Abstract
We report the first two cases of invasive human mycoses caused by the phaeoid ascomycete, Chaetomium perlucidum, and review the English literature regarding invasive Chaetomium infections. Fatal disseminated disease involving the brain, heart, lungs, and spleen is described in an acute myelogenous leukemia patient. A second patient with a history of asthma and chronic bronchiectasis experiencing right-middle-lobe syndrome grew C. perlucidum from lung tissue. This study adds C. perlucidum to the list of other known neurotropic Chaetomium species, C. atrobrunneum and C. strumarium, and also documents this organism's ability to disseminate beyond the central nervous system.
CASE REPORTS
Case 1.
A 45-year-old female patient with acute myelogenous leukemia was admitted to a hospital in Denver, Colo., for an unrelated, 4/5 HLA-matched umbilical cord blood transplant. On day −1, the patient was noted to be febrile. She was empirically placed on broad-spectrum antibiotic therapy, including liposomal amphotericin B (1 mg/kg/day). On day 10, the patient became disoriented and febrile, and a new pulmonary infiltrate was noted on a chest radiograph. Liposomal amphotericin B was increased to 3 mg/kg/day. Computed tomography of the chest revealed a 3- by 2-cm mass in the right lower lobe. Wide surgical resection of the right-lower-lobe lesion revealed infarction, and tissue analysis demonstrated hyphae presumed to represent aspergillosis. Six days later, the patient suffered a massive right-sided intraparenchymal hemorrhage and died 2 days later. Autopsy revealed disseminated invasive fungal infection involving the lungs, brain, and myocardium. Cultures obtained from the surgically obtained lung tissue grew Chaetomium perlucidum.
Case 2.
A 78-year-old female with a history of asthma and chronic bronchiectasis was admitted to a Denver hospital with pneumonia. Sputum cultures grew Pseudomonas and Proteus species. The patient was treated with broad-spectrum antibiotics but developed right-middle-lobe syndrome. She then underwent a lobectomy due to worsening symptoms despite presumed effective therapy. Cultures from the lung tissue grew C. perlucidum. No bacteria were isolated in culture. The histopathology of the lung wedge biopsy revealed honey-combing and nonspecific inflammation. Gomori methenamine silver (GMS) and Ziehl acid-fast bacillus stain analyses were negative. The patient did not receive antifungal therapy and had no further manifestations of disease after the lobectomy.
Autopsy findings.
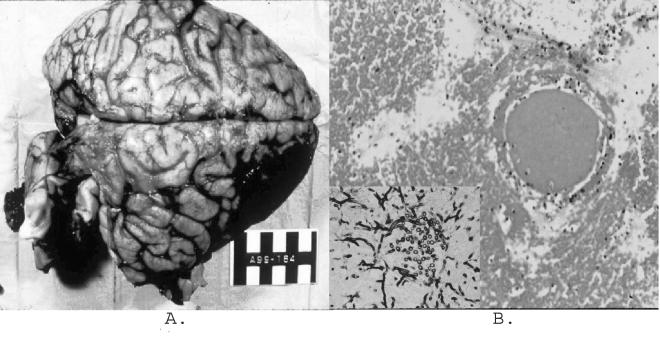
Permission was granted for a complete autopsy on patient 1. The examination of the cranial vault and brain revealed focal opacification of the meninges, with large areas of subarachnoid hemorrhage overlying the right frontal lobe and mid-brain and focally overlying the cerebellum. Serial frontal sections of the brain revealed a 6.7- by 4.6- by 3.8-cm hemorrhagic and necrotic infarction of the right posterior temporal lobe (Fig. 1A).
FIG. 1.
Gross anatomic and histologic findings. (A) Gross autopsy findings of a 6.7- by 4.6- by 3.8-cm hemorrhagic and necrotic infarction of the right posterior temporal lobe of the brain. (B) Histologic evidence of a cerebral abscess with surrounding hemorrhage and venous clot. (Inset) Tissue GMS stain with evidence of fungal hyphae.
Histologic sections from the hemorrhagic lesions within the heart, lungs, spleen, and brain were similar and revealed numerous blood vessels with fibrin and cellular debris occluding the vessel lumens. In addition, there was histological evidence of a cerebral abscess with surrounding hemorrhage and venous clot (Fig. 1B). The surrounding parenchyma of the involved organs displayed various amounts of necrosis and acute inflammation.
