Abstract
More than 1,000 cases of malaria are diagnosed each year in the United States. Reported numbers, however, may be artificially low because many clinicians fail to consider the diagnosis on presentation, U.S. hospital laboratory technologists have very limited experience in detecting and identifying malaria parasites, and reporting of malaria to state health departments is sporadic in many states. In this study, a rapid malaria diagnostic test, the OptiMAL test (DiaMed; under license from Flow Inc., Portland, Oreg.) was evaluated in six U.S. hospitals and compared with results of microscopy. The OptiMAL test is a 15-min rapid immunochromatographic test that both identifies and differentiates Plasmodium falciparum from non-P. falciparum malaria parasites on the basis of the detection of parasite lactate dehydrogenase in a drop of patient blood. A total of 216 specimens from patients suspected of having malaria were tested. Results indicated that 43 samples (20%) were positive for malaria parasites by microscopy (32 P. falciparum, 11 non-P. falciparum) while 42 (19%) were positive by OptiMAL (31 P. falciparum, 11 non-P. falciparum). The sensitivity of the OptiMAL test was 98%; its specificity was 100%, with positive and negative predictive values of 100 and 99%, respectively. Participating hospital physicians and laboratory directors independently reported that the OptiMAL rapid malaria test was accurate, easy to use, and well accepted by those working in their diagnostic laboratories. The overall conclusion was that integration of the OptiMAL rapid malaria test into the U.S. health care infrastructure would provide an important and easy-to-use tool for the timely diagnosis of malaria.
Malaria remains a major global health threat in the 21st century. The number of human infections continues to increase in countries where the disease is endemic; however, malaria also appears in regions where the disease is not endemic, such as the United States. In fact, cases of malaria have been steadily increasing in the United States because of increased international travel and immigration. It has recently become apparent that immigrants or refugees who return to their home country, termed visiting friends and relatives, are a very high-risk cohort compared to routine travelers (20). Cases of malaria in the United States have also been linked to blood transfusions, returning military personnel who were stationed in areas where the disease is endemic, and from local mosquito-borne transmission (1). Local transmission normally occurs in close proximity to an international airport, where an infected mosquito may have arrived on a flight from a country where the disease is endemic (2) or a recently arrived infected traveler is bitten by a local mosquito that then transmits the disease. Regardless of the source of infection, individuals with malaria in the United States must seek medical attention at local hospitals and clinics, where technical expertise in diagnosing malaria may be limited.
The ability of U.S. hospitals and clinics to accurately diagnose malaria is becoming increasingly important since cases of malaria detected in the United States have been on the increase (8). In the early 1990s, cases of malaria in the United States averaged 1,200 to 1,400/year. The Centers for Disease Control and Prevention (CDC) reported 1,544 cases in 1997, an increase of nearly 11% over the previous years (12). In 1999, this trend continued, with 1,540 cases; however, a small dip to 1,402 cases occurred in 2000 (2). Although it is not possible to predict malaria trends for the future, international travel, military conflicts, and continued immigration ensure that malaria will continue to be encountered in the United States.
Malaria caused by P. falciparum is a medical emergency, and timely, accurate detection and pathogen identification to the species level are imperative in order to provide appropriate treatment and supportive therapy. A majority of deaths attributable to malaria in the United States are deemed preventable, and delayed diagnosis and misdiagnosis are commonly implicated as causes, particularly in hospital emergency rooms (6, 9, 10, 11). Classically, diagnosis of malaria has been accomplished by examination of Giemsa-stained thin and thick blood smears under a microscope. This method is believed to be sensitive and specific when performed by those with a high level of expertise acquired from frequent examination of blood smears for malaria parasites. To develop reliable expertise, microscopists need to have frequent exposure to blood film examination from a large numbers of cases, as in many areas of the world where malaria is endemic, where several hundred persons suspected of having malaria appear in the hospital or clinic laboratory each week. In contrast, most U.S. hospital laboratories and clinics see a very limited number of malaria parasite-positive specimens and personnel have little experience in interpreting thin and thick blood films. Therefore, technologists have limited experience in identifying malaria parasites. Moreover, even though microscopy is considered the “gold standard,” it is not 100% sensitive and specific, even when practiced by skilled and experienced technologists in countries where malaria is endemic since low-level parasitemias and mixed infections are frequently not detected, interpretation of results is often ambiguous, and procedures for preparation of slides and enumeration of parasites are inconsistent (4, 17, 21).
