Abstract
We identified 29 yeast isolates from 22 patients using the API ID32C panel. Twenty-eight of these isolates were Candida norvegensis and one was C. inconspicua. Although C. norvegensis is considered a pseudohypha-producing species, only one isolate produced pseudohyphae. Restriction enzyme analysis of PCR-amplified ribosomal DNA with four different enzymes proved that all isolates were C. inconspicua.
The frequency of human infections caused by yeasts is increasing. Candida albicans is still the opportunistic pathogenic yeast most frequently isolated from humans with fungal diseases; however, several non-C. albicans Candida species are also cultured more and more frequently from different body sites (3, 15). Preliminary identification of non-C. albicans Candida species has been incorporated into the majority of routine laboratory algorithms (18). In doubtful cases, additional techniques are required to obtain a precise identification of the isolated species, e.g., the determination of different biochemical patterns with the API 20C and API ID32C systems (API/ATB package inserts, BioMerieux, 1996). Recently, molecular biology techniques have been introduced for the more precise identification of Candida isolates (21, 22). This urgent need is based on at least two factors: the spread of Candida species resistant to antifungal agents (6) and the threat of nosocomial Candida outbreaks (4). An additional advantage of these techniques is the ability to differentiate between closely related species or previously misidentified yeasts.
C. inconspicua and C. norvegensis are rarely isolated opportunistic pathogens (17, 20). During the past 5 years, the number of yeast isolates identified in the Laboratory for Bacteriological Diagnostics, University of Debrecen, as C. inconspicua or C. norvegensis increased from 3 to 4 per year to 29 per year (in 2001), while the overall increase in the rate of yeast isolation was only threefold (9). We could not reliably distinguish the two species by traditional identification methods (with the API ID32C system and detection of pseudohypha production) because the species identities obtained by different methods were contradictory. To precisely identify our isolates we applied ribosomal DNA (rDNA) analysis.
The genetic background of the method chosen can be summarized as follows. The tandemly repeated rDNA genes of yeasts contain highly conservative and less conservative regions. Sequences coding for the 18S, 5.8S, and 25S rRNAs evolved slowly during the phylogenesis of fungi; thus, they are suitable for use as tools for discrimination and identification at the interspecies or higher level. On the contrary, the internal transcribed spacer region 1 (ITS1) and ITS2 rDNA regions are more variable; thus, they can serve as a tool in the discrimination of closely related yeast species (21). Restriction enzyme analysis of PCR-amplified rDNA is a superior typing method suitable for correct identification, even with a large number of yeast isolates. In addition, this method can correct misidentification results, which frequently occur during routine identification based on morphological and biochemical tests (22).
Of 22 patients sampled, 5 were outpatients, while 17 were hospitalized (Medical School, University of Debrecen). The outpatients had serious vaginitis and mycotic tonsillitis. Fourteen of the 17 hospitalized patients had some condition that predisposed them to infection (i.e., organ transplantation, malignancy, cystic fibrosis, or diabetes mellitus). The inpatients were treated (and sampled) at different times (there was generally a time difference of at least a month between two patients) and at various clinics of the university, which are situated in separate buildings.
The samples yielding the yeast isolates studied were obtained for routine microbiological examination. The majority of the specimens were of the airway, with a few urine, wound, and genital swab samples also obtained. The specimens were plated directly onto Sabouraud dextrose agar (SDA) containing 500 μg of chloramphenicol per ml and were incubated for 24 h at 37°C, followed by an additional incubation for 13 days at room temperature. The colonies on SDA were transferred to CHROMagar Candida (Becton Dickinson). Preliminary identification was based on colony color. The germ tube test (19) was also performed. We also examined the colonies for pseudohypha formation on rice-agar-Tween (RAT) medium (Dalmau technique) (13). For species identification, carbohydrate assimilation profiles were determined by the using API ID32C panel (BioMerieux). We used C. inconspicua ATCC 16783, C. norvegensis ATCC 22977, and C. krusei ATCC 6258 type strains as reference strains. Yeast cultures were maintained on SDA at 4°C and in SDA-glycerol medium at −70°C. Fluconazole MICs were determined by use of the M27-A NCCLS standard (11).
