among the controls, 9 were sibling controls and 19 were random population-based controls.
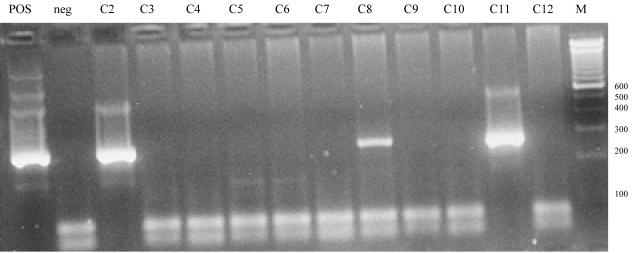
None of the Crohn's disease patients, one ulcerative colitis patient, and six controls were positive for M. avium. All controls who were positive were randomly selected, and none was a sibling of an IBD patient. Among the controls, only the cecal biopsy specimen set of samples from one individual was positive and only the rectal biopsy set of specimens from one individual was positive (Fig. 1). The specimens from both sites from the rest of the controls were positive. No tissues from any of the Crohn's disease patients, ulcerative colitis patients, or controls were positive for BVD virus, B. pilosicoli, B. hyodysenteriae, circovirus, C. psittaci, or C. burnetii.
FIG. 1.
Samples C2 and C11 retested positive two more times by nested PCR, and all other samples, including sample C8, retested negative two more times by nested PCR for M. avium. The band for sample C8 might have been weakly positive because the amount of the organism present was just barely at the lower detection limit of our nested PCR or the test might have had a false-positive result. In such situations, we reported sample C8 as being negative. All other samples that were processed on the same day before and after samples C2 and C11 were tested consistently retested negative. Several negative samples were randomly chosen and retested negative for M. avium by nested PCR. POS, positive control; neg, negative control. The numbers on the right are in base pairs.
In a separate study to test whether these organisms might be isolated from elderly patients with IBD, samples were obtained from 11 patients over the age of 50 years. This included four subjects with Crohn's disease (two males, two females; mean age, 55.5 ± 1.8 years) and seven subjects with ulcerative colitis (four males, three females; mean age, 61.1 ± 2.2 years). Among these elderly subjects, one patient with ulcerative colitis was positive for M. avium and one was positive for circovirus. This is the first reported identification of circovirus in human tissue.
DISCUSSION
M. avium has been cultured from a small number of patients with Crohn's disease (8, 17, 23), but generally, culture can be unreliable (M. avium has an extremely long incubation time, and there has been poor recovery of M. avium from the tissue of Crohn's disease patients by culture). Thus, investigators have used molecular techniques in search of gene sequences for M. avium. This has been greatly aided by the discovery of the IS900 gene sequence, which is highly specific for M. avium. This insertion sequence is present in multiple copies of 15 to 20 per genome (18). It is critical to distinguish M. avium subsp. paratuberculosis from other M. avium species, some of which can be ubiquitous in the environment and, therefore, in the human gut. Many studies probing for the IS900 gene sequence of M. avium in intestinal tissue have been conducted to date. The results of most of these studies have been negative (1, 6, 7, 10, 15, 30, 32; S. W. P. Wu, C. C. Pao, J. Chan, and T. S. B. Yen, Letter, Lancet 337:174-175, 1991) or similarly positive for cases and controls (36). Some, however, have been positive (13, 16, 25, 26, 33, 37).
In our study we found no association between the detection of M. avium DNA in the mucosas of patients with Crohn's disease and the presence of Crohn's disease and no association between the presence of the DNA of any zoonotic agent sought in this study and Crohn's disease. Circovirus was detected in one ulcerative colitis patient. This is the first report of the detection of this virus in humans and is of uncertain significance. Although the controls were statistically significantly older than the IBD patients, we doubt that this contributed to the higher rate of M. avium positivity for the control group. This higher rate also cannot be explained by a higher rate of residence in a rural area for the controls. In fact, the controls and cases were evenly matched by gender and residence. There is no obvious rationale for why the unrelated population-based controls had a higher rate of M. avium positivity (32%) than either the Crohn's disease or ulcerative colitis patients. We are not suggesting that the mucosal presence of M. avium is protective against the development IBD but are suggesting that these results may merely reinforce the negative association of the identification of M. avium in the mucosas of patient's with Crohn's disease. Alternatively, the detection of a marked disparity between the 32% rate of positivity for M. avium for the controls and the complete absence of positivity for M. avium for patients with Crohn's disease might suggest that patients with Crohn's disease may have been more likely to have eradicated any traces of M. avium, perhaps through repeated courses of antibiotics over the years. This finding might also provide clues to the etiology of Crohn's disease. If M. avium acquisition can ultimately be shown to be associated with childhood behaviors (such as drinking from specific water or milk sources), we may ultimately learn that those environmental influences are protective against the development of Crohn's disease (and, hence, that M. avium isolation is a proxy measure of specific protective lifestyles, without M. avium necessarily being protective itself). Another important consideration is that the standard DNA extraction approach that we used facilitates the detection of the vegetative form of M. avium. If this is not the form that ultimately resides in the intestines of Crohn's disease patients, it may not have been detected in our study. Elsewhere, a combined mechanical-enzymatic disruption technique for DNA extraction has been used to detect lysis-resistant (paucibacillary) forms of M. avium (5). In that study 26% of healthy controls were positive for M. avium (comparable to the 32% rate positivity for the controls in our study), whereas 92% of Crohn's disease patients were positive for M. avium (5). A possible explanation for the comparable rates of M. avium positivity among controls in both studies is that controls may harbor a vegetative form of M. avium, and in the two studies M. avium DNA was extracted by either the standard lysis technique, which we used, or the novel combined mechanical-enzymatic disruption technique. Perhaps recurrent courses of antibiotics or other drugs or simply altered intestinal function among Crohn's disease patients may select out or force a change in the M. avium organisms to a lysis-resistant, paucibacillary form. Whether Crohn's disease tissue that does harbor M. avium harbors it in a vegetative or a paucibacillary form, the existence of an enzyme lysis-resistant form remains to be proven.