In recent years, several rapid malaria tests have been developed. In addition to providing rapid diagnosis, several of these tests differentiate P. falciparum infections from non-P. falciparum infections. An in-depth review of recent developments in rapid malaria diagnostic tests has been presented by Moody (15). Here, we report on the results of a multicenter study evaluating the use of a rapid malaria diagnostic test, OptiMAL (DiaMed; under license from Flow, Inc., Portland, Oreg.), for the ability to increase the detection and identification of malaria parasites to the species level in selected U.S. hospitals. The objective of this study was to assess OptiMAL as an aid in rapid initial diagnosis of individuals presenting with symptoms consistent with malaria at health care facilities in areas where malaria is not endemic.
MATERIALS AND METHODS
Study design. (i) Study site selection.
Six major metropolitan hospitals in the United States participated in this study. Institutional Review Board (IRB) approval was obtained at the University of Florida and independently by each participant hospital from its IRB. The participating hospitals included the UCLA Medical Center, Los Angeles, Calif.; the Boston Medical Center, Boston, Mass.; the Washington Hospital Center, Washington, D.C.; the Kings County Hospital Center, Brooklyn, N.Y.; the Elmhurst Hospital Center, Elmhurst, N.Y; and the Regions Hospital/HealthPartners, St. Paul, Minn.
(ii) Patient selection.
A physician or laboratory director at each of the six participating hospitals served as the principal investigator at that site. In general, physicians identified individuals who presented with symptoms consistent with malaria and a history of travel to a country where malaria is endemic. Informed consent was obtained from each patient before participation in the study, except at two of the sites, where the study was granted an IRB exemption since no new blood was drawn and it was considered a laboratory comparative technique study. Blood samples of patients suspected of having malaria were collected in EDTA tubes, which were submitted to respective hospital laboratories for routine examination for malaria parasites. The patients were diagnosed and treated on the basis of the results of these standard microscopic examinations of thin and thick smears. A drop of blood from the same tube as that used for microscopy was used for the OptiMAL rapid malaria test. Different individuals were responsible for smear reading and performance of the OptiMAL test, and they were blinded to the results of the other technique. The OptiMAL test was not used as a diagnostic test for patient treatment or care as it has not been approved by the Food and Drug Administration.
(iii) Microscopic examination of blood smears.
Thin and thick blood smears were prepared from blood samples of patients suspected of having a malaria infection. Blood smears were examined microscopically after standard Giemsa staining (16). If a specimen was determined to be positive for malaria parasites by the hospital laboratory, the sample was sent for secondary confirmation, either to a hospital pathologist or to the respective State Department of Health. Half of the hospitals in this study did not routinely report parasitemia levels, and thus, parasitemia levels are not reported here.
OptiMAL test.
