Abstract
Proteus mirabilis compromises the care of many patients undergoing long-term indwelling bladder catheterization. It forms crystalline bacterial biofilms in catheters which block the flow of urine, causing either incontinence due to leakage or painful distention of the bladder due to urinary retention. If it is not dealt with, catheter blockage can lead to pyelonephritis and septicemia. We have examined the epidemiology of catheter-associated P. mirabilis infections by use of pulsed-field gel electrophoresis (PFGE) of NotI restriction enzyme digests of bacterial DNA. This technique was shown to be more discriminatory than the classical phenotypic Dienes typing technique. We demonstrated that each of 42 isolates from diverse environmental sources and 10 of 12 isolates from blood, wound swabs, and mid-stream urine samples of hospitalized patients had distinct genotypes. Examination of a set of 55 isolates of P. mirabilis, each from a different clinical or environmental source, identified 49 distinct genotypes and 43 Dienes types. The index of discrimination was 0.993 for the PFGE method and 0.988 for the Dienes method. Applying the PFGE method to isolates from catheter-associated urinary tract infections confirmed that the strains present in the crystalline catheter biofilms were identical to those isolated from the same patient's urine. An analysis of samples taken during a prospective study of infections in catheterized nursing home patients revealed that a single genotype of P. mirabilis can persist in the urinary tract despite many changes of catheter, periods of noncatheterization, and antibiotic therapy.
A common complication in the care of patients undergoing long-term bladder catheterization is recurrent encrustation and blockage of catheters. The problem stems from infection by Proteus mirabilis (12, 19). These bacterial cells colonize the catheter surfaces, forming biofilm communities embedded in a polysaccharide matrix. The bacterial urease enzyme generates ammonia and elevates the pH of the urine and the biofilm. Under these conditions, struvite (magnesium ammonium phosphate) and apatite (calcium phosphate) are formed and become trapped in the organic matrix which surrounds the cells (13). The continued development of this crystalline biofilm completely blocks the catheter lumen, obstructing the flow of urine and causing either incontinence due to leakage or painful distention of the bladder due to urinary retention. Bacteriuria is inevitably associated with the encrustation, so retention and vesico-ureteral reflux can induce ascending infection culminating in episodes of pyelonephritis, septicemia, and shock (9). All currently available types of Foley catheter are vulnerable to encrustation, and currently, there are no effective procedures for controlling the problem (1, 9, 14). Despite the clinical significance of these infections and the occurrence of outbreaks in hospitals and nursing homes, previous studies have not examined the molecular epidemiology of catheter-associated P. mirabilis infections.
A well-known characteristic of P. mirabilis is its ability to swarm over the surface of agar media. Many years ago, Dienes (3) described a test for discrimination between strains of this species based on the mutual inhibition of different strains as they swarm towards each other on a plate. If strains are different, a clear line will form as the swarming fronts repel each other. If the strains are related, there is no mutual repulsion, the swarming fronts merge, and no line of demarcation develops. This simple test can thus be used to determine whether isolates are the same or different (5, 17, 18). More recently, Pfaller et al. (16) evaluated the discriminatory power of the Dienes test compared to that of ribotyping, a method that detects polymorphisms in the DNA encoding the rRNA operons. They concluded that the Dienes method was just as discriminatory as the genotyping technique and had the advantages that it was simple, inexpensive, and easy to perform.
This paper reports the application of pulsed-field gel electrophoresis (PFGE) of restriction enzyme digests of P. mirabilis DNA to the study of catheter-associated infections. The discriminatory power of the method was investigated, and then we examined aspects of the molecular epidemiology of chronic catheter-associated urinary tract infections which may have implications for the management of patients.
MATERIALS AND METHODS
Bacterial strains.