A strength of our study was the case-control design, with subjects selected from the whole population. Had we found higher rates of positive PCR findings, there would have been an appropriate context in which to interpret these findings. Whereas colonoscopy provided relatively easy access to tissue specimens, the use of mucosal specimens may be a limitation of our study. Although ingested organisms would confront the mucosal epithelium first, it is plausible that for insidious infections, reactions are mainly established in mesenteric nodes. Furthermore, it remains plausible that Crohn's disease is a vasculitis; hence, if antigens circulate, they may be more likely to be found in the deeper layers of the bowel. Investigators with a keen interest in M. avium have shown that in patients with Crohn's disease M. avium may be a conventional spheroplast and that lysis of a tissue sample from a Crohn's disease patient in sodium dodecyl sulfate-proteinase K or 6 M guanidine thiocyanate, which would reliably release the DNA from most other bacteria, does not do so for M. avium (21).
Finally, these results do not refute the likelihood that animal-borne infections are responsible for Crohn's disease. M. avium was not identified in mucosal specimens of Crohn's disease patients in our study but was identified in mucosal specimens of control subjects. Hence, this may suggest that our findings reflect either a true absence of M. avium from the tissue of Crohn's disease patients (or simply the presence of a form of M. avium not identified by our DNA extraction technique). The use of surgical specimens from the deeper bowel layers and mesenteric lymph nodes is problematic, in that specimens from unbiased adequately matched controls would be difficult to access. However, recently, an M. avium detection system comprising a 10-week incubation of decontaminated tissue followed by IS900-specific PCR of the culture has identified M. avium in full-thickness surgical specimens from Crohn's disease patients (34). The finding of M. avium in breast milk does support the possibility that it can be a systemic infection in humans (27). Hence, the search for a systemic response to M. avium, for instance, by serological assays, may be necessary to establish an association between M. avium and Crohn's disease, if one exists. Nonetheless, at present, the finding of M. avium in 32% of our control specimens and the absence of M. avium from specimens from our Crohn's disease patients argue against an important role for this organism in the etiology of Crohn's disease.
Acknowledgments
Charles Bernstein is supported in part by a Crohn's and Colitis Foundation of Canada Research Scientist Award and a Canadian Institutes of Health Research Investigator Award. James Blanchard is supported by a Canadian Institutes of Health Research Investigator Award. This study was funded in part by a grant (grant MT-15537) from the Canadian Institutes of Health Research and by a grant from the Crohn's and Colitis Foundation of Canada.
We thank Michael Sargent, Cheryl Sachvie, Tracy Scammel-LaFleur, and Cherie Scammel for excellent technical assistance.
REFERENCES
- 1.Al-Shamali, M., I. Khan, B. Al-Nakib, F. Al-Hassan, and A. S. Mustafa. 1997. A multiplex polymerase chain reaction assay for the detection of Mycobacterium paratuberculosis DNA in Crohn's disease tissue. Scand. J. Gastroenterol. 32:819-823. [DOI] [PubMed] [Google Scholar]
- 2.Baca, O. G., and D. Paretsky. 1983. Q fever and Coxiella burnetii: a model for host-parasite interactions. Microbiol. Rev. 47:129-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernstein, C. N., and J. F. Blanchard. 2003. The epidemiology of inflammatory bowel disease, p. 17-32. In R. Cohen (ed.), Inflammatory bowel disease: diagnosis and therapeutics. Humana Press, Totowa, N.J.