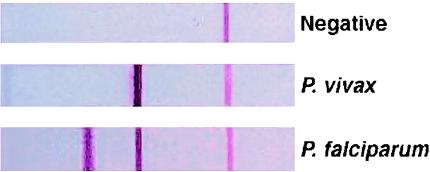
The OptiMAL rapid malaria test (Flow Inc.-Diamed) is a patient point-of-care immunochromatographic test that can be performed with a drop of finger stick blood. The test detects parasite lactate dehydrogenase, an enzyme produced by metabolizing malaria parasites. Briefly, a drop of blood is added to a well in a microtiter plate and mixed with a drop of buffer. An OptiMAL test strip is placed in the well, and the blood is wicked up by the nitrocellulose strip. After the blood is completely wicked up, the strip is transferred to the next well, which contains a few drops of wash buffer, and allowed to clear. The entire process takes approximately 15 min, and results are visually interpreted. A positive control line should always be present at the top of the strip to verify that the test strip is functional. If this is the only line that appears, then the test is considered negative for malaria. Appearance of a second line, adjacent to the positive control line, indicates the presence of a non-P. falciparum malaria parasite (P. vivax, P. ovalae, or P. malariae). When a third line is also present, this indicates a positive response for P. falciparum infection. Typical outcomes of the OptiMAL test are shown in Fig. 1.
FIG. 1.
Expected reaction patterns on the OptiMAL test strip for a negative patient, a patient with P. vivax malaria, and a patient with P. falciparum malaria.
Each hospital laboratory received on-site training in the performance of the OptiMAL rapid test and was provided several boxes of OptiMAL strips and reagents (lot 46050.18.04). After a half-day training session, the hospital personnel worked independently for the duration of the study. No communication or comparison of results occurred between individual hospitals. This was to prevent any bias on the test performance, result interpretation, or individual hospital opinion on the utility of the test as a laboratory tool.
Test interpretation parameters were as follows. Microscopy was used as the gold standard, except in instances in which there was disagreement between the microscopy and OptiMAL results. Discrepancies were resolved by PCR assay. The PCR result was considered correct in determining the test outcome. The PCR assay was done as described by Snounou et. al (22).
RESULTS
A total of 216 patients suspected of having malaria were tested by both microscopy and OptiMAL at the six different hospitals. Discrepant samples were resolved by PCR. Table 1 shows the data from the six hospitals. Results indicate that 43 (20%) of the samples were positive for malaria by blood film examination (32 P. falciparum, 11 non-P. falciparum) while 42 (19%) were positive by OptiMAL (31 P. falciparum, 11 non-P. falciparum). The sensitivity of the OptiMAL test was 98%; its specificity was 100%, with positive and negative predictive values of 100 and 99%, respectively (Table 2). The accuracy of the test was 100%.
TABLE 1.
OptiMAL and microscopy results from individual U.S. hospitals in the multicenter study
| Hospital | No. of samples tested | No. OptiMAL positive
|
No. blood film positivea
|
No. OptiMAL negative | No. Blood film negative | ||||
|---|---|---|---|---|---|---|---|---|---|
| P. falciparum | Non-P. falciparum | Total | P. falciparum | Non-P. falciparum | Total | ||||
| A | 111 | 4 | 7 | 11 | 4 | 7 | 11 | 100 | 100 |
| B | 36 | 9 | 1 | 10 | 9 | 1 | 10 | 26 | 26 |
| C | 41 | 4 | 1 | 5 | 4a | 1 | 5 | 36 | 36 |
| D | 15 | 9 | 0 | 9 | 9 | 0 | 9 | 6 | 6 |
| E | 8 | 5 | 1 | 6 | 6 | 1 | 7 | 2 | 1 |
| F | 5 | 0 | 1 | 1 | 0 | 1 | 1 | 4 | 4 |
| Totals | 216 | 31 | 11 | 42 | 32 | 11 | 43 | 174 | 173 |
Hospital C performed a PCR assay on samples when the microscopist could not identify the parasite to the species level (n = 2) or when the result was discrepant with respect to the OptiMAL result (non-P. falciparum diagnosed [n = 1], no parasites were observed [n = 1]).
TABLE 2.
Malaria results when microscopy and PCR were used to calculate statistics
| OptiMAL result | No. of microscropy and PCR results
|
Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 42 | 0 | 42 |
| Negative | 1 | 173 | 174 |
| Total | 43 | 173 | 216 |
Discrepancies.