A collection of 118 isolates of P. mirabilis from clinical and environmental sources was studied (Table 1). Isolates were obtained from the urine and encrusted catheters of patients being cared for in their own homes, in two spinal injury centers, and in a local nursing home in Glamorgan, Wales, United Kingdom. Isolates from mid-stream urine specimens, blood cultures, and wound swabs were also obtained from the microbiology laboratory of a large general hospital. Environmental samples were collected from river water, seawater, and sewage at a number of geographically distinct sites. A subcollection of 55 isolates was drawn from this main collection in order to evaluate the discriminatory power of typing methods (see below). Cultures were identified by Gram stain, indole reaction, and the BBL crystal identification system (Beckton Dickinson Europe, Meylan, France). Isolates were stored at −70°C in 5% (vol/vol) glycerol.
TABLE 1.
Sources of the 118 isolates used for the study
| Source | No. of isolates |
|---|---|
| Clinical samples | |
| Catheter biofilms | ....19 |
| Catheter urine samplesa | ....33 |
| Mid-stream urine samples | ....15 |
| Blood cultures | ....3 |
| Wound swabs | ....6 |
| Environmental samples | |
| Sewage | ....20 |
| Seawater | ....4 |
| River water | ....18 |
Fourteen of these isolates were collected over 121 days from a single patient.
PFGE.
Genotyping of P. mirabilis strains was conducted by macrorestriction of bacterial DNA followed by separation of the resulting fragments by PFGE. This was based on a method originally applied to Burkholderia cepacia complex bacteria (21) and adapted as follows. Overnight cultures of P. mirabilis in 3 ml of tryptone soy broth were centrifuged to harvest bacterial cells. These were then resuspended to an optical density at 600 nm of 1.0 in SE buffer (75 mM NaCl, 25 mM EDTA). Suspensions were warmed to 45°C and mixed with an equal volume of molten 2% low-gelling-temperature agarose (Type VII; Sigma Chemicals, Poole, United Kingdom). This mixture was then pipetted into 70-μl disposable plug molds (Bio-Rad, Hitchin, United Kingdom) and allowed to set at 4°C. Three plugs per strain were placed in 15-ml sterile tubes containing 10 ml of Pen buffer (0.5 M EDTA and 1% N-lauryl sarcosine) plus pronase (Roche Biochemicals, Lewes, United Kingdom) at 1 mg/ml and were subjected to 18 to 24 h of incubation at 37°C, with gentle rocking. The plugs were washed with five volume changes of TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0). Slices of the plugs (3-mm thick) were then subjected to restriction endonuclease digestion overnight at 37°C. The digestion mixture (150 μl) contained 5 U of NotI enzyme (see Results) and was set up in accordance with the manufacturer's recommendations (New England BioLabs, Inc., Hitchin, United Kingdom). The plugs were loaded into 1.2% agarose gels made with 1× TBE buffer (Tris-boric acid-EDTA) and sealed with 1.2% low-gelling-temperature agarose. Bacterial DNA fragments were then separated by electrophoresis at 6 V/cm for 8 h, with a switch time of 1 to 30 s, and then for 16 h, with a switch time of 30 to 70 s, at 14°C by use of a CHEF-DR II apparatus (Bio-Rad). Bacteriophage lambda DNA ladders were included as size standards. Gels were then stained with ethidium bromide, destained under running water, and photographed under UV light. The epidemiological relationship of strains to each other was assessed by use of the Tenover criteria (20).
Dienes typing.
Dienes typing was performed as previously described (18). Isolates of P. mirabilis were plated for single colonies on CLED agar (Oxoid Ltd., Basingstoke, United Kingdom) and incubated overnight at 37°C. Single colonies from up to eight isolates were then inoculated as macro-colonies onto tryptone soy agar plates (Oxoid Ltd.). After overnight incubation at 37°C, those isolates showing a clear band (Dienes demarcation line) between each other were designated different Dienes types, while those with no such line were regarded as the same Dienes type. Comparisons of each isolate to all other isolates were made in triplicate.
Discriminatory index analysis.