Total yeast DNA was isolated by the method of Hoffman and Winston (5). Primers NS3 and ITS4 were used for the amplification by PCR of an rDNA sequence approximately 1,900 bp long. The sequence comprises a part of small rDNA, the 5.8S rDNA, and the two ITS regions (21). The PCR was carried out in a 30-μl reaction mixture containing 3 μl of 10× PCR buffer, 1.5 μl of 50 mM MgCl2, 0.1 μl of 25 mM (each) deoxynucleoside triphosphates, 1.6 U of DyNAzyme II DNA polymerase (Finnzymes), 1 μl of primers (ca. 2 pmol), and 4 μl of template (yeast) DNA. Amplification was performed by using the PCR Sprint Thermal Cycler (Hybaid Ltd., Teddington, England) and standard conditions with 61.5°C as the annealing temperature. The amplified products were digested with the restriction enzymes MspI, RsaI, ScrFI, and HaeIII (New England BioLabs) in separate reactions. The DNA fragments generated by the restriction enzymes were separated by conventional agarose gel electrophoresis. The gel was digitized, photographed with Gel Documentation Equipment (Bio-Rad), and analyzed with the Molecular Analyst Program (Bio-Rad).
We isolated 29 yeasts from the 22 patients sampled. All formed round white colonies on CHROMagar Candida, similar to C. norvegensis ATCC 22977 and C. inconspicua ATCC 16783, while C. krusei ATCC 6258 grew as rough spreading pink colonies. The fluconazole MICs for the clinical isolates varied between 16 and 64 μg/ml; 25 isolates were dose-dependent susceptible to fluconazole, and 4 were resistant to fluconazole. Identification to the species level was performed by the API ID32C test (BioMerieux) and determination of pseudohypha production on RAT medium, as summarized in Table 1. Twenty-seven of the 29 isolates were identified as C. inconspicua/C. norvegensis, similar to the C. inconspicua and C. norvegensis reference strains. The biocodes for the remaining two isolates corresponded to an “acceptable identification” for the Candida genus only and suggested that the isolates were either C. krusei, C. inconspicua, or C. norvegensis. According to the API ID32C identification system, esculin hydrolysis should be tested to further differentiate among these three species. C. norvegensis hydrolyzes esculin, while C. inconspicua and C. krusei do not. Of our 29 clinical isolates, 28 hydrolyzed esculin, as did the C. norvegensis ATCC 22977 reference strain, while 1 clinical isolate, C. inconspicua ATCC 16783, and C. krusei ATCC 6258 did not (Table 1). On the basis of the results obtained with the API ID32C system, 28 clinical isolates were identified as C. norvegensis and one was identified as C. inconspicua. All three reference strains were correctly identified by the API ID32C system.
TABLE 1.
Phenotypic characteristics of clinical isolates
| Patients | Biocode | No. of isolates
|
|||
|---|---|---|---|---|---|
| Esculin hydrolysis
|
Pseudohypha production
|
||||
| + | − | + | − | ||
| Outpatients | 0200010005 | 4 | 0 | 0 | 4 |
| 0200010001 | 1 | 0 | 0 | 1 | |
| Inpatients | 0200010005 | 22 | 1 | 1a | 22 |
| 0200010001 | 1 | 0 | 0 | 1 | |
| Total | 28 | 1 | 1 | 28 | |
This isolate also hydrolysed esculin.
All Candida isolates failed to produce chlamydospores and germ tubes. Pseudohypha formation on Dalmau plates was negative for all isolates except the C. krusei reference strain and one clinical isolate, which became positive on the 2nd and 9th days after inoculation, respectively. As C. krusei and C. norvegensis have been described to produce pseudohyphae on Dalmau plates (7, 8) and C. inconspicua has not (10), the pseudohypha production findings contradict the results of the biochemical tests mentioned above.
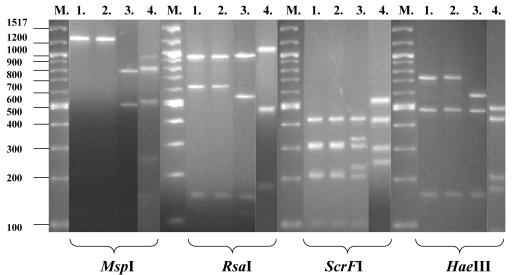
Restriction enzyme analysis of the PCR-amplified rDNA regions produced three typical patterns with any of the four enzymes applied. One pattern was typical for the C. inconspicua ATCC 16783 strain and all 29 clinical isolates, the second pattern was typical for the C. norvegensis ATCC 22977 reference strain, and the third pattern was typical for the C. krusei ATCC 6258 reference strain. Typical patterns are illustrated in Fig. 1. This result clearly indicates that the 28 isolates identified as C. norvegensis by the API ID32C system belong to C. inconspicua.