GMS stains were performed on sections from all of the involved organs. Sections revealed numerous fungal organisms within the occluded vessels, penetrating the vascular walls, and within the surrounding parenchyma. The fungal elements were septate and displayed acute angle branching that was morphologically consistent with Aspergillus species. Masson-Fontana staining for melanin was also done on sections from the involved organs demonstrating hyphae, and this test demonstrated weak staining of the fungal walls.
Microbiological studies.
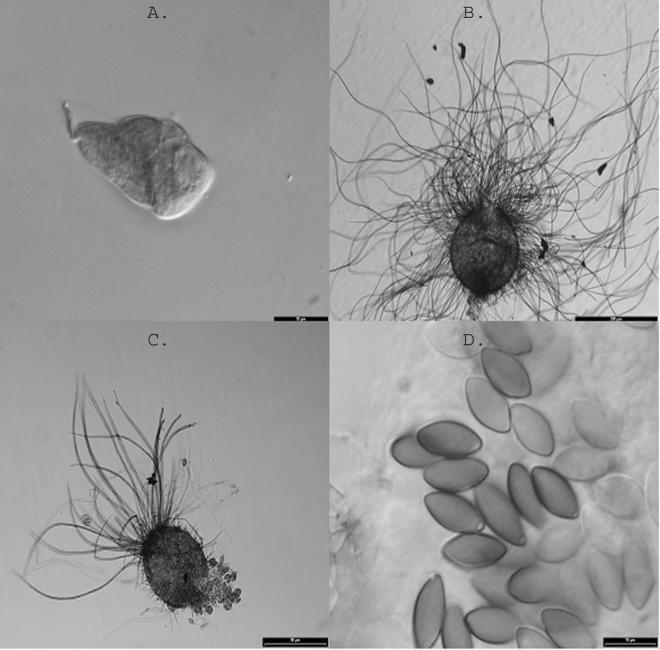
Isolates from both cases, originally submitted to the Fungus Testing Laboratory, San Antonio, Tex., were subsequently referred to the Universitat Rovira I Virgili, Tarragona, Spain, for species identification. Studies at the Fungus Testing Laboratory indicated that both isolates grew well at 35°C. At 42°C, the colony diameter (not measured) for the case 2 isolate was equivalent to that at 35°C, whereas the colony diameter for the case 1 isolate was reduced by approximately one-half. Young ascomata were evident with both isolates after 6 to 10 days of incubation at 25°C on potato flake agar prepared in house and appeared to be mature at between 12 and 18 days. Isolates resembled Chaetomium atrobrunneun macroscopically but produced perithecia and ascospores larger than those described for this species. The final species identification was provided in the laboratory of J. Guarro by comparison of the cases' isolates with reference strains from the Centraal bureau voor Schimmelcultures, Utrecht, The Netherlands. Temperature studies in Spain corroborated those done in San Antonio, Tex., with the best growth occurring at 37°C, growth at 42°C consisting only of sterile mycelium, and no growth at 50°C. To induce mature perithecia, a suspension of ascospores was inoculated onto sterilized plant material. Numerous well-developed ascomata were produced on the surface of the substrate, and mature ascomata and ascospores were evident within 16 to 20 days. No differences in perithecal size or time for maturation were noted between 25 and 35°C. Both patients' isolates showed globose (spherical) to subglobose or ovoid ascomata (108 to 220 μm by 90 to 200 μm), with undulate hairs and a wide ostiole (30 to 50 μm in diameter) and eight-spored asci (20 to 40 μm by 7 to 18 μm; Fig. 2). The ascospores were elliptical and olive brown, measured 12.0 to 15.0 μm in length by 6 to 7.5 μm in width, and contained a subapical germ pore. The ascomatal hairs were less profuse and less undulate than those displayed in an earlier study (19); however, this feature was observed on media slightly different than that used in the original description of the species and, in the case of patient 2, in an immature ascoma. Other important features fundamental to the identification of Chaetomium species, including the size and shape of ascospores and the presence and position of germ pores, remained constant, permitting identification. Similar species include C. gangligerum, C. raii, C. jodhpurense, and C. fusisporum. C. gangligerum produces ascospores that are clearly broader (12 to 15 μm by 7.5 to 9.5 μm), and chlamydospores are usually formed; C. raii has smaller ascospores (10 to 13 μm by 5.5 to 7.5 μm), and C. jodhpurense and C. fusisporum have larger ascospores (14 to 19 μm by 6 to 8 μm and 14 to 17 μm by 7 to 8 μm, respectively). Case 1 and 2 isolates have been deposited in the University of Alberta Microfungus Collection and Herbarium, Devonian Botanic Garden, Edmonton, Alberta, Canada, as UAMH 9705 (UTHSC 99-1994) and UAMH 9706 (UTHSC 98-2214), respectively. The features that differentiate C. perlucidum and the other invasive species of Chaetomium are described in Table 1.
