Abstract
Dengue type 3 viruses were isolated from patients in Martinique between 1999 and 2002. This serotype had not been detected on the island in the last 20 years. Genomic sequence determination and analysis showed great stability of the virus during the period studied.
Dengue virus (DENV) infection is recognized as a major public health problem. An estimated 100 millions humans are infected each year worldwide (13), and the incidence of the severe, sometimes lethal, forms of the disease is increasing (5). DENV is a mosquito-borne flavivirus with a single-stranded, nonsegmented, positive-sense RNA genome approximately 11 kb long. There are four antigenically distinct DENV serotypes, 1 to 4 (17). Infection with any of these serotypes leads to a large clinical spectrum, ranging from subclinical infection or classical dengue fever (DF) to severe forms (dengue hemorrhagic fever [DHF] and dengue shock syndrome [DSS]). Possible roles of antibody-dependent enhancement of infection (6), increased pathogenicity of viral strains (19), or other possible unidentified factors in promoting DHF and DSS have been proposed.
Like other Caribbean islands, Martinique experiences dengue epidemics. Since the Cuban epidemic in 1981, DHF and DSS have been frequently reported in the region (the Caribbean, northern South America, Central America, and Mexico). However, since the first report of DHF and DSS in Martinique in 1995 (12), DHF has been rarely reported, contrary to observations from other countries in the region (24; http://www.carec.org/data/dengue).
However, factors potentially responsible for an increase in severe forms of dengue infections have been identified in Martinique. DENV type 2 (DENV-2) was principally responsible for the 1998 epidemic. Sequence determination established that it belongs to a recently identified genotype of the American region whose presence correlates with the increase in DHF and DSS in South and Central America (18, 22). Between November 1999 and December 2001, DENV-3 reappeared on the island and became prominent. During this period, 97 French soldiers presenting with dengue-like syndromes were received in the Infirmerie du Bataillon d'infanterie de marine. A total of 147 blood samples withdrawn at early and late stages of the infection were received in our laboratory in Marseilles. All of the samples were found to be positive for dengue infection by the presence of immunoglobulin M (IgM), the elevation of specific IgG, or both, using dengue virus-specific enzyme-linked immunosorbent assay (ELISAs) developed in house (MAC ELISA for IgM and sandwich ELISA for IgG). Virus isolation by coincubation of white blood cells with C6/36 cells (22) was positive in 29 patients. DENV-3 was identified by indirect immunofluorescence assay with serotype-specific monoclonal antibodies (kindly provided by Nick Karabatsos, Centers for Disease Control and Prevention, Fort Collins, Colo.). The 97 patients developed DF and recovered fully.
The population of Martinique also suffered from the disease, and between September 2001 and January 2002, DENV transmission became epidemic, with 24,000 patients suspected of having dengue (3). Of these patients, 217 were hospitalized with at least one symptom of severe infection (elevated temperature, malaise, or thrombocytopenia). Serum samples from hospitalized patients underwent reverse transcription (RT)-PCR at the Fort de France University Hospital in accordance with a previously published protocol (9), as did another 154 serum samples from outpatients. Of the 371 RT-PCR assays performed, 133 indicated DENV-3 infection. A DENV-2-positive RT-PCR result was found only once. Despite the existence of symptoms of severe infection in the 217 hospitalized patients, only three fatal cases were classified as DHF or DSS in accordance with World Health Organization criteria (1). In relation to the total number of suspected cases (clinical evidence of dengue infection without virological confirmation), the incidence of DHF during the epidemic was lower than 0.02%, an unexpected value in view of the presence of potentially more pathogenic DENV-2 and the recent reintroduction of DENV-3.
The virus strain isolated in December 1999 from a military patient was the first DENV-3 strain isolated in Martinique in 20 years (3, 15). The complete sequence of this DENV strain, named D3Mart1243, was determined as previously described (22) (Table 1). Between November 1999 and December 2001, the 97 military cases constituted a homogeneous group with regard to the clinical course and basic virological parameters (serotype, cytopathogenic effect on Vero cells) when virus isolation was positive. Of the 28 isolates, 5 were chosen randomly for partial sequencing, 2 of the year 2000 isolates and 3 of the year 2001 isolates (Table 1). Nucleotide and amino acid sequence positions were numbered by reference to the sequence of strain D3PhilH87 (16). Alignments of nucleotide and deduced amino acid sequences were carried out with the ClustalW1.7 software (21).