There were five instances in which the results of microscopic examination did not correlate with those of the OptiMAL test. Two of these samples were found to be positive for malaria parasites by microscopy, but the Plasmodium species could not be determined by the microscopist. Both the OptiMAL and PCR results for these two specimens indicated a P. falciparum infection. One patient sample was determined to be negative by microscopy and positive for P. falciparum by both OptiMAL and PCR. A fourth patient specimen was read as P. vivax by microscopy and P. falciparum by both OptiMAL and PCR. Because PCR was used to determine the final result in cases in which the microscopy and OptiMAL results disagreed and since in these four instances the PCR result was in agreement with the OptiMAL result, Table 2 reflects the PCR result in the microscopy column as agreeing with the OptiMAL test result. There was not sufficient leftover blood to perform PCR on the fifth discrepant sample, and thus, results of microscopy alone (microscopy positive for P. falciparum, OptiMAL malaria negative) were used in tabulating the data, indicating a false-negative result for the OptiMAL test. It should be noted that workers at this laboratory reported that they observed only two parasites (ring forms) on the entire slide (<0.01%) and had repeated the OptiMAL test three times to verify the negative result. The negative OptiMAL result may be attributed to the fact that the patient had begun antimalarial treatment 2 days before this specimen was tested and the parasites may have been killed (and no longer producing detectable lactate dehydrogenase) but not yet eliminated from the bloodstream.
Reexamination of the data by tabulation of the level of agreement between microscopy and the OptiMAL test if you ask only whether or not the patient has malaria, regardless of the pathogen species, shows that the sensitivity of the OptiMAL test remains 98% while the specificity drops slightly to 99% (Table 3). There is also a slight drop in the positive predictive value (98%) and accuracy (99%) but no change in the negative predictive value (99%).
TABLE 3.
Results of microscopy compared to those of OptiMAL for detection of malaria regardless of the species
| OptiMAL result | No. of microscopy results
|
Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 41 | 1 | 42 |
| Negative | 1 | 173 | 174 |
| Total | 42 | 174 | 216 |
Initial misdiagnoses.
Three (50%) of the six participating hospitals had cases in which a blood sample was initially diagnosed as malaria positive by blood films. When the sample was submitted for mandatory secondary confirmation by the pathologist or State Department of Health, the result was determined to be a Babesia sp. infection and not malaria. In these cases, the OptiMAL and PCR results were negative for malaria.
Epidemiological data from U.S. malaria cases in this study.
The travel history of the participating patients encompassed 32 different countries around the globe. These countries included Angola, Bangladesh, Cambodia, Cameroon, Congo, the Dominican Republic, Ecuador, El Salvador, Ethiopia, Gambia, Ghana, Guadeloupe, Haiti, India, Indonesia, Iraq, Ivory Coast, Kenya, Korea, Laos, Liberia, Mexico, Nigeria, Peru, Senegal, Sierra Leon, Somalia, Sudan, Tanzania, Thailand, Uganda, and Vietnam.
DISCUSSION
The results of this study suggest that the OptiMAL test could help to improve the diagnosis of malaria at U.S. health care facilities. Because of the delayed diagnoses, occasional misdiagnoses, and potential mortality associated with malaria, an improved diagnostic method would be welcomed. Malaria carries a high risk of serious morbidity and possible mortality, and it has been demonstrated that delayed diagnosis or misdiagnosis frequently contributes to poor patient outcome (23, 24). In half of the participating hospitals, Babesia sp. parasites were misidentified as malaria parasites at least once during the study period. This is not surprising, as some stages of the Babesia parasite may appear similar to malaria parasites under a microscope. Importantly, patients infected with Babesia sp. were negative in the OptiMAL test. For future use in U.S. hospitals, the finding of ring forms on a smear and a negative OptiMAL result could alert the laboratory to possible Babesia infection.