FIG. 2.
C. perlucidum isolates. (A) Young clavate (club-shaped) ascus in an isolate from patient 1. Bar, 10 μm. (B) Ascoma with undulate hairs in patient 1. Bar, 200 μm. (C) Isolate from patient 2 showing a young ascoma with immature asci in the basal part. Bar, 50 μm. (D) Isolate from patient 2 showing ascospores. Note the conspicuous subapical germ pores. Bar, 10 μm.
TABLE 1.
Features differentiating clinically significant Choetomium speciesa
Antifungal susceptibility studies.
Isolates were tested utilizing the National Committee for Clinical Laboratory Standards broth macrodilution method M38-P (12). Briefly, isolates were grown on potato flake agar for 7 days at 30°C. Case isolates and the Paecilomyces variotii control strain, UTHSC 90-459, were overlaid with sterile distilled water, and suspensions were made by gently scraping the colonies with the tip of a sterile Pasteur pipette. Heavy hyphal fragments were allowed to settle and the upper, homogenous suspensions were removed. The conidial suspension of the control strain was standardized by a hemocytometer count. The case isolates were standardized spectrophotometrically to 95% transmission at 530 nm. Antifungal agents were prepared as previously described (12). Final drug concentrations were 0.03 to 16 μg/ml for amphotericin B (AMB; Bristol Myers Squibb Pharmaceuticals, Wallingford, Conn.); 0.015 to 8 μg/ml for itraconazole (ITC; Janssen, Beerse, Belgium), voriconazole (VRC; Pfizer Pharmaceuticals, New York, N.Y.), and posaconazole (POC; Schering-Plough, Kenilworth, N.J.); and 0.125 to 64 μg/ml for caspofungin (CAS; Merck Research Laboratories, Rahway, N.J.). Testing parameters for the isolates and control included 35°C incubation, a final inoculum standardization of 1 × 104 to 5 × 104 CFU/ml, Antibiotic Medium 3 for AMB and CAS, and RPMI 1640 buffered with morpholinepropanesulfonic acid (Angus, Niagara Falls, N.Y.) for the triazoles. Endpoint determinations were optically clear for AMB or 80% inhibition compared to the growth control for ITC, VRC, POC, and CAS. The 80% inhibition for CAS was roughly equivalent to the minimal ef-fective concentration proposed by Arikan et al. for CAS endpoints (3). Minimum lethal concentrations (MLC) for AMB were determined by subculturing 100-μl aliquots from the growth control and optically clear tubes to Sabouraud dextrose agar (Remel, Lenexa, Kans.). Plates were incubated until growth was seen in the growth control culture. The MLC was the lowest drug concentration that resulted in either no growth or fewer than three colonies (99.9% killing). The results are summarized in Table 2.
TABLE 2.
In vitro antifungal susceptibility data for C. perlucidum isolatesa
| Antifungal agent | Patient | MIC (μg/ml) at 48/72 h | MLC (μg/ml) at 48/72 h |
|---|---|---|---|
| AMB | 1 | 0.25/0.25 | 0.25/0.25 |
| 2 | 0.25/0.25 | 0.25/0.25 | |
| ITC | 1 | ≤0.015/0.06 | |
| 2 | 0.06/0.06 | ||
| VRC | 1 | 0.5/0.5 | |
| 2 | 0.5/0.5 | ||
| POS | 1 | 0.125/0.125 | |
| 2 | 0.06/0.125 | ||
| CAS | 1 | 8/16 | |
| 2 | 16/32 |
As determined by the NCCLS M-38P method.
Discussion.