- 4.Bernstein, C. N., J. F. Blanchard, P. Rawsthorne, and A. Wajda. 1999. The epidemiology of Crohn's disease and ulcerative colitis in a central Canadian province: a population-based study. Am. J. Epidemiol. 149:916-924. [DOI] [PubMed] [Google Scholar]
- 5.Bull, T. J., E. J. McMinn, K. Sidi-Boumedine, A. Skull, D. Durkin, P. Neild, G. Rhodes, R. Pickup, and J. Herman-Taylor. 2003. Detection and verification of Mycobacterium avium subsp. paratuberculosis in fresh ileocolonic mucosal biopsy specimens from individuals with and without Crohn's disease. J. Clin. Microbiol. 41:2915-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cellier, C., H. DeBeenhouwer, A. Berger, C. Penna, F. Carbonnel, R. Parc, P. H. Cugnec, Y. LeQuintrec, J. P. Gendre, J. P. Barbier, and F. Portaels. 1998. Mycobacterium paratuberculosis and Mycobacterium avium subsp. silvaticum DNA cannot be detected by PCR in Crohn's disease tissue. Gastroenterol. Clin. Biol. 22:675-678. [PubMed] [Google Scholar]
- 7.Chiba, M., T. Fukushima, Y. Horie, M. Iizuki, and O. Masamune. 1998. No Mycobacterium paratuberculosis detected in intestinal tissue, including Peyer's patches and lymph follicles, in Crohn's disease. J. Gastroenterol. 33:482-487. [DOI] [PubMed] [Google Scholar]
- 8.Chiodini, R. J., H. J. Van Kruiningen, W. R. Thayer, R. S. Merkal, and J. A. Coutu. 1984. Possible role of mycobacteria in inflammatory bowel disease. I. An unclassified Mycobacterium species isolated from patients with Crohn's disease. Dig. Dis. Sci. 29:1073-1079. [DOI] [PubMed] [Google Scholar]
- 9.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 10.Clarkston, W. K., M. E. Presti, P. F. Petersen, P. E. Zachary, W. X. Fan, C. L. Leonardi, A. M. Vernava, W. E. Longo, and J. M. Kreeger. 1998. Role of in Crohn's disease. A prospective controlled study using polymerase chain reaction. Dis. Colon Rectum 41:195-199. [DOI] [PubMed] [Google Scholar]
- 11.Council for Agricultural Science and Technology. 2001. Johne's disease in cattle, p. 1-10. Issue paper 17. Council for Agricultural Science and Technology, Ames, Iowa.
- 12.Dalziel, T. K. 1913. Chronic interstitial enteritis. BMJ ii:1068-1070. [Google Scholar]
- 13.Dell'Isola, B., C. Poyart, O. Goulet, J. F. Mougenot, E. Sadoun-Journo, N. Brousse, J. Schmitz, C. Ricour, and P. Berche. 1994. Detection of Mycobacterium paratuberculosis by polymerase chain reaction in children with Crohn's disease. J. Infect. Dis. 169:449-451. [DOI] [PubMed] [Google Scholar]
- 14.Duhamel, G. 2001. Comparative pathology and pathogenesis of naturally acquired and experimentally induced colonic spirochetosis. Anim. Health Res. Rev. 2:3-17. [PubMed] [Google Scholar]
- 15.Dumonceau, J. M., A. Van Gossum, M. Adler, P. A. Fonteyne, J. P. Van Vooren, J. Deviere, and F. Portaels. 1996. No Mycobacterium paratuberculosis found in Crohn's disease using the polymerase chain reaction. Dig. Dis. Sci. 41:421-426. [DOI] [PubMed] [Google Scholar]
- 16.Fidler, H. M., W. Thurrell, N. M. Johnson, G. A. W. Rook, and J. J. McFadden. 1994. Specific detection of Mycobacterium paratuberculosis DNA associated with granulomatous tissue in Crohn's disease. Gut 35:506-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gitnick, G., J. Collins, B. Beaman, D. Brooks, M. Arthur, T. Imaeda, and M. Palieschesky. 1989. Preliminary report on isolation of mycobacteria from patients with Crohn's disease. Dig. Dis. Sci. 34:925-932. [DOI] [PubMed] [Google Scholar]
- 18.Green, E. P., M. L. V. Tizard, M. T. Moss, J. Thompson, D. J. Winterbourne, and J. J. McFadden. 1989. Sequence and characteristics of IS900, an insertion element identified in human Crohn's disease isolate of Mycobacterium paratuberculosis. Nucleic Acids Res. 17:9063-9073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamel, A. L., L. Lin, C. Sachvie, E. Grudeski, and G. P. S. Nayar. 2000. 2000. PCR detection and characterization of type-2 porcine circovirus. Can. J. Vet. Res. 64:44-52. [PMC free article] [PubMed] [Google Scholar]
