Abstract
Assessment of cure of Trypanosoma cruzi infection by antibody seroconversion usually involves several years of follow-up. Parasitological negativity is useless for cure assessment, since even untreated patients mostly show negative results; conversely, positive tests are of great value because they indicate treatment failure. Here, PCR was used to assess the rate of specific chemotherapy failure in a well-characterized Brazilian cohort of T. cruzi-seropositive children, who were enrolled in a field trial of benznidazole (Bz) efficacy. Paired blood samples from 111 children were taken at baseline and 36 months after treatment with either Bz (n = 58) or a placebo (n = 53). DNA extraction and PCR amplification were carried out as previously described, and hybridization was performed with all PCR products. At the end of follow-up, PCR was positive for 39.6% of the patients in the Bz group versus 64.2% in the placebo group (P = 0.01). Untreated patients had a 1.6-fold-higher chance of remaining positive by PCR than those in the Bz group (P < 0.05). We conclude that PCR is a useful tool for revealing therapeutic failure of T. cruzi infection on a short-term basis.
Trypanosoma cruzi infection affects about 15 million people in the Americas. Thirty percent of these patients may develop myocardial disease early in life. Improved triatominal vector control is being achieved in the South Cone and in some countries of Central America (32). Nevertheless, currently infected individuals should receive medical attention, and in the past few years etiological treatment has been proposed as an effective intervention, especially during the acute phase and in childhood, when its efficacy may reach 60% (1, 27). The tools for treatment assessment are mainly serological, with an expected negative seroconversion in those “cured,” after some years of follow-up.
The use of conventional parasitological tests (xenodiagnosis and hemoculture) is hampered by their low sensitivity, since more than half of untreated individuals will have a negative result (11, 14, 15, 18, 22). Moreover, these tests are time-consuming and are not commercially available. The recent introduction of the PCR for Chagas' disease (3, 4, 30) has opened wider perspectives, because this test has demonstrated higher sensitivity than hemoculture or xenodiagnosis (5, 12, 19, 23, 24, 31). PCR has been used to detect T. cruzi in the blood of chronic chagasic patients (8, 13, 17, 19) and may be a promising tool for evaluating parasitologic failure after specific etiologic treatment in chronic infections (6, 9, 10, 20), especially at early stages.
The purpose of the present study was to use PCR to assess the rate of specific chemotherapy failure in a well-characterized Brazilian cohort of T. cruzi-seropositive children, who were enrolled in a previously reported field trial of benznidazole (Bz) efficacy (1).
MATERIALS AND METHODS
Study population and data collection.
A population-based serological survey was carried out in a rural setting of high endemicity for Chagas' disease in central Brazil in order to select participants for a double-blind field trial to assess the efficacy of Bz among T. cruzi-infected children. Details of this methodology have been described previously (1). At entry into the trial, all participating children were concurrently positive by three conventional serological tests: indirect immunofluorescence (IIF) (≥1/40), indirect hemagglutination (IHA) (≥1/16), and enzyme-linked immunosorbent assay (ELISA) (index, ≥1.2), as published elsewhere (1). The trial was launched in 1991, and patients were followed up until 1997. Seropositive schoolchildren were randomly allocated to receive either Bz or a placebo (Pl) preparation. Tablets of the drug (Bz or Pl) were administered daily under the supervision of the classroom teacher at the beginning and end of the school day. During the weekends, the children received the exact number of tablets corresponding to the Saturday and Sunday doses for self-administration, under their mothers' supervision.
