Abstract
We have developed a rapid colorimetric method for testing the susceptibility of M. tuberculosis to isoniazid (INH) and rifampin (RIF) based on incorporation of nitrate in broth cultures containing growth supplements. The performance of this colorimetric nitrate reductase-based antibiotic susceptibility (CONRAS) test was compared with that of the radiometric BACTEC 460TB system in determining the susceptibilities of 74 M. tuberculosis strains to INH and RIF. By using the BACTEC 460TB system as the “gold standard,” the sensitivity (i.e., the ability to detect true drug resistance) and specificity (i.e., the ability to detect true drug susceptibility) of the CONRAS test were 100 and 95% for INH and 94 and 100% for RIF, respectively. The repeatability of the CONRAS test was excellent (for INH, kappa = 1 and P < 0.001; for RIF, kappa = 0.88 and P < 0.001). For the majority of strains, results were obtained within 5 days. The CONRAS test is rapid, accurate, and inexpensive and is an adequate alternative, particularly for resource-poor countries.
The resurgence of tuberculosis (TB) worldwide has been accompanied by an increase in the incidence of drug-resistant TB and, more importantly, also in that of multidrug-resistant (MDR) TB (strains resistant to at least isoniazid [INH] and rifampin [RIF], the two most important first-line drugs). The spread of MDR strains of Mycobacterium tuberculosis has become a major public health problem (24). Current TB diagnostic tests are expensive (automated liquid-based culture systems and molecular methods) or slow (culture on solid media and biochemical tests). Standard methods for antibiotic susceptibility testing (AST) of M. tuberculosis such as the proportion method and the absolute-concentration method depend on culture on solid media and are also time-consuming. The BACTEC 460TB system (Becton Dickinson, Sparks, Md.) using liquid media is faster but involves radioactive substrates and expensive technology (17). The radiometric BACTEC 460TB system is being replaced by the BACTEC Mycobacteria Growth Indicator Tube (MGIT) 960 (Becton Dickinson) and MB/Bact (bioMérieux, Inc., Durham, N.C.) automated liquid-based systems for detection and AST. The turnaround times for the latter systems are comparable to that for the radiometric BACTEC 460TB system, but reagents for automated systems are expensive. The manual BACTEC MGIT system is nonautomated and thus does not require additional instrumentation (22). Several studies have validated the performance of this system for AST (19, 22). Molecular methods for AST have also been described (14) but need substantial investments in equipment and quality control.
A number of low-cost colorimetric AST assays, such as the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (3, 13), the Alamar blue assay (15), and an assay based on microscopic detection of cord-like growth by M. tuberculosis (4), have been described. However, these tests have limitations; mycobacteria other than M. tuberculosis can produce cord factor (10), and INH can interfere with the formazan production in the MTT assay and give rise to false-resistant results (13). Moreover, the use of a liquid medium in a microtiter plate format in these tests may be disadvantageous not only as a biohazard but also due to possible contamination between wells.
Recently, an AST for M. tuberculosis based on a nitrate reductase assay performed in nitrate-containing Löwenstein-Jensen (LJ) solid medium was described (2). The test was comparable to the BACTEC 460TB system in detecting INH and RIF resistance, and the AST results were available in 10 days for 87% of the strains tested (2).
In the present study, the nitrate reductase-based test was adapted for use as a colorimetric nitrate reductase-based antibiotic susceptibility (CONRAS) test for M. tuberculosis in Middlebrook 7H9 broth cultures. The method is based on the ability of M. tuberculosis to reduce nitrate to nitrite by using the nitrate reductase enzyme (12). Most apathogenic mycobacteria lack this enzyme. The presence of nitrite is detected by a color change to pink upon the addition of specific reagents. The reduction of nitrate to nitrite was detected visually and, on a subset of samples, also by spectrophotometry. The performance of the CONRAS test was evaluated against those of two commercial liquid-based systems, the radiometric BACTEC 460TB and the manual MGIT, for determining the susceptibilities of 74 M. tuberculosis strains to INH and RIF.