FIG. 1.
RFLP patterns of PCR-amplified rDNA obtained from one clinical isolate and the type strains of C. inconspicua, C. norvegensis, and C. krusei. Lane M, molecular size standard; lane 1, isolate N-22838; lane 2, C. inconspicua ATCC 16783; lane 3, C. norvegensis ATCC 22977; lane 4, C. krusei ATCC 6258. MspI, RsaI, ScrFI, and HaeIII were the restriction enzymes used to digest the PCR products.
The dramatic increase in human fungal infections is well documented (4, 9). However, judgment of the etiological role of an isolated fungus is very difficult and requires consideration of the individual isolate (9). This report represents a further example of the importance of correct identification of clinical yeast isolates.
Use of only the esculin hydrolysis probe from the 31 biochemical tests of the API ID32C panel is recommended to discriminate between C. inconspicua and C. norvegensis. According to our results, this characteristic is variable among the members of the species C. inconspicua; only one of the clinical isolates and the type strain gave the expected result. C. krusei can cause an additional complication in routine laboratory work, because this yeast frequently shows assimilation profiles similar to those of C. inconspicua and C. norvegensis (2, 12). Fortunately, the commercial medium CHROMagar Candida greatly facilitates the identification of C. krusei.
Interestingly, one clinical isolate of C. inconspicua produced pseudohyphae on RAT medium. Thus, pseudohypha production also seems to be an unreliable diagnostic tool for discrimination of esculin-negative C. inconspicua and C. krusei strains, which has been described earlier (13). Nho et al. (12) have also demonstrated the unreliability of pseudohypha production.
As the two phenotype-based identification methods applied gave contradictory results, we used rDNA analysis for definitive species identification. A similar approach to the identification of these biochemically similar three yeast species has been described by Nho et al. (12), who examined C. inconspicua, C. norvegensis, and C. krusei strains obtained from the BioMerieux stock collection. They demonstrated that HhaI cuts the PCR products generated by the ITS1- and ITS4-specific primers into six small restriction fragments for both C. inconspicua (esculin negative) and C. norvegensis (esculin positive), and differences in restriction fragment lengths determined by sequencing were used for discrimination of the two species. They obtained four fragments for C. krusei. Our method, which uses different primers and restriction enzymes, proved to be capable of distinguishing the three species without sequencing of the restriction fragments.
Several investigators have postulated that the observed shift in the distribution of yeast isolates in clinical samples may be due to the widespread use of fluconazole, which leads to the emergence of yeasts less susceptible to fluconazole (14). In addition to C. krusei, C. inconspicua and C. norvegensis are also considered to possess decreased susceptibility to fluconazole (1, 16, 20), which is confirmed by our results for C. inconspicua.
While a significant amount of data on C. krusei has been gathered in the past few years, the information available on C. inconspicua and C. norvegensis is scant. To address the question of the occurrences and the roles of C. inconspicua and C. norvegensis in human disease as well as the importance of their fluconazole resistance, exact identification and reliable differentiation of the two species are required. These facts also underline the growing need for the application of molecular biology methods during identification.
Acknowledgments
We thank our colleagues Cecília Miszti and Erzsébet Falusi for valuable help in performing the experiments.
REFERENCES
- 1.Baily, G. G., C. B. Moore, S. M. Essayag, S. de Wit, J. P. Burnie, and D. W. Denning. 1997. Candida inconspicua, a fluconazole-resistant pathogen in patients infected with human immunodeficiency virus. Clin. Infect. Dis. 25:161-163. [DOI] [PubMed] [Google Scholar]
- 2.Essayag, S. M., G. G. Baily, D. W. Denning, and J. P. Burnie. 1996. Karyotyping of fluconazole resistant yeasts with phenotype reported as Candida krusei or Candida inconspicua. Int. J. Syst. Bacteriol. 46:35-40. [DOI] [PubMed] [Google Scholar]
- 3.Fidel, P. L., Jr., J. A. Vazquez, and J. D. Sobel. 1999. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin. Microbiol. Rev. 12:80-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fridkin, S. K., and W. R. Jarvis. 1996. Epidemiology of nosocomial fungal infections. Clin. Microbiol. Rev. 9:499-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffman, C., and F. Winston. 1987. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 57:267-272. [DOI] [PubMed] [Google Scholar]
- 6.Kontoyiannis, D. P., and R. E. Lewis. 2002. Antifungal drug resistance of pathogenic fungi. Lancet 359:1135-1144. [DOI] [PubMed] [Google Scholar]
- 7.Kurtzman, C. P. 1998. Issatchenkia Kudryavtsev emend. Kurtzman, Smiley and Johnson, p. 221-226. In C. P. Kurtzmann and J. W. Fell (ed.), The yeasts, a taxonomic study. Elsevier, Amsterdam, The Netherlands.