- 20.Hamel, A. L., M. D. Wasylyshen, and G. P. S. Nayar. 1995. Rapid detection of bovine viral diarrhea virus by using RNA extracted directly from assorted specimens and a one-tube reverse transcription PCR assay. J. Clin. Microbiol. 33:287-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hermon-Taylor, J. 1998. The causation of Crohn's disease and treatment with antimicrobial drugs. Ital. J. Gastroenterol. Hepatol. 30:607-610. [PubMed] [Google Scholar]
- 22.Johnston, W. B., and the Committee of the National Association of State Public Health Veterinarians. 2000. Compendium of measures to control Chlamydia psittaci infection among humans (psittacosis) and pet birds (avian chlamydiosis). Morb. Mortal. Wkly. Rep. 49(RR-8):3-17. [Google Scholar]
- 23.McFadden, J. J., P. D. Butcher, R. Chiodini, and J. Herman-Taylor. 1987. Crohn's disease-isolated mycobacteria are identical to Mycobacterium paratuberculosis, as determined by DNA probes that distinguish between mycobacterial species. J. Clin. Microbiol. 25:796-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNab, W. B., A. H. Meek, J. R. Duncan, S. W. Martin, and A. A. Van Dreumel. 1991. An epidemiological study of paratuberculosis in dairy cattle in Ontario: study design and prevalence estimates. Can. J. Vet. Res. 55:246-251. [PMC free article] [PubMed] [Google Scholar]
- 25.Mishina, D., P. Katsel, S. T. Brown, E. C. A. M. Gilberts, and R. J. Greenstein. 1996. On the etiology of Crohn's disease. Proc. Natl. Acad. Sci. USA 93:9816-9820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moss, M. T., J. D. Sanderson, M. L. V. Tizard, J. Herman-Taylor, F. A. K. El-Zaatari, D. C. Markesich, and D. Y. Graham. 1992. Polymerase chain reaction detection of Mycobacterium paratuberculosis and Mycobacterium avium subsp. silvaticum in long term cultures from Crohn's disease and control tissues. Gut 33:1209-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naser, S., D. Schwartz, and I. Shafran. 2000. Isolation of Mycobacterium avium subsp. paratuberculosis from breast milk of Crohn's disease patients. Am. J. Gastroenterol 95:1094-1095. [DOI] [PubMed] [Google Scholar]
- 28.National Animal Health Monitoring System. 1997. Johne's disease on U.S. dairy operations. Animal Plant and Inspection Service, Veterinary Services, U.S. Department of Agriculture, Fort Collins, Colo.
- 29.Neumaier, M., A. Braun, and C. Wagener. 1998. Fundamentals of quality assessment of molecular amplification methods in clinical diagnostics. Clin. Chem. 44:12-16. [PubMed] [Google Scholar]
- 30.Riggio, M. P., J. Gibson, A. Lennon, D. Wray, and D. G. MacDonald. 1997. Search for Mycobacterium paratuberculosis DNA in orofacial granulomatosis and oral Crohn's disease tissue by polymerase chain reaction. Gut 41:646-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roos, L. L., C. A. Mustard, and J. P. Nicol. 1993. Registries and administrative data: organization and accuracy. Med. Care 3:201-212. [DOI] [PubMed] [Google Scholar]
- 32.Rowbotham, D. S., N. P. Mapstone, L. K. Trejdosiewicz, P. D. Howdle, and P. Quirke. 1995. Mycobacterium paratuberculosis DNA not detected in Crohn's disease tissue by fluorescent polymerase chain reaction. Gut 37:660-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanderson, J. D., M. T. Moss, M. L. V. Tizard, and J. Herman-Taylor. 1992. Mycobacterium paratuberculosis DNA in Crohn's disease tissue. Gut 33:890-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz, D., I. Shafran, C. Romero, C. Piromalli, J. Biggerstaff, N. Naser, W. Chamberlin, and S. A. Naser. 2000. Use of short-term culture for identification of Mycobacterium avium subsp. paratuberculosis in tissue from Crohn's disease patients. Clin. Microbiol. Infect. 6:303-307. [DOI] [PubMed] [Google Scholar]
- 35.Stabel, J. R. 1998. Johne's disease: a hidden threat. J. Dairy Sci. 81:283-288. [DOI] [PubMed] [Google Scholar]
- 36.Suenaga, K., Y. Yokoyama, K. Okazaki, and Y. Yamamoto. 1995. Mycobacteria in the intestine of Japanese patients with inflammatory bowel disease. Am. J. Gastroenterol. 90:76-80. [PubMed] [Google Scholar]
- 37.Tivelung, A., J. D. Soderholm, G. Olaison, J. Jonasson, and H. J. Monstein. 1999. Presence of eubacteria in biopsies from Crohn's disease inflammatory lesions as determined by 16S rRNA gene-based PCR. J. Med. Microbiol. 48:263-268. [DOI] [PubMed] [Google Scholar]