(A preliminary report of this work was presented at the 4th World Congress on Tuberculosis, Washington, D.C., 3 to 5 June 2002.)
MATERIALS AND METHODS
Strains.
A total of 74 M. tuberculosis strains were included in the study. Of these, 21 strains were obtained from the Norwegian Institute of Public Health, Oslo, Norway, 13 strains were obtained from Russia (20), 20 strains (and in addition a panel of duplicates from 18 of these strains) were obtained from the World Health Organization/International Union against Tuberculosis and Lung Disease supranational laboratory network (11), 8 were obtained from Myanmar (16), and 11 were obtained from the Haukeland University Hospital, Bergen, Norway. In addition, the M. tuberculosis strain H37Rv (ATCC 27294) was also included. Forty-two of the strains were susceptible to INH and RIF, whereas 32 strains were resistant to one or both of the drugs (15 were INH resistant, and 17 were resistant to both INH and RIF). The nitrate-positive reference strain M. tuberculosis H37Rv (ATCC 27294) and a nitrate-negative clinical isolate of the Mycobacterium avium-intracellulare complex were used as controls in the CONRAS test. The 74 M. tuberculosis strains and the panel of 18 duplicates, of which 3 were INH resistant and 7 were resistant to both INH and RIF, were coded and tested blindly. The strains were stored at −70°C and cultured on LJ medium before use.
Preparation of inocula for use in the CONRAS test.
Several loopfuls of growth from 14-day-old cultures on LJ medium were transferred to sterile tubes with glass beads containing 4 ml of Middlebrook 7H9 broth (Difco, Detroit, Mich.) supplemented with 10% oleic acid-albumin-dextrose-catalase (OADC), 0.5% glycerol, 0.05% Tween 80, and 850 μg of NaNO3/ml (7H9-S broth). The tubes were vortexed for 2 to 3 min to break up larger clumps. The suspension was allowed to stand for 20 min before transfer of the supernatant to another sterile tube. The supernatant fluid was allowed to stand for another 15 min before transfer of the supernatant to a third sterile tube. The suspensions were then adjusted to turbidities equal to those of no. 0.5 and no. 1 McFarland standards and were used in the CONRAS test. The 1 McFarland standard suspensions were used for rapidly screening the 74 M. tuberculosis strains for nitrate positivity. The test was performed in 110- by 16-mm round-bottom, screw-cap sterile polystyrene tubes (Nunc, Roskilde, Denmark). The purity of each mycobacterial suspension was checked on blood agar plates (Colombia agar base [Oxoid, Basingstoke, England] with 5% sheep blood), which were incubated at 37°C for 48 h.
Nitrate reduction test.
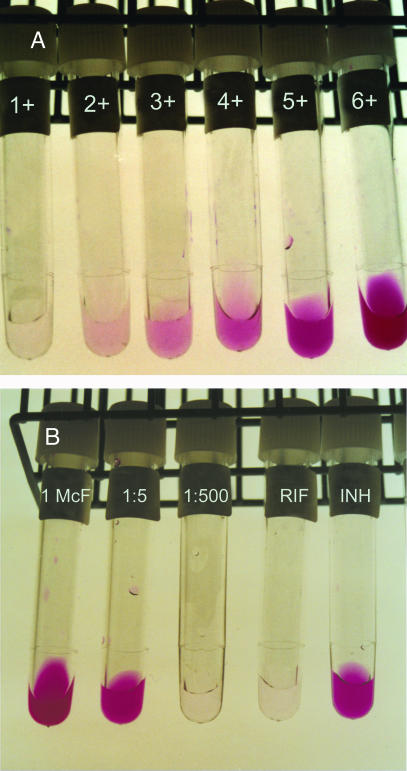
The nitrate test was performed as described previously (12), with some modifications. Thus, the following reagents were added sequentially to the AST tubes: 25 μl of concentrated hydrochloric acid (HCl), 50 μl of 0.2% sulfanilamide, and 50 μl of 0.1% N-1-naphthyl-ethylenediamine dihydrochloride. The resulting color changes were read visually after 5 min. In addition, on a subset of 24 isolates (of which 8 were INH resistant and 9 were resistant to both INH and RIF), absorbance was recorded using a spectrophotometer at 570 nm (Novaspec II photometer; Pharma Biotech, Cambridge, United Kingdom). In order to detect nitrite that may have been further reduced to nitric oxide (which cannot be detected by the reagents used in the nitrate test), a small amount of powdered zinc (Merck and Co., Inc., Rahway, N.J.) was added to each negative tube to detect false-negative results. Six standard tubes, prepared by serial twofold dilutions of a 1:128 dilution of a nitrite stock solution (10 mM sodium nitrite), were used as reference standards for visually recording color intensity (graded from 1+ through 6+) (Fig. 1A).