To compare the efficacy of PFGE fingerprinting and Dienes testing for P. mirabilis strain typing, a representative subset of 55 isolates was analyzed. The subset comprised 36 clinical and 19 environmental isolates, each from a distinct source (Table 2). Four isolates were chosen to be representatives of a single known PFGE strain type. The remaining 51 were chosen at random from the collection. All isolates were then assigned an identification number and typed blindly by both Dienes testing and PFGE. In order to evaluate the discriminatory power of PFGE typing in comparison to Dienes testing, a discriminatory index was calculated as previously described (6).
TABLE 2.
Sources of the 55 isolates used for comparison of Dienes and PFGE typing
| Source | No. of isolates |
|---|---|
| Clinical samples | |
| Catheter biofilms | ....15 |
| Catheter urine samples | ....10 |
| Mid-stream urine samples, wound swabs, and blood | ....11 |
| Total clinical samples | ....36 |
| Environmental samples | |
| Sewage | ....7 |
| Seawater | ....2 |
| River water | ....10 |
| Total environmental samples | ....19 |
RESULTS
Macrorestriction PFGE analysis for P. mirabilis typing.
Efficient PFGE typing of bacteria is heavily dependent on the choice of a restriction enzyme that produces relatively few DNA fragments. Previous studies have used the enzymes SmaI, XbaI, SfiI, and NotI for genotyping P. mirabilis (2, 11, 15, 16). Since P. mirabilis DNA has a G+C content of 39% (4), our initial experiments aimed at identification of enzymes optimal for typing of P. mirabilis used enzymes having G+C-rich recognition sequences. AvaI, BamHI, and SmaI were shown to cut too frequently and to produce many small-molecular-weight fragments, resulting in profiles that were unsuitable for typing. NotI and SfiI both produced clear restriction profiles, but we decided to optimize the method by using NotI, as this enzyme produced profiles that were more readily interpretable.
Typing of environmental and clinical P. mirabilis isolates.
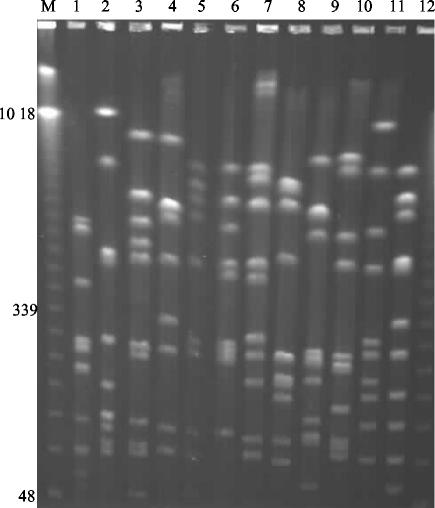
The efficacy of the NotI PFGE typing method was tested on representative collections of P. mirabilis recovered from raw sewage, seawater, river water, and clinical specimens. The environmental isolates were examined as representatives of potentially highly diverse and unrelated strains. The resulting profiles produced by PFGE of the NotI digests of P. mirabilis DNA isolated from raw sewage are presented in Fig. 1. It is clear that each isolate produced distinct macrorestriction profiles. A further 30 environmental isolates were typed, and all were unique strain types (data not shown).
FIG. 1.
PFGE profiles of 12 isolates of P. mirabilis from raw sewage. Lane M, molecular size markers. Relevant sizes of bands are indicated in kilobases.
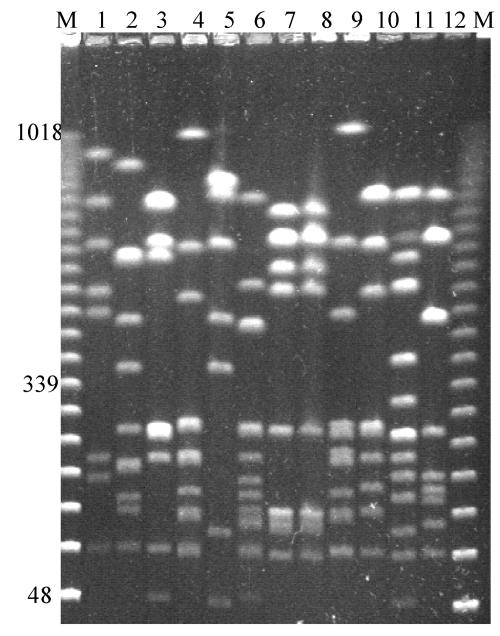
An analysis of 12 clinical isolates obtained from blood, mid-stream urine samples, or wound swabs from patients in a general hospital showed 10 distinct profiles (Fig. 2). Two of the isolates that gave identical profiles were from the blood and urine samples of the same patient. The other 12 isolates from mid-stream urine samples, blood cultures, and wound swabs (Table 1) gave 11 distinct profiles (data not shown).