Chaetomium species belong to a large genus of saprobic ascomycetes found on dung, straw, paper, bird feathers, seeds, plant debris, and in soil (8). Although Chaetomium species are rarely implicated in human disease, their spectrum of mycoses includes onychomycosis (15) and sinusitis (4) in immunocompetent individuals and empyema (11), pneumonia (10, 21), and fatal disseminated cerebral disease in immunocompromised hosts (2, 9, 17) and intravenous drug users (1). Earlier studies have demonstrated that C. atrobrunneum (1, 9, 17) and C. strumarium (1) are invasive, neurotropic species. C. globosum has been reliably shown to be an occasional agent of onychomycosis (15). We present here the first reports of C. perlucidum infections occurring in a bone marrow transplant patient that resulted in death and in an otherwise healthy patient with chronic pneumonia. We also add C. perlucidum to the neurotropic species of Chaetomium and review invasive Chaetomium infections in the English literature.
The genus Chaetomium includes about 80 species, most of which grow best at between 25 and 35°C (18). Those that have been reported to cause invasive human disease grow well at 35 to 37°C, and those with a predilection for the central nervous system often display growth at up to 42 to 45°C. Table 3 summarizes the reported cases of invasive Chaetomium infections to date.
TABLE 3.
Summary of reported cases of invasive Chaetomium infections
| Patient or reference | Age (y)/sex | Underlying medical conditiona | Site of infection | Speciesb | Treatment | Outcome |
|---|---|---|---|---|---|---|
| Patient 1 | 47/F | Leukemia/umbilical cord blood transplant | Multiple organs | C. perlucidum | L-AMB | Death |
| Patient 2 | 78/F | Asthma/chronic bronchiectasis | Lung | C. perlucidum | RML lobectomy | Cure |
| 11 | 19/F | Lymphoma/autologous BMT | Lung pleura | C. globosum* | Tienamycin, vancomycin, amikacin, AMB | Death |
| 21 | 19/M | AML | Lung | Chaetomium sp.** | Liposomal AMB | Death |
| 9, 17 | 31/M | Multiple myeloma/allogeneic BMT | Brain, lung | C. atrobrunneum | AMB, ITC | Death |
| 1 | 28/M | IVDU | Brain | C. strumarium | Oxacillin, cefotaxime, metronidazole | Death |
| 1 | 25/M | IVDU | Brain | C. strumarium | Amoxicillin, chloramphenicol, acyclovir, AMB, rifampin, isoniazid | Death |
| 1 | 20/M | IVDU | Brain | C. strumarium | Ceftriaxone, penicillin, acyclovir | Death |
| 10 | 24/M | ALL | Lung | C. globosum† | AMB | Death |
| 2 | 32/M | Renal transplant | Brain | C. atrobrunneum‡ | Unknown | Death |
| 4 | 73/F | None | Left maxillary sinus | Chaetomium sp. | Infundibulectomy | Cure |
Abbreviations: BMT, bone marrow transplant; RML, right middle lobe; IVDU, intravenous drug user; AML, acute myelogenous leukemia; ALL, acute lymphocytic leukemia.
*, there was no tissue invasion, and the authors were unable to determine the role of the organism in the death of the patient; **, the identification of this strain as C. globosum in the original reference has been questioned (7); †, this species is considered to be synonymous with C. cochliodes (18) (the isolate failed to produce fertile acomata for species identification); ‡, this strain was previously identified as C. globosum (1).
Although three cases of life-threatening Chaetomium infection have occurred in association with injection drug use (thought to have resulted from direct inoculation from injection drugs and subsequent hematogenous spread), the majority of reports have involved patients with hematologic malignancies and/or immunosuppression secondary to bone marrow or solid organ transplantation. The clinical findings in each of these patients resembled aspergillosis both radiographically and histopathologically. One of four patients with lung involvement had evidence of pneumonia with cavitating lesions (21), and one had evidence of a lung abscess (9, 17). Of the patients with neurological involvement, single or multiple cerebral abscesses were common, although one case demonstrated diffuse cerebral involvement (1, 2, 9, 17). In all reports in which death was the outcome, identification of the etiologic agent as a Chaetomium species occurred subsequent to the patient's demise. This illustrates the difficulty in identifying members of this genus by routine methods. The neurotropism of certain Chaetomium species is noteworthy. C. atrobrunneum and C. strumarium have previously been documented as neurotropic agents. The present study adds C. perlucidum to the list, and the ability of these species to grow at elevated temperatures may contribute to their neurotropic potential.