FIG. 1.
(A) Serial twofold dilutions of a standard stock solution of sodium nitrite (NaNO2) resulting in an increasing color intensity graded from 1+ through 6+. (B) Example of a strain examined by the CONRAS test. The color intensity of the 1:500 dilution of the bacterial suspension was compared with the color intensity in the standard tubes (panel A). A strain was considered to be resistant if the color intensity of the antibiotic-containing tube was equal to or greater than two color intensity gradations above that of the 1:500 tube. This strain is resistant to INH.
CONRAS test.
The CONRAS test was performed with an initial concentration of 0.1 μg/ml for INH and 1.0 μg/ml for RIF. If a strain was resistant to an antibiotic by visual recording, the test was repeated with a higher concentration of the drug in question (0.4 μg/ml for INH and 2.0 μg/ml for RIF). After the code was broken, AST results that were discrepant between the CONRAS test and the BACTEC 460TB system were retested by the CONRAS test. Thus, for each strain tested, panels of 10 tubes with 1 ml of 7H9-S broth were used: 1 tube each with INH and RIF (inoculated with 110 μl of the bacterial suspension with a turbidity equal to the no. 0.5 McFarland standard) and 8 tubes without any antibiotic. Three of the antibiotic-free tubes were inoculated with 110 μl of the bacterial suspension (turbidity equal to the no. 1 McFarland standard), three were inoculated with 110 μl of a 1:5 dilution of the bacterial suspension (turbidity equal to the no. 0.5 McFarland standard), and two were inoculated with 110 μl of a 1:500 dilution of the bacterial suspension (turbidity equal to the no. 0.5 McFarland standard). A control tube containing 7H9-S broth alone was also included. The tubes were incubated at 37°C with gentle shaking. Thus, for each strain tested, the nitrate test was performed on one 1 McFarland tube every second day, starting on the third day after inoculation. If a color intensity equal to or greater than that of the 4+ standard tube was obtained (Fig. 1A), the corresponding 1:5 tube was also tested by the nitrate test. The antibiotic-containing tubes were tested if the color intensity in the 1:5 tube was greater than or equal to that of the 4+ standard tube. If the 1:5 tube showed a color intensity less than that of the 4+ standard tube, the antibiotic-containing tubes were incubated for an additional two days. In an initial pilot study performed on 30 strains tested for susceptibility to INH and RIF, the cutoff for detection of resistant strains by the CONRAS method was determined. Thus, for visual recording, the color intensity of the 1:500 tube of a strain was compared with the intensity in the color standard tubes. A strain was classified as resistant if the color intensity in the antibiotic-containing tube was ≥2 color intensity gradations above that of the 1:500 tube. By use of spectrophotometry, a strain was classified as resistant if the antibiotic-containing tube had an optical density at 570 nm (OD570) >0.03 OD unit above the OD570 recorded in the 1:500 tube.
Manual MGIT.
AST was performed according to the manufacturer's instructions (BACTEC MicroMGIT; Becton Dickinson). The following critical concentrations were used: 0.1 μg/ml for INH and 1.0 μg/ml for RIF.
Radiometric BACTEC 460TB.
Antibiotic susceptibility was determined by standard procedures in a BACTEC 460TB instrument (Becton Dickinson) (17) with the following critical concentrations: 0.2 μg/ml for INH and 2.0 μg/ml for RIF.
Resolution of discrepant results.