FIG. 2.
PFGE profiles of 12 isolates of P. mirabilis from mid-stream urine samples, blood samples, and wound swabs from patients in a district general hospital. The isolates in lanes 7 and 8 were from the urine and blood, respectively, of the same patient. Lanes M, molecular size marker. Relevant sizes of bands are indicated in kilobases.
Comparison of PFGE typing and Dienes testing.
Strain typing by PFGE is time-consuming and requires special equipment. The Dienes test, a simple culture-based phenotypic inhibition test, has been widely applied to differentiate P. mirabilis isolates. To evaluate strain typing efficacy, the discriminatory power of PFGE of NotI digests of bacterial DNA was compared to that of the classical Dienes typing technique. A random subset of 55 isolates of P. mirabilis, each from a different clinical or environmental source, were selected. With the Dienes method, 43 distinct types were identified. The same set of isolates produced 49 distinct PFGE fingerprint types (Table 3). The discriminatory index for each typing method was calculated using the generalized formula proposed by Hunter (6) and was found to be 0.988 for the Dienes test and 0.993 for PFGE.
TABLE 3.
Comparison of Dienes and PFGE typing of 55 P. mirabilis isolates from different sources
| Isolate reference no.a | Source | Dienes type | PFGE type |
|---|---|---|---|
| NP1 | Catheter biofilm | 1 | 1 |
| NP2 | Catheter urine | 1 | 1 |
| NP3 | Catheter urine | 1 | 1 |
| NP4 | Catheter biofilm | 1 | 1 |
| NP5 | Catheter urine | 2 | 2 |
| NP6 | Catheter biofilm | 2 | 2 |
| NP22 | River water | 3 | 3 |
| NP38 | Catheter biofilm | 3 | 4 |
| NP56 | Sewage | 3 | 5 |
| NP33 | Catheter urine | 4 | 6 |
| NP50 | Catheter biofilm | 4 | 7 |
| NP34 | Seawater | 5 | 8 |
| NP20 | Catheter urine | 5 | 9 |
| NP41 | Catheter biofilm | 6 | 10 |
| NP44 | Catheter biofilm | 6 | 11 |
| NP46 | Catheter biofilm | 6 | 11 |
| NP42 | Catheter urine | 7 | 12 |
| NP45 | Catheter urine | 7 | 12 |
| NP47 | Blood culture | 8 | 13 |
| NP51 | Catheter urine | 8 | 13 |
| Remaining 35 isolates | Various sources | 9-43 | 14-49 |
NP1 and NP2, NP3 and NP4, NP5 and NP6, and NP47 and NP51 were isolated from pairs of samples from four different patients.
Molecular epidemiology of catheter-associated infections.