TABLE 1.
Sequenced DENV-3 strains and corresponding GenBank accession numbers
| Identification no. | Sequenced region | GenBank accession no. | Isolation date |
|---|---|---|---|
| D3Mart1243 | Complete | AY099337 | December 1999 |
| D3Mart1567 | 5′ end, 1-2878 | AY099338 | April 2000 |
| 3′ end, 8201-10707 | AY099343 | ||
| D3Mart1706 | 5′ end, 1-2878 | AY099339 | October 2000 |
| 3′ end, 8201-10707 | AY099344 | ||
| D3Mart2012 | 5′ end, 1-2878 | AY099340 | September 2001 |
| 3′ end, 8201-10707 | AY099345 | ||
| D3Mart2023 | 5′ end, 1-2878 | AY099341 | October 2001 |
| 3′ end, 8201-10707 | AY099346 | ||
| D3Mart2336 | 5′ end, 1-2878 | AY099342 | December 2001 |
| 3′ end, 8201-10707 | AY099347 | ||
| D3SriLa1266 | Complete | AY099336 | September 2000 |
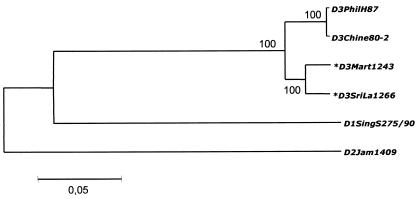
Genetic comparison and phylogenetic analyses including the complete sequences of viruses of different origins available in the GenBank database showed a close relationship between D3Mart1243 and DENV-3 reference strain D3PhilH87, despite a 43-year time lapse and the geographical distance separating the locations where they were isolated (Fig. 1). Moreover, only 310 nucleotide mutations and 39 amino acid substitutions differentiated D3Mart1243 and D3SriLa1266, a strain that was isolated from a tourist infected in Sri Lanka in 2000.
FIG. 1.
Phylogenetic tree of DENV-3 based on complete nucleotide sequences. Phylograms were constructed with the MEGA program (8) by using the Jukes-Cantor algorithm and the neighbor-joining method. The percentage of successful bootstrap replicates (500 bootstrap replications, confidence probability greater than 90%) is indicated at nodes. The length of branches is proportional to the number of nucleotide changes (percent divergence). The strains sequenced in this work are indicated by asterisks.
Comparison of partial nucleotide sequences showed a high level of identity between the strains from Martinique (99.5 to 99.9% paired identity), which all have an 11-nucleotide-long insertion between positions 10275 and 10276 in the 3′ untranslated region (AGTGAAAAAGA). Interestingly, the same insertion was found in D3SriLa1266, with a one-base difference (AGTGGAAAAGA), indicating that it probably has a recent ancestor in common with the Martinique viruses.
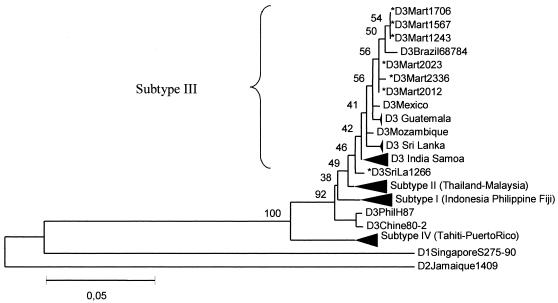
A phylogenetic tree was constructed on the basis of partial sequences of the prM/M-E gene region (positions 437 to 1144) of the genomes, including the strains from Martinique and several others from different origins previously characterized (Fig. 2) (7, 10, 23). DENV isolates from Martinique and Sri Lanka were grouped into subtype 3, as defined by Lanciotti (10), which also includes isolates from India, Samoa, Mozambique, Brazil (17), Guatemala (23), and Mexico. The six isolates from Martinique clustered together. An analysis of the deduced amino acid sequences generated a similar phylogram. Altogether, these results could indicate the existence of a genotype specific to Martinique, close to the Brazil isolate. They also indicate a common origin for all of the DENV-3 strains circulating in the American and Caribbean region, their ancestor probably originating from Southeast Asia. New sequences of American DENV-3 strains, encompassing the insertion site, should help to confirm this hypothesis and identify the factors influencing the evolution of the virus in the area, as was recently done for DENV-4 (4).