Strains with AST results that were discrepant between the CONRAS test and the BACTEC 460TB system were retested by additional methods. Thus, the Etest (AB BIODISK, Solna, Sweden) (23) providing quantitative drug susceptibility results for RIF and INH and the INNO-LiPA Rif. TB test (Innogenetics N.V., Ghent, Belgium) were used according to the manufacturers' instructions. For determination of susceptibility categories by the Etest, breakpoints of ≤1 and 0.2 μg/ml for RIF and INH, respectively, were utilized (Etest application sheet; AB BIODISK). The INNO-LiPA Rif. TB test identifies mutations associated with RIF resistance in the gene encoding the β-subunit of RNA polymerase (rpoB) (18).
Data analysis.
The performance of the CONRAS test and the manual MGIT system in comparison with the BACTEC 460TB system was evaluated in terms of sensitivity, specificity, and positive and negative likelihood ratios for the two drugs tested. A positive likelihood ratio above 10 or a negative likelihood ratio below 0.1 was considered to indicate excellent test-performance, whereas ratios above 5 and below 0.2 were taken to indicate adequate performance (5, 8). The independent sample t test was used to compare the times needed for CONRAS test completion for susceptible versus resistant strains. The agreement between the CONRAS results, the manual MGIT results, and the BACTEC 460TB AST results, as well as CONRAS repeatability, were estimated by the kappa statistic. The kappa value, a measure of test reliability, was interpreted as follows: <0.2, poor; 0.21 to 0.4, fair; 0.41 to 0.6, moderate; 0.61 to 0.8, good; ≥0.81, excellent (1).
Costs.
Costs (shipping, custom taxes, and labor not included) were calculated by using purchase records.
RESULTS
Comparison between CONRAS, manual MGIT, and BACTEC 460TB tests.
All 74 M. tuberculosis strains were nitrate positive and were therefore tested for susceptibility to RIF and INH at low antibiotic concentrations by the CONRAS test. Strains that were resistant at the low drug concentrations were tested at high concentrations (39 tests for INH; 22 tests for RIF). The sensitivity (i.e., the ability to detect true drug resistance) and specificity (i.e., the ability to detect true drug susceptibility) of the CONRAS test at low critical concentrations of the drugs, by using the BACTEC 460TB system as the reference, were 100 and 83% for INH and 94 and 89% for RIF, respectively. The corresponding sensitivities and specificities at high antibiotic concentrations were 100 and 95% for INH and 94 and 100% for RIF, respectively (Table 1). At low concentrations, there was excellent agreement for INH and good agreement for RIF between the CONRAS test and the BACTEC 460TB system (kappa values, 0.81 for INH and 0.76 for RIF; P < 0.001). However, at high concentrations, there was excellent agreement for both INH and RIF between the two tests (kappa, 0.95 and 0.96, respectively; P < 0.001).
TABLE 1.
Performance of the CONRAS test compared with the BACTEC 460TB as a reference method for 74 M. tuberculosis strains
| Drug | CONRASa result | No. of isolates with the following BACTEC 460TBb result:
|
Sensitivity (%) | Specificity (%) | Likelihood ratio
|
||
|---|---|---|---|---|---|---|---|
| Susceptible | Resistant | Positive | Negative | ||||
| INH | Susceptible | 40 | 0 | 100 | 95 | 21.0 | 0.00 |
| Resistant | 2 | 32 | |||||
| RIF | Susceptible | 57 | 1 | 94 | 100 | Infinite | 0.06 |
| Resistant | 0 | 16 | |||||
Drug concentrations, 0.4 μg/ml for INH and 2.0 μg/ml for RIF.
Drug concentrations, 0.2 μg/ml for INH and 2.0 μg/ml for RIF.