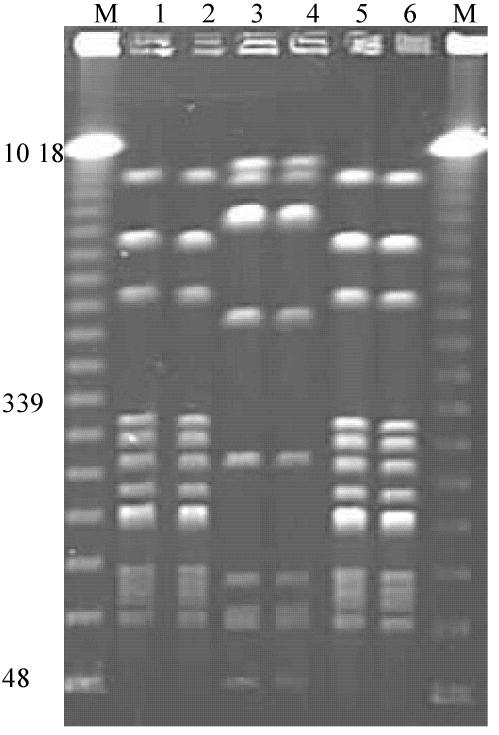
In order to determine the epidemiology of catheter-associated urinary tract infections, genetic typing was performed on a collection of 19 isolates from catheter biofilms and 33 isolates from catheter urine samples (Table 1). The results of the analysis of six P. mirabilis isolates from the urine and crystalline biofilms on catheters of three patients residing in a local nursing home are presented in Fig. 3. The macrorestriction profiles of the P. mirabilis strains isolated from the urine and catheter of each patient were identical. In addition, two of the patients were infected with organisms possessing identical PFGE fingerprints.
FIG. 3.
PFGE profiles of pairs of isolates of P. mirabilis from urine samples and catheter biofilms of catheterized patients residing in a nursing home. Lanes 1 and 2, 3 and 4, and 5 and 6 are pairs of isolates from three patients. Lanes M, molecular size markers. Relevant sizes of bands are indicated in kilobases.
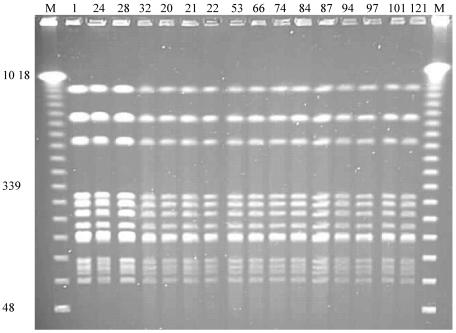
During the course of a prospective study on the urinary flora of patients with indwelling catheters at a local nursing home, we isolated P. mirabilis repeatedly from the urine of one patient over a period of 121 days. Results of PFGE analysis of the DNA from 14 of these isolates and 2 isolates from catheters removed from this patient are shown in Fig. 4. These patterns indicate that the same strain was colonizing the urinary tract of this patient, even though over the 121 days the patient had undergone eight changes of catheter, an 8-day course of antibiotics (during which P. mirabilis was isolated from the urine on the first 2 days but not thereafter), and a 20-day period during which the bladder was not catheterized.
FIG. 4.
PFGE profiles of DNA from P. mirabilis isolated over 121 days from a catheterized patient residing at a nursing home. Lane numbers indicate the days on which the bacteria were isolated. Catheters were changed on days 2, 17, 31, 50, 56, 85, 93, and 101. The patient received antibiotic treatment for a respiratory infection during days 65 to 72 and was not catheterized between days 101 and 119. Isolates on days 32 and 101 were from catheter biofilms, and all others were from urine specimens. Lanes M, molecular size markers. Relevant sizes of bands are indicated in kilobases.
DISCUSSION
The lack of effective procedures for the control and prevention of catheter encrustation by crystalline P. mirabilis biofilms means that currently the problem is handled by management of the clinical crises as they arise (7). The usual approach is simply to replace the blocked catheter. The subsequent catheters commonly block rapidly, however, and the patient gains a reputation as a “blocker” (10). While there is clearly a need to improve catheter design and to develop novel biomaterials that are less vulnerable to colonization by P. mirabilis (8), it is also important to gain a better understanding of the epidemiology of these infections. To facilitate this approach, we used a PFGE-based genotyping method for P. mirabilis and demonstrated that it was capable of distinguishing unrelated strains isolated from diverse environmental and clinical sources (Fig. 1 and 2). The NotI macrorestriction profiles of P. mirabilis DNA obtained by PFGE proved to be highly discriminatory, giving a slightly better discrimination index than the classical Dienes method (Table 3).