Another diagnostic difficulty observed in our study was the inability of a microscopist to identify the Plasmodium parasite to the species level. This problem holds true in other U.S. hospitals as well since in nearly 9% (n = 134) of the 1,544 malaria cases reported in 1997, the Plasmodium species causing malaria could not be determined (12). Reports from the year 2000 also showed that 161 (8.7%) of the 1,402 malaria cases were not identified to the species level (2). Accurate identification of malaria parasites to the species level is imperative so that the patient receives appropriate therapy, particularly when the patient has relapsing malaria (caused by P. vivax and P. ovale). Identification to the species level is also important because of the severe morbidity and mortality associated with P. falciparum and growing resistance to antimalarial therapy. It is also vital to obtain follow-up specimens from malaria-positive patients to monitor therapy outcome and detect drug failure.
A major benefit of using the OptiMAL rapid malaria test is its potential to quickly and confidently identify high-parasitemia P. falciparum infections, allowing a quick decision on treatment options, including hospitalization. The duration of hospitalization can thus be reduced significantly and the patient can be followed up as an outpatient. Currently, patients are generally kept in the emergency department of the hospital until the results of blood smear tests for malaria are known, which may take 4 h or longer. Until further data are available and OptiMAL receives Food and Drug Administration approval, smears remain the standard method for laboratory diagnosis of malaria. The OptiMAL test is suggested not as a replacement for blood films but as a complementary adjunct to the tools available for the diagnosis of malaria and in particular as a rapid point-of-care screening test.
Since few laboratories in this country would be expected to have a high level of expertise in malaria diagnosis, a rapid, inexpensive, sensitive, and specific screening test would be a welcome addition to the malaria diagnostic armamentarium. By offering a rapid preliminary diagnosis in relevant cases, the OptiMAL rapid test would improve patient care by increasing the detection of malaria, improving identification to the species level, decreasing the time to initiation of appropriate therapy, and substantially decreasing the length of stay in the emergency department. This is particularly important for hospital emergency rooms. A study in Los Angeles concluded that only 12 of 20 cases of malaria were identified in the emergency room and of those, identification of the parasite to the species level was accomplished in only 2 cases (10). The authors of that report stated that hepatitis and gastroenteritis were the most common misdiagnoses for malaria patients. While it is suggested that emergency room and other physicians improve malaria diagnosis by ensuring that they obtain travel histories from patients with clinical features of infectious disease, having access to a rapid malaria test that is not subjective, is easy to use, and is able to at least differentiate P. falciparum malaria from non-P. falciparum malaria would significantly improve the diagnosis and subsequent immediate treatment of malaria in the United States.
Patients enrolled in this study acquired malaria in 32 countries, with 50% of the travelers having a history of travel to countries in Africa, 28% having a history of travel to Southeast Asia, India, and the Middle East, and 22% having a history of travel to countries in the Americas. This diversity of countries where malaria is transmitted highlights the importance of identification of malaria parasites to the species level, especially in light of the rising drug resistance patterns. Given the rising drug resistance patterns of both P. falciparum and P. vivax, close monitoring of antimalarial drug therapy is important for the detection of early drug failures caused by drug-resistant malaria parasites. There have been several reports on the use of the OptiMAL rapid malaria diagnostic test to successfully monitor antimalarial drug therapy in countries other than the United States (14, 19). We are in the process of initiating a second phase of this study wherein patients at U.S. hospitals will be followed up posttherapy with the OptiMAL test.
This is the first reported study using a rapid malaria test in a clinical setting in the United States. OptiMAL performed well against the gold standard, and the results are encouraging and indicate that OptiMAL may be an excellent diagnostic screening tool for malaria in U.S. hospital settings. The test has been in the global market for nearly 6 years. There have been a plethora of studies on the OptiMAL test by investigators all around the world (3, 5, 7, 13, 14, 18). The sensitivity and specificity of the test when used by others have ranged from a low of 25% to a high of 100%. It is difficult to explain why there is such a large range of results. Factors that may contribute to these diverse findings include test kit storage conditions in the field, inadequate adherence to the test protocol, or levels of parasitemia below the detection limit of the OptiMAL test. Our parasitemia levels were generally 0.1% or higher, which is above the lower detection limit of the OptiMAL test (which is stated to be 50 parasites/μl).