There were three discordant single-drug susceptibility results between the high-antibiotic-concentration CONRAS test and the BACTEC 460TB system (Table 1). Thus, one strain tested for single-drug susceptibility was determined to be RIF susceptible by the CONRAS test as well as the manual MGIT test but was resistant by the BACTEC 460TB system (the strain was resistant to INH by all three methods). A repeat CONRAS test confirmed the original result. The Etest showed that the RIF MIC for the strain was 0.38 μg/ml. The INNO-LiPA Rif. TB test classified the strain as RIF resistant (ΔS2 mutation). Furthermore, two strains tested for single-drug susceptibility were determined to be resistant in both low and high concentrations of INH by the CONRAS test but were susceptible in the BACTEC 460TB system (both strains were susceptible to RIF). The Etest confirmed that the strains were susceptible to INH (MIC, <0.016 μg/ml).
There were seven discordant single-drug susceptibility results between the manual MGIT and the BACTEC 460TB system (Table 2). Three strains tested for single-drug susceptibility were characterized as susceptible for RIF by the manual MGIT but as resistant by the BACTEC 460TB system (Table 2). Four strains tested for single-drug susceptibility were determined to be INH resistant by the manual MGIT but were susceptible in the BACTEC 460TB system (Table 2). There was excellent agreement between the manual MGIT system and the BACTEC 460TB system for INH and RIF (kappa, 0.89 and 0.88, respectively; P < 0.001). There were six discordant single-drug susceptibility results between the CONRAS test and the manual MGIT (Table 3), and the agreement between the two tests for INH and RIF was excellent (kappa, 0.89 and 0.92, respectively; P < 0.001 for both).
TABLE 2.
Performance of the manual MGIT compared with the BACTEC 460TB as a reference method for 74 M. tuberculosis strains
| Drug | MGITa result | No. of isolates with the following BACTEC 460TBb result:
|
Sensitivity (%) | Specificity (%) | Likelihood ratio
|
||
|---|---|---|---|---|---|---|---|
| Susceptible | Resistant | Positive | Negative | ||||
| INH | Susceptible | 38 | 0 | 100 | 90 | 10.5 | 0.00 |
| Resistant | 4 | 32 | |||||
| RIF | Susceptible | 57 | 3 | 82 | 100 | Infinite | 0.18 |
| Resistant | 0 | 14 | |||||
Drug concentrations, 0.1 μg/ml for INH and 1.0 μg/ml for RIF.
Drug concentrations, 0.2 μg/ml for INH and 2.0 μg/ml for RIF.
TABLE 3.
Performance of the CONRAS test compared with the manual MGIT as a reference method for 74 M. tuberculosis strains
| Drug | CONRASa result | No. of isolates with the following manual MGITb result:
|
Sensitivity (%) | Specificity (%) | Likelihood ratio
|
||
|---|---|---|---|---|---|---|---|
| Susceptible | Resistant | Positive | Negative | ||||
| INH | Susceptible | 37 | 3 | 92 | 97 | 34.8 | 0.09 |
| Resistant | 1 | 33 | |||||
| RIF | Susceptible | 58 | 0 | 100 | 97 | 30.0 | 0.00 |
| Resistant | 2 | 14 | |||||
Drug concentrations, 0.4 μg/ml for INH and 2.0 μg/ml for RIF.
Drug concentrations, 0.1 μg/ml for INH and 1.0 μg/ml for RIF.
Spectrophotometric reading.
Susceptibility results were also recorded spectrophotometrically on a subset of 24 isolates. Excellent agreement was achieved between the spectrophotometric and visual recordings of the CONRAS test when high concentrations of RIF and INH were used (kappa, 1 and 0.89, respectively; P < 0.001 for both).
Repeatability.
The repeatability of the CONRAS test as determined in the panel of 18 duplicates was 100% (18 of 18) for INH and 94% (17 of 18) for RIF. The repeatability of the CONRAS test for the two key anti-TB drugs was excellent (for INH, kappa = 1 and P < 0.001; for RIF, kappa = 0.88 and P < 0.001).
Turnaround time.