A recent study by Pfaller et al. (16) used the Dienes test and the genotyping method of ribotyping to discriminate between 63 clinical isolates of P. mirabilis. They reported that both methods worked well, producing discrimination indexes of 0.980 for the Dienes test and 0.979 for ribotyping. Isolates that were indistinguishable by the Dienes test and/or ribotyping were characterized further by a PFGE method based on the use of the restriction enzyme SfiI. Forty of the isolates represented 40 different ribotypes and Dienes types. The other 23 isolates were grouped into 12 ribotypes, 13 Dienes types, and 14 PFGE types. Pfaller et al. (16) concluded that as the Dienes test is simple, inexpensive, and easy to perform and has good discriminatory powers, it should be the method of choice for the epidemiological characterization of P. mirabilis isolates. The results summarized in Table 3, however, indicate that PFGE based on the use of the NotI enzyme is more discriminatory than the Dienes test for P. mirabilis. While strains designated different by Dienes typing were all confirmed to have distinct genotypes, there were several cases for which Dienes typing did not distinguish between strains from diverse sources that had been shown to be distinct by PFGE. The isolates NP22, -38, and -56, for example, which were from river water from France, a catheter biofilm, and sewage from South Wales, respectively, were designated the same strain by Dienes but were clearly distinct by PFGE (Table 3). A further potential advantage of the genotyping method is that analysis of the DNA profiles by specialized software could be used to construct dendrograms showing the relatedness of all the isolates. This could provide epidemiological data that would not be available by Dienes typing.
Having established the discriminatory power of the NotI PFGE-based method, we went on to examine for the first time the molecular epidemiology of P. mirabilis catheter-associated urinary tract infections. The results confirmed that the organisms present on the crystalline biofilms encrusting catheters were identical to those isolated from the same patient's urine (Fig. 3). In several instances, Dienes analysis corroborated the epidemiological observations made by PFGE. For example, both methods confirmed that isolates (NP1 to NP4) from the catheter biofilms and urine samples of two catheterized patents residing in a nursing home were identical (Fig. 3 and Table 3). Isolates NP5 and NP6, from the catheter and urine, respectively, of a third patient at the nursing home, possessed different Dienes types, which was also confirmed by PFGE fingerprinting (Fig. 3 and Table 3).
Problems with potential cross-contamination of catheters were identified, as was the failure of both replacing catheters and antibiotic therapy as a means to control chronic infections (Fig. 3 and 4). The remarkable stability and persistence of a strain of P. mirabilis in a catheterized urinary tract were also revealed. These novel molecular epidemiological observations highlight challenges in the clinical management of P. mirabilis catheter-associated infections.
Many questions about the epidemiology and pathogenesis of P. mirabilis infections of the catheterized bladder need answers. Are the strains that cause catheter encrustation a particular subset of the species? Are the patients who suffer from recurrent catheter encrustation fecal carriers of the strain of P. mirabilis that forms the crystalline biofilms? Many patients who suffer recurrent catheter encrustation have been found to have bladder stones. Do these stones harbor the P. mirabilis strain that colonizes successive catheters? Application of the PFGE typing method to examine these questions could lead to the development of more effective strategies for controlling the complications caused by P. mirabilis in the care of the many patients enduring long-term indwelling bladder catheterization.