FIG. 2.
Phylogenetic tree of DENV-3 strains based on prM/M and partial E nucleotide sequences (nucleotides 437 to 1144). For the Mexican isolate, only the sequence between positions 437 and 766 was available. The strains sequenced in this work are indicated by asterisks. Dark triangles correspond to viruses of the same subtype clustering together. DENV-1 and -2 sequences were introduced for correct rooting of the tree.
Two decades ago, DENV-3 strains prevalent in the Caribbean belonged to subtype 4 (20). Viruses belonging to subtype 3 were first reported in Nicaragua and Panama in 1994 (2). They were then identified in Guatemala between 1996 and 1998 and in Puerto Rico, Barbados, and Jamaica in 1998 (15, 23). As was feared, importation of DENV-3 into Martinique occurred rapidly and was first detected in 1999. As the DENV-3 strain isolated in 1999 was the first isolated in Martinique in 20 years, it was likely imported recently. However, the virus seems to have quickly spread and almost completely replaced the previous DENV-1 and -2 serotypes. Alternatively, the persistence of DENV-3 through the 2-year period studied, overlapping epidemic and interepidemic periods, may be due to reimportation of closely related viruses from the neighboring islands or countries. In any case, the identified virus genotype exhibits great stability and good adaptation to local conditions. In this regard, sequencing of the DENV-3 strains recently detected in Brazil (11), Paraguay (http://www.promedmail.org), or Argentina (http://www.promedmail.org) should lead to specification of the geographic distribution of the Martinique genotype.
Acknowledgments
We are indebted to F. Tock, H. Pugelli, B. Pastorino, and A. Buguet for assistance in virus isolation, primer synthesis, sequence comparisons, and manuscript revision.
This work was partially funded by the French Armed Forces Medical Service and the French “Délégation Générale pour l'Armement” (contract 02 CO 013).
The opinions and assertions contained herein are those of the authors and are not to be construed as official or reflecting the views of the French Armed Forces Medical Service or the French Army at large.
REFERENCES
- 1.Anonymous. 1986. La dengue hémorragique: diagnostic, traitement et moyens de lutte p. 8-18. World Health Organization, Geneva, Switzerland.
- 2.Balmaseda, A., E. Sandoval, L. Perez, C. M. Gutierrez, and E. Harris. 1999. Application of molecular typing techniques in the 1998 dengue epidemic in Nicaragua. Am. J. Trop. Med. Hyg. 61:893-897. [DOI] [PubMed] [Google Scholar]
- 3.Cicchelero, V., and A. Yebakima. 2002. Bilan de l'épidémie de dengue en Martinique septembre 2001-janvier 2002. Technical report. DSDS, inspection de la Santé, Fort de France, Région Martinique, France.
- 4.Foster, J. E., S. N. Bennett, H. Vaughan, V. Vorndam, W. O. McMillan, and V. F. Carrington. 2003. Molecular evolution and phylogeny of dengue type 4 virus in the Caribbean. Virology 306:126-134. [DOI] [PubMed] [Google Scholar]
- 5.Guzman, M. G., and G. Kouri. 2002. Dengue: an update. Lancet Infect. Dis. 2:33-42. [DOI] [PubMed] [Google Scholar]
- 6.Halstead, S. B. 1970. Observations related to pathogenesis of dengue hemorrhagic fever. VI. Hypotheses and discussion. Yale J. Biol. Med. 42:350-362. [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi, N., R. Thayan, C. Sugimoto, K. Oda, Z. Saat, B. Vijayamalar, M. Sinniah, and A. Igarashi. 1999. Type-3 dengue viruses responsible for the dengue epidemic in Malaysia during 1993-1994. Am. J. Trop. Med. Hyg. 60:904-909. [DOI] [PubMed] [Google Scholar]
- 8.Kumar, S., K. Tamura, and M. Nei. 1993. Molecular evolutionary genetics analysis, version 1.01. Pennsylvania State University, University Park.