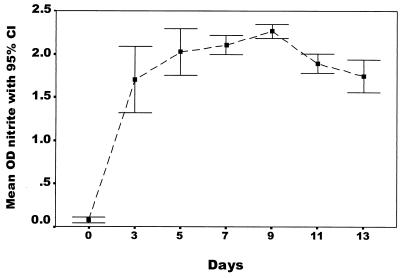
Nitrite production increased rapidly during the first 3 days of growth and reached a plateau after day 5 (Fig. 2). The average time required for obtaining a susceptibility result by the CONRAS test was 5.4 days (range, 3 to 9), compared to 5.1 days (range, 5 to 8) for the BACTEC 460TB and 5.0 days (range, 5 to 6) for the manual MGIT system. Susceptibility results by the CONRAS test were available in 5 days for 66% of the strains, in 7 days for 93% of the strains, and in 9 days for 100% of the strains. The results for fully susceptible strains were significantly likely to be available before those for strains with any resistance (means, 4.9 and 5.7 days, respectively; P < 0.001).
FIG. 2.
Nitrite production (y axis) measured in 24 M. tuberculosis strains during 2 weeks' growth in Middlebrook 7H9 broth with supplements.
DISCUSSION
Rapid AST results for M. tuberculosis are a prerequisite for initiating effective anti-TB treatment. Rapid and low-cost AST procedures for the testing of INH and RIF, the two anti-TB drugs that define MDR TB, are urgently needed, particularly in poor countries where TB is endemic.
In this study, we describe the evaluation of a nitrate reductase-based assay in a liquid medium as a rapid and reliable AST for RIF and INH. More than 99% (9) of M. tuberculosis strains possess nitrate reductase and are capable of reducing nitrate to nitrite. Some other mycobacterial species (M. kansasii, M. smegmatis, M. flavescens, and M. fortuitum) also possess this enzyme. However, these strains are not frequently encountered in human infections, and they can be identified by morphological and biochemical tests. It has been shown that the peak in the nitrate reductase activity of M. tuberculosis takes place after 3 to 4 weeks of bacterial incubation in LJ medium (12). The time to achieve peak activity can be considerably shortened by growing the bacterium in a liquid medium (Fig. 2).
The CONRAS test was evaluated against two commercial systems: the radiometric BACTEC 460TB semiautomated system (Table 1) and the manual liquid system (MGIT) (Table 3). Seventy-four M. tuberculosis strains, including 20 well-characterized strains, and, in addition, a panel of 18 duplicates (to assess repeatability) were used in the evaluation. The average time for a susceptibility result by the CONRAS test was 5.4 days, which was comparable to turnaround times for the BACTEC 460TB and manual MGIT systems. The repeatability of the CONRAS test for the two key anti-TB drugs was excellent. Susceptibility data obtained by the CONRAS method for these drugs compared excellently with those from the BACTEC 460TB (Table 1) and manual MGIT (Table 3), suggesting that the CONRAS method is useful in providing rapid and reliable AST results for these antibiotics. Previous studies have shown that the manual MGIT is a reliable, rapid, and convenient method for performing AST; however, cost is a factor prohibiting widespread implementation of this test (6, 19, 22). This study shows that the CONRAS test matches the manual MGIT system in the reliability of susceptibility results and the turnaround time.
The CONRAS test was first performed at low concentrations of the antibiotics, and strains that were found resistant at these concentrations were then retested at high antibiotic concentrations. The overall agreement of the CONRAS test with the BACTEC 460TB system was 91% (134 out of 148 drug susceptibility tests) when the strains were tested at low antibiotic concentrations and 98% (145 out of 148 tests) when the resistant strains were retested with the high concentration of the antibiotic in question (Table 1). Thus, it is recommended that the CONRAS test be performed using critical concentrations of 0.4 μg/ml for INH and 2.0 μg/ml for RIF.