REFERENCES
- 1.Capewell, A. E., and N. S. Morris. 1993. Audit of catheter management provided by district nurses and continence advisors. Br. J. Urol. 71:259-264. [DOI] [PubMed] [Google Scholar]
- 2.Decre′, D., C. Verdet, L. Raskine, H. Blanchard, B. Burghoffer, A. Philippon, M. J. Sanson-Le-Pors, J. C. Petit, and G. Arlet. 2002. Characterization of CMY-type β-lactamases in clinical strains of Proteus mirabilis and Klebsiella pneumoniae isolated in four hospitals in the Paris area. J. Antimicrob. Chemother. 50:681-688. [DOI] [PubMed] [Google Scholar]
- 3.Dienes, L. 1946. Reproductive processes in Proteus cultures. Proc. Soc. Exp. Biol. Med. 63:265-270. [DOI] [PubMed] [Google Scholar]
- 4.Falkow, S., I. R. Ryman, and O. Washington. 1964. Deoxyribonucleic acid base composition of Proteus and Providence organisms. J. Bacteriol. 83:1318-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hickman, F. W., and J. J. Farmer III. 1976. Differentiation of Proteus mirabilis by bacteriophage typing and the Dienes reaction. J. Clin. Microbiol. 3:350-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter, D. 1990. Reproducibility and indices of discriminatory power of microbial typing methods. J. Clin. Microbiol. 28:1903-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohler-Ockmore, J., and R. C. Feneley. 1996. Long-term catheterisation of the bladder: prevalence and morbidity. Br. J. Urol. 77:347-351. [DOI] [PubMed] [Google Scholar]
- 8.Kunin, C. M. 1988. Can we build a better urinary catheter? N. Engl. J. Med. 319:365-366. [DOI] [PubMed] [Google Scholar]
- 9.Kunin, C. M. 1997. Urinary tract infections: detection, prevention and management, 5th ed., p. 226-278. Williams and Wilkins, Baltimore, Md.
- 10.Kunin, C. M., Q. F. Chin, and S. Chambers. 1987. Formation of encrustations on indwelling urinary catheters in the elderly: a comparison of different types of catheter materials in “blockers” and “non-blockers.” J. Urol. 138:899-902. [DOI] [PubMed] [Google Scholar]
- 11.Maslow, J. N., M. E. Mulligan, and R. D. Arbeit. 1993. Molecular epidemiology: application of contemporary techniques to the typing of microorganisms. Clin. Infect. Dis. 17:153-162. [DOI] [PubMed] [Google Scholar]
- 12.Mobley, H. L. T., and J. W. Warren. 1987. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J. Clin. Microbiol. 25:2216-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris, N. S., D. J. Stickler, and R. J. McLean. 1999. The development of bacterial biofilms on indwelling catheters. World J. Urol. 17:345-350. [DOI] [PubMed] [Google Scholar]
- 14.Morris, N. S., D. J. Stickler, and C. Winters. 1997. Which indwelling urethral catheters resist encrustation by Proteus mirabilis biofilms? Br. J. Urol. 80:58-63. [DOI] [PubMed] [Google Scholar]
- 15.Neuwirth, C., E. Siebor, A. Pechinot, J.-M. Duez, M. Pruneaux, F. Garel, A. Kazmierczak, and R. Labia. 2001. Evidence of in vivo transfer of a plasmid encoding the extended-spectrum β-lactamase TEM-24 and other resistance factors among different members of the family Enterobacteriaceae. J. Clin. Microbiol. 39:1985-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfaller, M. A., I. Mujeeb, R. J. Hollis, R. N. Jones, and G. V. Doern. 2000. Evaluation of the discriminatory powers of the Dienes test and ribotyping as typing methods for Proteus mirabilis. J. Clin. Microbiol. 38:1077-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senior, B. W. 1977. Typing of Proteus strains by proticene production and sensitivity. J. Med. Microbiol. 10:7-17. [DOI] [PubMed] [Google Scholar]
- 18.Skirrow, M. B. 1969. The Dienes (mutual inhibition) test in the investigation of Proteus infections. J. Med. Microbiol. 2:471-477. [DOI] [PubMed] [Google Scholar]
- 19.Stickler, D. J., L. Ganderton, J. King, J. Nettleton, and C. Winters. 1993. Proteus mirabilis biofilms and the encrustation of urethral catheters. Urol. Res. 21:407-411. [DOI] [PubMed] [Google Scholar]
- 20.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelson, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vandamme, P., E. Mahenthiralingam, B. Holmes, T. Coenye, B. Hoste, P. De Vos, D. Henry, and D. P. Speert. 2000. Identification and population structure of Burkholderia stabilis sp. nov. (formerly Burkholderia cepacia genomovar IV). J. Clin. Microbiol. 38:1042-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]