In addition, patient 1 had evidence of fungal dissemination, an attribute not previously reported with other species of Chaetomium. Reports of C. globosum inciting invasive disease are inadequately documented. Its restricted growth at 35°C, lack of growth at elevated temperatures (1), and lemon-shaped ascospores set it apart from invasive species. The isolate from one report of C. globosum from a brain abscess (2) was later reidentified by Abbott et al. to be C. atrobrunneum (1). Three other literature reports are also questionable. One isolate (10) grew well at 37°C, suggesting a species other than C. globosum, and it failed to produce fertile ascomata for species identification. A photograph of narrowly fusoidal ascospores from an acute myelogenous leukemia pneumonia patient reported by Yeghen et al. (21) suggests that this organism is also probably not C. globosum. The isolate from a bone marrow transplant patient with sepsis reported by Lesire et al. (11), although isolated from a sterile site, lacked evidence of tissue invasion, and these authors state they were unable to determine the role of this organism in the death of the patient.
The optimal management of cerebral phaeohyphomycosis due to dematiaceous molds other than Chaetomium is not clear. There are reports of successful treatment of phaeohyphomycosis with neurosurgical intervention, with or without concurrent antifungal therapy (5, 6, 13, 20). Antifungal therapy alone was successful in a single case (14); however, in general, morbidity and mortality remains unacceptably high (16).
The appropriate treatment for Chaetomium infections is unknown. Published in vitro susceptibility data for Chaetomium species has revealed resistance to flucytosine and fluconazole. Although ITC, ketoconazole, and miconazole demonstrated inhibitory activity (8), none of these agents, including AMB, demonstrated fungicidal activity. Most patients with reported invasive disease received either conventional or lipid-based AMB empirically during their treatment course (Table 3).
Despite surgical debridement and treatment with liposomal AMB, patient 1 in our series died with disseminated disease. While patient 2 responded to surgical treatment, the patient was not immunocompromised. Whether early surgical intervention is effective in controlling local disease is unknown.
In vitro susceptibility data for the two cases presented here, determined retrospectively at the Fungus Testing Laboratory, are shown in Table 2. Since the established breakpoints for filamentous fungi are not yet defined, one can only presume susceptibility based upon yeast endpoints and/or MICs that fall within normally achievable concentrations in serum for the agents being evaluated. Based upon these assumptions, both isolates appeared susceptible to AMB, ITC, VRC, and POC. The isolates appeared to be resistant to CAS.
The issue of appropriate patient management is also compounded by the difficulty in identifying Chaetomium species. The Masson-Fontana stain, a melanin-specific stain, was useful in identifying this organism as a melanized organism, thereby distinguishing it from most Aspergillus species. This type of stain may be a useful diagnostic adjunct to routine histologic stains for fungi. In addition, the cases presented illustrate the importance of obtaining microbiological cultures as part of the routine management of patients suspected of having a fungal infection, since patient 1 was suspected of having aspergillosis.
We recommend that radiographic screening for central nervous system invasion be performed in immunocompromised patients when Chaetomium has been identified from other sites. In addition, given the similarities between this mycosis and aspergillosis, we encourage clinicians to obtain specimens for fungal culture and for continued communications to elucidate the spectrum of disease associated with unusual molds.
Acknowledgments
We thank Dora McCarthy for performing the antifungal susceptibility testing of the case isolates.