Venous blood was collected at baseline and also after 3 years of follow-up for serological testing and PCR evaluation. Blood samples (5 ml) were collected and mixed with 5 ml of 0.2 M EDTA-6 M guanidine hydrochloride (Sigma Chemical Co., St. Louis, Mo.) in sterile 15-ml Falcon centrifuge tubes (Becton Dickinson). After gentle mixing, samples were transported on ice at 4°C from the field to the Chagas' Disease Laboratory, in GoiÂnia city, where they were stored at −20°C until the end of the field study. Aliquots (1 to 2 ml) were transferred to sterile 2-ml polypropylene vials (Nunc) and sent at the end of follow-up to the Laboratório de Biologia do Trypanosoma cruzi, Departamento de Parasitologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil, where PCR was performed. All samples were coded and assigned a number before being sent for PCR evaluation. This blinded procedure was adopted in order to avoid observer bias during the PCR assay. Of 127 blood samples collected at the trial baseline, 111 (87.4%) were matched with a second sample 36 months after intervention, yielding a total of 222 blood specimens. Codes were broken after the final serological and PCR results were provided. All the study area was sprayed with insecticide every 6 to 8 months for 6 years, starting with the beginning of the trial.
Preparation of DNA for PCR.
The blood samples stored were maintained 1 week at room temperature and then boiled at 100°C for 15 min to shear the DNA molecules, when a 200-μl aliquot was collected from each sample and DNA extraction was performed (7, 19). DNA extraction and detection of the ≈330-bp fragment of T. cruzi kinetoplast DNA (kDNA) by PCR were performed at the same time, 3 years after the intervention. PCR hybridization was repeated up to three times for 32 (13.4%) out of 238 samples, which presented doubtful results.
PCR conditions.
PCR amplification was performed in a total volume of 20 μl containing 0.1% Triton X-100, 10 mM Tris-HCl (pH 9.0), 75 mM KCl, 5 mM Cl2Mg3, 0.2 mM each dATP, dTTP, dGTP, and dCTP (Sigma Chemical Co.), 1 μl of Taq DNA polymerase (Promega Corp.), 20 pmol of primers 121 [5′-AAATAATGTACGG(T/G)-GAGATGCATGA-3′] and 122 (5′-GGTTCGATTGGGGTTGGTGTAATATA-3′) (Operon Technologies Inc.), and 2 μl of DNA from each sample (16, 19). The reaction mixture was overlaid with 30 μl of mineral oil (Sigma) to prevent evaporation and was subjected to 35 cycles of amplification in an automatic thermocycler (MJ Research Programmable Thermal Controller PTC-100) using 0.5-ml plastic microtubes. The temperature profile was as follows: 95°C for 1 min for denaturation (with a longer initial time of 5 min at 95°C), 65°C for 1 min for primer annealing, and 72°C for 1 min for extension, with a final incubation at 72°C for 10 min to extend the annealed primers. The PCR products were visualized by 6% polyacrylamide gel electrophoresis and silver stained (26). All DNA extraction steps and reaction mixtures used for PCR were monitored and compared with positive and negative controls. To avoid contamination, the reaction steps were performed in separate environments using equipment and reagents destined exclusively for each stage. The sizes of the amplified bands were monitored by using a 100-bp molecular size marker ladder (Promega Corp.).
Slot blot hybridization.
All samples were submitted to hybridization in a slot blot (Hoefer Scientific Instruments) with a specific probe that hybridized internally to the 330-bp fragment amplified by PCR. This probe consisted of the oligonucleotide 5′-TGGTTTTGGGAGGGGCGTTCAAATTT-3′ labeled with alkaline phosphatase (28) and was synthesized by Life Technologies. This technique confirms the specificity of the amplified product and/or increases the sensitivity of the protocol. When PCR alone is employed, 10 fg of parasite DNA may be detected in the polyacrylamide gel, while hybridization permits detection of as little as 0.1 fg (19).
Data analysis.
To estimate the relative sensitivity of PCR and the respective 95% confidence intervals (95% CI), concurrently positive results by all three conventional serological techniques (IIF, IHA, and ELISA) at baseline were assumed a priori to constitute the “gold standard.” The “intention-to-test” approach was used, as a borderline PCR result was considered a negative result. The PCR results were analyzed for the 111 paired samples available both at baseline and after the 3 year follow-up. The failure rate of Bz, based on PCR results, was assessed as the ratio of the number of children with positive PCR results to the total number of samples available at the end of follow-up. P values of <5% or 95% CI that did not overlap were considered to be statistically significant.
Comparison between PCR and antibody titers.