- 9.Lanciotti, R. S., C. H. Calisher, D. J. Gubler, G. J. Chang, and A. V. Vorndam. 1992. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J. Clin. Microbiol. 30:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanciotti, R. S., J. G. Lewis, D. J. Gubler, and D. W. Trent. 1994. Molecular evolution and epidemiology of dengue-3 viruses. J. Gen. Virol. 75(Pt. 1):65-75. [DOI] [PubMed] [Google Scholar]
- 11.Lourenco-de-Oliveira, R., N. A. Honorio, M. G. Castro, H. G. Schatzmayr, M. P. Miagostovich, J. C. Alves, W. C. Silva, P. J. Leite, and R. M. Nogueira. 2002. Dengue virus type 3 isolation from Aedes aegypti in the municipality of Nova Iguacu, State of Rio de Janeiro. Mem. Inst. Oswaldo Cruz 97:799-800. [DOI] [PubMed] [Google Scholar]
- 12.Mansuy, J. M., R. Delor, H. Mehdaoui, and L. Elizabeth. 1996. First case of dengue hemorrhagic fever with shock syndrome in Martinique. Bull. Soc. Pathol. Exot. 89:243-244. [PubMed] [Google Scholar]
- 13.McBride, W. J., and H. Bielefeldt-Ohmann. 2000. Dengue viral infections: pathogenesis and epidemiology. Microbes Infect. 2:1041-1050. [DOI] [PubMed] [Google Scholar]
- 14.Miagostovich, M. P., F. B. dos Santos, T. S. de Simone, E. V. Costa, A. M. Filippis, H. G. Schatzmayr, and R. M. Nogueira. 2002. Genetic characterization of dengue virus type 3 isolates in the State of Rio de Janeiro, 2001. Braz. J. Med. Biol. Res. 35:869-872. [DOI] [PubMed] [Google Scholar]
- 15.Nadeau, Y., A. Yébakima, A. Blateau, and P. Chaud. 1999. Spécial dengue 1998. Technical report. DDASS Martinique. Bulletin du réseau des médecins sentinelles no. 10. DDASS Martinique, Fort de France, Région Martinique, France.
- 16.Osatomi, K., I. Fuke, D. Tsuru, T. Shiba, Y. Sakaki, and H. Sumiyoshi. 1988. Nucleotide sequence of dengue type 3 virus genomic RNA encoding viral structural proteins. Virus Genes 2:99-108. [DOI] [PubMed] [Google Scholar]
- 17.Rice, C. M. 1996. Flaviviridae: The viruses and their replication, p. 931-1034. In B. N. Field, D. M. Knipe, P. M. Howley, R. M. Chanock, J. L. Melnick, T. P. Monath, B. Roizman, and S. E. Straus (ed.), Virology. Lippincott-Raven, Philadelphia, Pa.
- 18.Rico-Hesse, R., L. M. Harrison, R. A. Salas, D. Tovar, A. Nisalak, C. Ramos, J. Boshell, M. T. de Mesa, R. M. Nogueira, and A. T. da Rosa. 1997. Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas. Virology 230:244-251. [DOI] [PubMed] [Google Scholar]
- 19.Rosen, L. 1977. The Emperor's New Clothes revisited, or reflections on the pathogenesis of dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 26:337-343. [DOI] [PubMed] [Google Scholar]
- 20.Sherer, W. F., R. W. Dickerman, and J. V. Ordonez. 1977. Serologic surveys for antibodies to western, eastern, California and St. Louis encephalitis and dengue 3 arboviruses in middle America. Bull. Pan Am. Health Organ. 11:212-223. [PubMed] [Google Scholar]
- 21.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tolou, H., P. Couissinier-Paris, V. Mercier, M. R. Pisano, X. de Lamballerie, P. de Micco, and J. P. Durand. 2000. Complete genomic sequence of a dengue type 2 virus from the French West Indies. Biochem. Biophys. Res. Commun. 277:89-92. [DOI] [PubMed] [Google Scholar]
- 23.Usuku, S., L. Castillo, C. Sugimoto, Y. Noguchi, Y. Yogo, and N. Kobayashi. 2001. Phylogenetic analysis of dengue-3 viruses prevalent in Guatemala during 1996-1998. Arch. Virol. 146:1381-1390. [DOI] [PubMed] [Google Scholar]
- 24.Uzcategui, N. Y., D. Camacho, G. Comach, R. Cuello de Uzcategui, E. C. Holmes, and E. A. Gould. 2001. Molecular epidemiology of dengue type 2 virus in Venezuela: evidence for in situ virus evolution and recombination. J. Gen. Virol. 82:2945-2953. [DOI] [PubMed] [Google Scholar]