REFERENCES
- 1.Abbott, S. P., L. Sigler, R. McAleer, D. A. McGough, M. G. Rinaldi, and G. Mizell. 1995. Fatal cerebral mycoses caused by the ascomycete Chaetomium strumarium. J. Clin. Microbiol. 33:2692-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anandi, V., T. J. John, A. Walter, et al. 1989. Cerebral phaeohyphomycosis caused by Chaetomium globosum in a renal transplant recipient. J. Clin. Microbiol. 27:2226-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arikan, S., M. Lozano-Chiu, V. Paetznick, and J. H. Rex. 2001. In vitro susceptibility testing methods for caspofungin against Aspergillus and Fusarium isolates. Antimicrob. Agents Chemother. 45:327-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aru, A., L. Munk-Nielsen, and B. H. Federspiel. 1997. The soil fungus Chaetomium in the human paranasal sinuses. Eur. Arch. Otorhinolaryngol. 254:350-352. [DOI] [PubMed] [Google Scholar]
- 5.Baddley, J. W., S. A. Moser, D. A. Sutton, and P. G. Pappas. 2000. Microascus cinereus (anamorph Scopulariopsis) brain abscess in a bone marrow transplant recipient. J. Clin. Microbiol. 38:395-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon, D. M., T. J. Walsh, W. G. Merz, and M. R. McGinnis. 1989. Infections due to Xylohypha bantiana (Cladosporium trichoides). Rev. Infect. Dis. 11:515-525. [DOI] [PubMed] [Google Scholar]
- 7.Guarro, J. 1998. Comments on recent human infections caused by ascomycetes. Med. Mycol. 36:349-350. [PubMed] [Google Scholar]
- 8.Guarro, J., L. Soler, and M. G. Rinaldi. 1995. Pathogenicity and antifungal susceptibility of Chaetomium species. Eur. J. Clin. Microbiol. Infect. Dis. 14:613-618. [DOI] [PubMed] [Google Scholar]
- 9.Guppy, K. H., C. Thomas, K. Thomas, and D. Anderson. 1998. Cerebral fungal infections in the immunocompromised host: a literature review and a new pathogen—Chaetomium atrobrunneum: case report. Neurosurgery 43:1463-1469. [DOI] [PubMed] [Google Scholar]
- 10.Hoppin, E. C., E. L. McCoy, and M. G. Rinaldi. 1983. Opportunistic mycotic infection caused by Chaetomium in a patient with acute leukemia. Cancer 52:555-556. [DOI] [PubMed] [Google Scholar]
- 11.Lesire, V., E. Hazouard, P. F. Dequin, M. Delain, M. Therizol-Ferly, and A. Legras. 1999. Possible role of Chaetomium globosum in infection after autologous bone marrow transplantation. Intensive Care Med. 25:124-125. [DOI] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. 1998. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Proposed standard M38-P. NCCLS, Wayne, Pa.
- 13.Nieto-Rodriguez, J. A., and S. Kusne. 1996. Successful therapy for cerebral phaeohyphomycosis due to Dactylaria gallopava. Clin. Infect. Dis. 23:211. [DOI] [PubMed] [Google Scholar]
- 14.Salama, A. D., T. Rogers, G. M. Lord, R. I. Lechler, and P. D. Mason. 1997. Multiple Cladosporium brain abscesses in a renal transplant patient: aggressive management improves outcome. Transplantation 63:160-162. [DOI] [PubMed] [Google Scholar]
- 15.Stiller, M. J., S. Rosenthal, R. C. Summerbell, J. Pollack, and A. Chan. 1992. Onychomycosis of the toenails caused by Chaetomium globosum. J. Am. Acad. Dermatol. 26:775-776. [DOI] [PubMed] [Google Scholar]
- 16.Sutton, D. A., M. Slifkin, R. Yakulis, and M. G. Rinaldi. 1998. U.S. case report of cerebral phaeohyphomycosis caused by Ramichloridium obovoideum (R. mackenziei): criteria for identification, therapy, and review of other known dematiaceous neurotropic taxa. J. Clin. Microbiol. 36:708-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas, C., D. Mileusnic, R. B. Carey, M. Kampert, and D. Anderson. 1999. Fatal Chaetomium cerebritis in a bone marrow transplant patient. Hum. Pathol. 30:874-879. [DOI] [PubMed] [Google Scholar]
- 18.von Arx, J. A., J. Guarro, and M. J. Figueras. 1986. The ascomycete genus Chaetomium. Beih. Nova Hedwigia 84:1-162. [Google Scholar]
- 19.von Arx, J. A., M. J. Figueras, and J. Guarro. 1988. Sordariaceous ascomycetes without ascospore ejaculation. Beih. Nova Hedwigia 94:1-104. [Google Scholar]
- 20.Vukmir, R. B., S. Kusne, P. Linden, et al. 1994. Successful therapy for cerebral phaeohyphomycosis due to Dactylaria gallopava in a liver transplant recipient. Clin. Infect. Dis. 19:714-719. [DOI] [PubMed] [Google Scholar]
- 21.Yeghen, T., L. Fenelon, C. K. Campbell, et al. 1996. Chaetomium pneumonia in a patient with acute myeloid leukaemia. J. Clin. Pathol. 49:184-186. [DOI] [PMC free article] [PubMed] [Google Scholar]