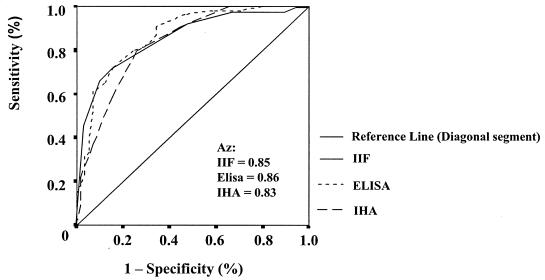
Receiver operating characteristics (ROC) curves were used to compare the performances of the three conventional serology tests (IIF, IHA, and ELISA), after a 3-year-follow-up, in detecting treated (Bz) and untreated (Pl) individuals. The ROC curve was constructed by plotting sensitivity against 1 − specificity, and the best predictor was the test giving the highest area under the curve (Az). The Az's and their 95% CI were computed to compare the overall diagnostic performances of the serological tests in distinguishing between treated (Bz) and untreated individuals (21), since the Az of a diagnostic test (rather than the sensitivity and specificity at a single cutoff) represents a summary statistic of the overall accuracy of the test. CI of the Az that excluded 0.5 were considered to indicate significant results. The serological test with the highest Az was chosen for purposes of comparison with PCR.
RESULTS
Detection of T. cruzi in human blood samples by PCR hybridization.
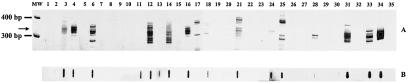
Amplification of the ≈330-bp T. cruzi kDNA fragment in some blood samples collected from infected children at baseline and 3 years posttreatment showed different patterns in polyacrylamide gel electrophoresis (Fig. 1A). Several blood samples showed weak or no kDNA amplification in the region of 330 bp, and positive results were obtained for them only after they were submitted to hybridization (Fig. 1B, lanes 11, 13, and 30). Paired samples of the blood of the same patient before and after intervention showed the following different patterns: (i) both samples negative (pairs [designated according to lane numbers] 8-9, 22-23, and 26-27), (ii) the first sample negative and the second positive (pairs 2-3, 10-11, 20-21, and 32-33), (iii) the first sample positive and the second negative (exemplified in pairs 4-5, 6-7, 14-15, 16-17, 18-19, and 28-29), and (iv) both samples positive (as in pairs 12-13, 24-25, and 30-31), regardless of Pl or Bz treatment.
FIG. 1.
Representative polyacrylamide gel electrophoresis results for blood samples from the Pl and Bz groups of chagasic patients at baseline and 3 years posttreatment analyzed by PCR. (A) MW, molecular weight marker (100-bp ladder; Promega); lane 1, DNA extracted from the blood of a nonchagasic individual (negative control); lanes 2, 4, 6, 8, 10, 12, 14, and 16, DNA from the blood of the Pl group at baseline; lanes 3, 5, 7, 9, 11, 13, 15, and 17, DNA from the blood of the Pl group 3 years later; lanes 18, 20, 22, 24, 26, 28, 30, and 32, DNA from the blood of the Bz group at baseline; lanes 19, 21, 23, 25, 27, 29, 31, and 33, DNA from the blood of the Bz group 3 years posttreatment; lane 34, DNA from the blood of a chronic chagasic patient (positive control); lane 35, no DNA in the reaction mixture for PCR amplification. Arrow indicates T. cruzi-specific products of 330 bp. (B) Slot blot hybridization of PCR products of blood samples from the same individuals.
PCR sensitivity.
According to Table 1, the relative sensitivity of PCR measured at baseline was 84.3% (95% CI, 77.1 to 89.8). False-negative results (20 of 127) were observed in 15.7% of cases (95% CI, 1.9 to 23.3). Among subjects who were negative or borderline at baseline and who had a complete follow-up (n = 13), five persisted as negative whereas eight shifted to a positive PCR result. PCR performed in blood amplified the 330-bp fragment corresponding to T. cruzi kDNA in 85.9% (55 of 64) of subjects in the Bz group and in 82.5% (52 of 63) of subjects in the Pl group (P > 0.05).
TABLE 1.