The emergence of MDR strains of M. tuberculosis poses a serious problem in TB control. Resistance to RIF is almost always associated with MDR (21). Thus, RIF resistance can serve as a surrogate marker for the detection of MDR strains in resource-poor settings. In our study, there were 17 strains that were resistant to both INH and RIF. The CONRAS assay correctly identified 16 of these 17 strains. The remaining strain was resistant to INH and RIF by the BACTEC 460TB system but was susceptible to RIF by the CONRAS test and the manual MGIT method. The sensitivity of the CONRAS test depends on the metabolic activity of viable cells in order to achieve measurable nitrate reduction. It has been suggested that drug-resistant isolates may have lower metabolic activity (7); for example, rpoB mutations leading to RIF resistance might affect the expression levels of particular enzymes. This strain could have a low level of resistance that could not be detected by the CONRAS test or the manual MGIT. This assumption was corroborated by the Etest, which revealed that the MIC for the strain was 0.38 μg/ml, i.e., close to the breakpoint between susceptible and resistant strains (the RIF MIC range for the fully susceptible strain H37RV is 0.06 to 0.25) (23). The INNO-LiPA test revealed that the strain had a relatively uncommon resistant genotype (ΔS2 mutation in the rpoB gene) (18).
There was excellent agreement between the visual and spectrophotometric recording of AST results by the CONRAS method for both drugs. Thus, the CONRAS test is not particularly vulnerable to observer misinterpretation, and visual recording of results can replace spectrophotometric readings.
We calculated the cost for AST by the CONRAS test for the two first-line anti-TB drugs per strain to be approximately U.S. $3. This is substantially less expensive than the BACTEC 460TB (U.S. $21) and the manual MGIT system (U.S. $23). Thus, the CONRAS test is a cheap and reliable alternative for the rapid detection of MDR TB.
Acknowledgments
We thank E. Ulvestad for providing excellent laboratory facilities, H. Sommerfelt and A. Digranes for valuable comments, and E. Rønnild and H. Valvatne for assistance in the laboratory.
This study was funded in part by the Norwegian Research Council, University of Bergen, and the Meltzer Høyskolefond.
REFERENCES
- 1.Altman, D. G. 1999. Inter-rater agreement, p. 403-409. In D. G. Altman (ed.), Practical statistics for medical research. Chapman & Hall/CRC, London, United Kingdom.
- 2.Ängeby, K. A. K., L. Klintz, and S. E. Hoffner. 2002. Rapid and inexpensive drug susceptibility testing of Mycobacterium tuberculosis with a nitrate reductase assay. J. Clin. Microbiol. 40:553-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caviedes, L., J. Delgado, and R. H. Gilman. 2002. Tetrazolium microplate assay as a rapid and inexpensive colorimetric method for determination of antibiotic susceptibility of Mycobacterium tuberculosis. J. Clin. Microbiol. 40:1873-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caviedes, L., T. Lee, R. H. Gilman, P. Sheen, E. Spellman, E. H. Lee, D. E. Berg, S. Montenegro-James, and The Tuberculosis Working Group in Peru. 2000. Rapid, efficient detection and drug susceptibility testing of Mycobacterium tuberculosis in sputum by microscopic observation of broth cultures. J. Clin. Microbiol. 38:1203-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deeks, J. J. 2001. Systematic reviews of evaluations of diagnostic and screening tests. BMJ 323:157-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goloubeva, V., M. Lecocq, P. Lassowsky, F. Matthys, F. Portaels, and I. Bastian. 2001. Evaluation of Mycobacteria Growth Indicator Tube for direct and indirect drug susceptibility testing of Mycobacterium tuberculosis from respiratory specimens in a Siberian prison hospital. J. Clin. Microbiol. 39:1501-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inderlied, C. B., and M. Salfinger. 1999. Antimycobacterial agents and susceptibility test, p. 1601-1623. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 8.Jaeschke, R., G. H. Guyatt, D. L. Sackett, et al. 1994. Users' guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? JAMA 271:703-707. [DOI] [PubMed] [Google Scholar]
- 9.Kent, P. T., and G. P. Kubica. 1985. Public health mycobacteriology. A guide for the level III laboratory, p. 96-103. U.S. Department of Health and Human Services, Atlanta, Ga.