In conclusion, the strength of the present study is that it was performed equally well at six separate hospitals with no communication between the sites, each of which performed the test under common U.S. hospital conditions. Therefore, these results represent a thorough evaluation of the ability of this test to be a useful diagnostic tool for detection of malaria in U.S. hospitals. A poststudy survey of all of the personnel directly involved in the performance of the OptiMAL test found the unanimous opinion that the test was easy to use, decreased stress and potential error in the laboratory, and had outstanding potential to improve patient care in the United States. The overwhelming sentiment was that the manufacturers of the OptiMAL test should seek approval from the Food and Drug Administration for its use in diagnosing malaria in U.S. hospitals.
Acknowledgments
We especially thank the laboratory directors and technologists at the six hospitals who worked with us on this study. Special thanks goes to Robyn Shimizu, UCLA Medical Center; James Feldman, Boston Medical Center; Ruth Ann Bates, Washington Hospital Center; Josephine Mora, Kings County Medical Center; Jeoffrey Clover, Elmhurst Hospital; and Doug Olson, Region's Hospital/HealthPartners.
This project and report were supported by CDC award U50 CCU421845.
The contents of this report are solely our responsibility and do not necessarily represent the official views of the CDC.
REFERENCES
- 1.Anonymous. 2002. Local transmission of Plasmodium vivax malaria—Virginia, 2002. Morb. Mortal. Wkly. Rep. 51:921-923. [PubMed] [Google Scholar]
- 2.Causer, L. M., R. D. Newman, A. M. Barber, J. M. Roberts., G. Stennies, P. B. Bloland, M. E. Parise, and R. W. Steketee. July 2002. Malaria surveillance—United States, 2000. Morb. Mortal. Wkly. Rep. 51:9-21. [Google Scholar]
- 3.Cho, D., K. H. Kim, S. C. Park, Y. K. Kim, K. N. Lee, C. S. Lim. 2001. Evaluation of rapid immunocapture assays for diagnosis of Plasmodium vivax in Korea. Parasitol. Res. 87:445-448. [DOI] [PubMed] [Google Scholar]
- 4.Craig, M. H., and B. I. Sharp. 1997. Comparative evaluation of four techniques for the diagnosis of Plasmodium falciparum infections. Trans. R. Soc. Trop. Med. Hyg. 91:279-282. [DOI] [PubMed] [Google Scholar]
- 5.Ferro, B., I. J. Gonzalez, F. de Carvajal, G. I. Palma, and N. G. Saravia. 2002. Performance of OptiMAL in the diagnosis of Plasmodium vivax and Plasmodium falciparum infection sin a malaria referral center in Colombia. Mem. Inst. Oswaldo Cruz 97:731-735. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg, A., and A. Lobel. 1990. Mortality from Plasmodium falciparum in travelers from the United States: 1959-1987. Ann. Intern. Med. 113:326-327. [DOI] [PubMed] [Google Scholar]
- 7.Iqbal, J., A. Sher, P. R. Hira, and R. Al-Owaish. 1999. Comparison of the OptiMAL test with PCR diagnosis of malaria in immigrants. J. Clin. Microbiol. 37:3644-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jerrard, D. A., J. S. Broder, J. R. Hanna, J. E. Colletti, K. A. Grundmann, A. J. Geroff, and A. Mattu. 2002. Malaria: a rising incidence in the United States. J. Emerg. Med. 23:23-33. [DOI] [PubMed] [Google Scholar]