Sensitivity of PCR in T. cruzi-seropositivea children enrolled in the Bz trial
| Assigned intervention | No. (%) of samples with the following PCR result at baseline:
|
Total no. (%) | ||
|---|---|---|---|---|
| Positive | Negative | Borderline | ||
| Bz | 55 (85.9) | 6 (9.4) | 3 (4.7) | 64 (100.0) |
| Pl | 52 (82.5) | 9 (14.3) | 2 (3.2) | 63 (100.0) |
| Totalb | 107 (84.3) | 15 (11.8) | 5 (3.9) | 127 (100.0) |
Seropositive by IIF, IHA, and ELISA.
Relative sensitivity, 107 of 127, or 84.3% (95% CI, 77.1 to 89.8).
PCR for monitoring failure posttreatment.
After PCR results were decoded, the paired samples were found to represent 58 Bz recipients and 53 Pl recipients, for a total of 111. The percentage of positive PCR results in the Bz group 3 years after treatment (39.6%) was significantly lower (P = 0.01) than that in the Pl group (64.2%), as shown in Table 2. This significant difference persisted even after adjustment for age and sex.
TABLE 2.
PCR results for T. cruzi-infected children 3 years after Bz or Pl treatment
| Assigned intervention | No. (%) of samples with the following PCR result:
|
Total no. (%) | |
|---|---|---|---|
| Positive | Negative | ||
| Bz | 23 (39.6) | 35 (60.4) | 58 (100.0) |
| Pl | 34 (64.2) | 19 (36.8) | 53 (100.0) |
| Total | 57 (51.4) | 54 (48.6) | 111 (100.0) |
Comparison between PCR and antibody titers.
According to Fig. 2, no significant difference was found when the areas under the ROC curve yielded by IIF (0.85%; 95% CI, 0.78 to 0.92), IHA (0.83%; 95% CI, 0.76 to 0.91), and ELISA (0.86%; 95% CI, 0.80 to 0.93) were compared, since the corresponding 95% CI of the areas overlapped. The closer the curve is to the upper left corner of the graph (100% sensitivity and 100% specificity), the higher the overall predictive accuracy of the model. Since the three serological tests distinguished similarly between treated (Bz) and untreated (Pl) individuals, the results provided by IIF antibody titers were used for comparison with PCR. A decrease in (3) titers in antibody concentrations measured as the inverse of the titer (log2) after 3 years was assumed to be a favorable outcome. Thus, among those who received Bz, 22.6% did not present a decrease in IIF titers and PCR continued to be positive for 39.6% (P > 0.05), pointing out the proportion of Bz failure after a 3-year follow-up. On the other hand, in analysis of the Pl group, a high proportion of PCR positivity (64.2%) was detected, in agreement with the absence of a decrease in IIF titers (77.4%).
FIG. 2.
ROC curve of IIF titers, IHA titers, and ELISA index for the identification of children treated with Bz after 3 years of follow-up.
DISCUSSION
In the present study the PCR technique was applied to a cohort of T. cruzi-seropositive children who had been exposed to Bz chemotherapy or Pl 3 years earlier. We found that PCR positivity for T. cruzi was significantly lower in the Bz group. While in the majority of studies the PCR technique is performed as a diagnostic tool against a reference panel (gold standard), this study has the advantage of performing PCR under field conditions, where, at a population level, there is not always a clear separation between positive and negative individuals. Moreover, we used the intention-to-test analysis as an analogy to the intention-to-treat approach, which represents a conservative way to estimate the performance of PCR. Thus, borderline results were assumed to indicate an unfavorable outcome.
The results obtained by PCR at baseline (84.2% positivity) in our data set are comparable to those published by other groups, mainly when the target population is composed of children (2, 6). A recent study has shown that PCR confirmed chagasic infection in about 84% of seropositive patients including children, teenagers, and adults living in a Bolivian region of endemicity (6). It is well known that parasitemia measured by xenodiagnosis and/or hemoculture is higher in children than in adults. Due to the low sensitivity of any parasitological tool, all the strength of the PCR test resides in the detection of a positive result. At the 3 year-follow-up, the untreated patients had a 1.6-fold-higher chance of remaining PCR positive (34 of 53 versus 23 of 58) than those who had received the specific chemotherapy (P = 0.01). It should be remembered that the environment of the study area was submitted to insecticide spraying immediately before and throughout follow-up to guarantee the absence of triatominal vectors and hence to rule out the possibility of reinfection.