- 10.Kilburn, J. O., and K. Takayama. 1981. Effects of ethambutol on accumulation and secretion of trehalose mycolates and free mycolic acids in Mycobacterium smegmatis. Antimicrob. Agents Chemother. 20:401-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laszlo, A., M. Rahman, M. Raviglione, and F. Bustreo. 1998. Quality assurance programme for drug susceptibility testing of Mycobacterium tuberculosis in the WHO/IUATLD Supranational Laboratory Network: first round of proficiency testing. Int. J. Tuberc. Lung Dis. 2:69-70. [PubMed] [Google Scholar]
- 12.Master, R. N. 1992. Identification tests for mycobacteria, part 3.122, p. 1-29. In H. O. Isenberg (ed.), Clinical microbiology procedures handbook, vol. 1. American Society for Microbiology, Washington, D.C.
- 13.Mshana, R. N., G. Tadesse, G. Abate, and H. Miørner. 1998. Use of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide for rapid detection of rifampin-resistant Mycobacterium tuberculosis. J. Clin. Microbiol. 36:1214-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nachamkin, I., C. Kang, and M. P. Weinstein. 1997. Detection of resistance to isoniazid, rifampin, and streptomycin in clinical isolates of Mycobacterium tuberculosis by molecular methods. Clin. Infect. Dis. 24:894-900. [DOI] [PubMed] [Google Scholar]
- 15.Palomino, J. C., and F. Portaels. 1999. Simple procedure for drug susceptibility testing of Mycobacterium tuberculosis using a commercial colorimetric assay. Eur. J. Clin. Microbiol. Infect. Dis. 18:380-383. [DOI] [PubMed] [Google Scholar]
- 16.Phyu, S., T. Ti, R. Jureen, T. Mmun, H. Myint, A. Tun, H. M. S. Grewal, and B. Bjorvatn. 2003. Drug resistant tuberculosis among new patients in Yangon, Myanmar. Emerg. Infect. Dis. 9:274-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts, G. D., N. L. Goodman, L. Heifets, H. W. Larsh, T. H. Linder, J. K. McClatchy, M. R. McGinnis, S. H. Siddiqi, and P. Wright. 1983. Evaluation of the BACTEC radiometric method for recovery of mycobacteria and drug susceptibility testing of Mycobacterium tuberculosis from acid-fast smear-positive specimens. J. Clin. Microbiol. 18:689-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossau, R., H. Traore, H. De Beenhouwer, W. Mijs, G. Jannes, P. De Rijk, and F. Portaels. 1997. Evaluation of the INNO-LiPA Rif. TB assay, a reverse hybridization assay for the simultaneous detection of Mycobacterium tuberculosis complex and its resistance to rifampin. Antimicrob. Agents Chemother. 41:2093-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rüsch-Gerdes, S., C. Domehl, G. Nardi, M. R. Gismondo, H.-M. Welscher, and G. E. Pfyffer. 1999. Multicenter evaluation of the mycobacterial growth indicator tube for testing susceptibility of Mycobacterium tuberculosis to first-line drugs. J. Clin. Microbiol. 37:45-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toungoussova, O. S., P. Sandven, A. O. Mariandyshev, N. I. Nizovtseva, G. Bjune, and D. A. Caugant. 2002. Spread of drug-resistant Mycobacterium tuberculosis strains of the Beijing genotype in the Archangel Oblast, Russia. J. Clin. Microbiol. 40:1930-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vareldzis, B. P., J. Grosset, I. De Kantor, J. Crofton, A. Laszlo, M. Felten, M. C. Raviglione, and A. Kochi. 1994. Drug-resistant tuberculosis: laboratory issues. World Health Organization recommendations. Tuber. Lung Dis. 75:1-7. [DOI] [PubMed] [Google Scholar]
- 22.Walters, S. B., and B. A. Hanna. 1996. Testing of susceptibility of Mycobacterium tuberculosis to isoniazid and rifampin by mycobacterium growth indicator tube method. J. Clin. Microbiol. 34:1565-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wanger, A., and K. Mills. 1996. Testing of Mycobacterium tuberculosis susceptibility to ethambutol, isoniazid, rifampin, and streptomycin by using Etest. J. Clin. Microbiol. 34:1672-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Global tuberculosis control: surveillance, planning, financing. WHO Report 2002. WHO/CDS/TB/2002.295. World Health Organization, Geneva, Switzerland.