- 8.Kurtzman, C. P. 1998. Pichia E. C. Hansen emend. Kurtzman, p. 273-352. In C. P. Kurtzmann and J. W. Fell (ed.), The yeasts, a taxonomic study. Elsevier, Amsterdam, The Netherlands.
- 9.Majoros, L., G. Kardos, I. Pócsi, and B. Szabó. 2002. Distribution and susceptibility of Candida species isolated in the Medical University of Debrecen. Acta Microbiol. Immunol. Hung. 49:351-361. [DOI] [PubMed] [Google Scholar]
- 10.Meyer, S. A., R. W. Payne, and D. Yarrow. 1998. Candida Berkhout, p. 454-573. In C. P. Kurtzmann and J. W. Fell (ed.), The yeasts, a taxonomic study. Elsevier, Amsterdam, The Netherlands.
- 11.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard M27-A. National Committee for Clinical Laboratory Standards Wayne, Pa.
- 12.Nho, S., M. J. Anderson, C. B. Moore, and D. W. Denning. 1997. Species differentiation by internally transcribed spacer PCR and HhaI digestion of fluconazole-resistant Candida krusei, Candida inconspicua, and Candida norvegensis strains. J. Clin. Microbiol. 35:1036-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Odds, F. C., M. G. Rinaldi, C. L. Cooper, Jr., A. Fothergill, L. Pasarell, and M. R. McGinnis. 1997. Candida and Torulopsis: a blinded evaluation of use of pseudohypha formation as basis for identification of medically important yeasts. J. Clin. Microbiol. 35:313-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orozco, A. S., L. M. Higginbotham, C. A. Hitchcock, T. Parkinson, D. Falconer, A. S. Ibrahim, M. A. Ghannoum, and S. G. Filler. 1998. Mechanism of fluconazole resistance in Candida krusei. Antimicrob. Agents Chemother. 42:2645-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samaranayake, Y. H., and L. P. Samaranayake. 1994. Candida krusei: biology, epidemiology, pathogenicity and clinical manifestations of an emerging pathogen. J. Med. Microbiol. 41:295-310. [DOI] [PubMed] [Google Scholar]
- 16.Sandven, P., K. Nilsen, A. Digranes, T. Tjade, and J. Lassen. 1997. Candida norvegensis: a fluconazole-resistant species. Antimicrob. Agents Chemother. 41:1375-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan, D. J., M. C. Henman, G. P. Moran, L. C. O'Neill, D. E. Bennett, D. B. Shanley, and D. C. Coleman. 1996. Molecular genetic approaches to identification, epidemiology and taxonomy of non-albicans Candida species. J. Med. Microbiol. 44:399-408. [DOI] [PubMed] [Google Scholar]
- 18.Szabó, B., L. Majoros, and C. Miszti. 2000. Microbiological diagnosis of fungal infections, p. 198-213. In L. Maródi (ed.), Emerging infectious diseases in Eastern-Central Europe. Medicina Publishing House Co., Budapest, Hungary.
- 19.Warren, N. G., and H. J. Shadomy. 1991. Yeasts of medical importance, p. 617-629. In A. Balows, W. J. Hausler, Jr., K. L. Herrmann, H. D. Isenberg, and H. J. Shadomy (ed.), Manual of clinical microbiology, 5th ed. American Society for Microbiology, Washington, D.C.
- 20.White, T. C., K. A. Marr, and R. A. Bowden. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White, T. J., T. Bruns, S. Lee, and J. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols: a guide to methods and applications. Academic Press, Inc., San Diego, Calif.
- 22.Williams, D. W., M. J. Wilson, M. A. O. Lewis, and J. C. Potts. 1995. Identification of Candida species by PCR and restriction fragment length polymorphism analysis of intergenic spacer regions of ribosomal DNA. J. Clin. Microbiol. 33:2476-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]