- 9.Kain, K. C., M. A. Harrington, S. Tennyson, and J. S. Keystone. 1998. Imported malaria: prospective analysis of problems in diagnosis and management. Clin. Infect. Dis. 27(1):142-149. [DOI] [PubMed] [Google Scholar]
- 10.Kyriacou, D. N., A. M. Spira, D. A. Talan, and D. C. Mabey. 1996. Emergency department presentation and misdiagnosis of imported falciparum malaria. Ann. Emerg. Med. 27:696-699. [DOI] [PubMed] [Google Scholar]
- 11.Kyriacou, D. N., and J. H. Coben. 2000. Errors in emergency medicine: research strategies. Acad. Emerg. Med. 7:1201-1203. [DOI] [PubMed] [Google Scholar]
- 12.MacArthur, J., A. Levin, and J. Roberts. 2001. Malaria surveillance—United States, 1997. Morb. Mortal. Wkly. Rep. 50:25-29. [Google Scholar]
- 13.Mason, D. P., F. Kawamoto, K. Lin, A. Laoboonchai, and C. Wongsrichanalai. 2002. A comparison of two rapid field immunochromatographic tests to expert microscopy in the diagnosis of malaria. Acta Trop. 82(1):51-59. [DOI] [PubMed] [Google Scholar]
- 14.Moody, A., A. Hunt-Cooke, E. Gabbett, and P. Chiodini. 2000. Performance of the OptiMAL malaria antigen capture dipstick for malaria diagnosis and treatment monitoring at the Hospital for Tropical Diseases, London. Br. J. Haematol. 109:891-894. [DOI] [PubMed] [Google Scholar]
- 15.Moody, A. 2002. Rapid diagnostic tests for malaria parasites. Clin. Microbiol. Rev. 15:66-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.NCCLS. 2000. Laboratory diagnosis of blood borne parasitic diseases. M15-A, vol. 20. NCCLS, Villanova, Pa.
- 17.Ohrt, C., M. Purnomo, M. Sutamihardja, D. Tang, and K. C. Kain. 2002. Impact of microscopy error on estimates of protective efficacy in malaria-prevention trials. J. Infect. Dis. 186:540-546. [DOI] [PubMed] [Google Scholar]
- 18.Palmer, C. J., J. F. Lindo, W. I. Klaskala, J. Quesada, R. Kaminsky, M. Baum, and A. L. Ager. 1998. Evaluation of the OptiMAL® test for the rapid diagnosis of Plasmodium vivax and Plasmodium falciparum malaria. J. Clin. Microbiol. 36:203-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer, C. J., L. Validum, J. Lindo, A. Campa, C. Validum, M. Makler, R. R. Cuadrado, and A. Ager. 1999. Field Evaluation of the OptiMAL Rapid Malaria Diagnostic Test in Tracking Patient Outcomes during Anti-malarial Therapy in Guyana. Trans. R. Soc. Trop. Med. Hyg. 93:517-518. [DOI] [PubMed] [Google Scholar]
- 20.Ryan, E. T., and K. C. Kain. 2000. Health Advice and Immunizations for Travelers. N. Engl. J. Med. 342:1716-1725. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz, E., F. Paul, H. Pener, S. Almog, M. Rotenberg, and J. Golenses. 2001. Malaria antibodies and mefloquine levels among United Nations Troops in Angola. J. Travel Med. 8:113-116. [DOI] [PubMed] [Google Scholar]
- 22.Snounou, G., S. Viriyakosol, and X. P. Zhu. 1993. High sensitivity detection of human malaria parasites by the use of nested polymerase chain reaction. Mol. Biochem. Parasitol. 61:315-320. [DOI] [PubMed] [Google Scholar]
- 23.Stauffer, W. M., and D. Kamat. 2003. Special challenges in the prevention and treatment of malaria in children. Curr. Infect. Dis. Rep. 5:43-52. [DOI] [PubMed] [Google Scholar]
- 24.Stauffer, W. M., and P. R. Fischer. Diagnosis and treatment of malaria in children. Clin. Infect. Dis., in press. [DOI] [PubMed]