According to our results, two issues should be addressed. First, the fact that almost 40.0% of treated individuals remained PCR positive after specific chemotherapy reflects the failure of Bz to clear the parasites. Second, among the 35 treated children presenting negative PCR results, some are expected to shift to positive PCR results during long-term follow-up, since there is no guarantee that a single “flash” of a negative parasitological test means parasitological cure, especially when the well-known waves of parasitemia during the long course of Chagas' disease are taken into account. Some examples may be seen in Fig. 1, in which pairs of blood samples showed different patterns of parasitemia, from the absence of parasites in samples both before and after intervention to their presence in both samples, and even negative samples before intervention which became positive later. These profiles may occur in nontreated (Pl) or Bz-treated patients, reinforcing the principle that the value of parasitological tests lies only in the positive results they yield. Another fact that should be stressed is the need for hybridization of all samples in order to confirm the positivity of PCR, as exemplified by three samples that were negative by PCR but for which the presence of parasites was confirmed by positive hybridization, as can be observed in Fig. 1B.
An adequate correlation could be found between a high proportion of negative PCR test results and a decrease in antibody titers in the Bz group. But, most significantly, PCR positivity occurred in patients without reductions in antibody titers. Although there was no significant difference between Bz failure assessed by PCR (39.6%) and by the decrease in IIF titers (22.6%), we should keep in mind the clinical significance of these results at the individual level. A recent publication evaluating chagasic patients after specific chemotherapy demonstrated that PCR is more reliable and sensitive than xenodiagnosis for assessment of cure and should be carried out together with serological testing (10). A positive PCR result may reflect the detection of intact parasites or circulating DNA, and the elimination of circulating DNA could mean the absence of parasites, since after intramuscular injection of large quantities of kDNA, PCR was positive only for 2 days after the injection and not thereafter. These data support the hypothesis that parasite DNA detected by PCR originates from intact extracellular or recently lysed parasites (29). In this respect, PCR can be used as an early marker of resistance to specific chemotherapy years before a conclusion can be drawn by serological analysis. In using serology to assess cure, it is necessary to achieve long-lasting sustainability of the negative seroconversion (25), even though T. cruzi recombinant complement regulatory protein detected by ELISA may be a candidate antigen for monitoring chagasic patients after specific therapy, as recently reported (24). In the Pl group, as expected, the positivity of PCR was higher, as well as sustained antibody concentrations.
Once again, we confirmed that, although serological tests are very useful in the long run for demonstrating cure, parasitological tests are of paramount importance in demonstrating therapeutic failure during the follow-up of T. cruzi-infected individuals. Here, a good respondent target population was evaluated by a parasitological test, which gives reliable results in terms of positivity, within a relatively short time after specific treatment. Thus, we propose PCR as a helpful tool for detection of early therapeutic failure in Bz-treated children, providing a rapid result that may allow switching to an alternative drug.
Acknowledgments
This work was supported by the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Disease (TDR grant no. 900278), Financiadora de Estudos e Projetos 41/96/0894/00 (FINEP/PRONEX), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), and fellowships from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
We are grateful to Renato M. Oliveira for the field work coordination and to Orlando Carlos Magno and Afonso Da Costa Viana of the Departamento de Parasitologia, Universidade Federal de Minas Gerais, for technical assistance.
REFERENCES
- 1.Andrade, A. L. S. S., F. Zicker, R. M. Oliveira, S. Almeida Silva, A. Luquetti, L. R. Travassos, I. C. Almeida, S. S. De Andrade, J. G. De Andrade, and C. M. Martelli. 1996. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet 348:1407-1413. [DOI] [PubMed] [Google Scholar]
- 2.Antas, P. R., N. Medrano-Mercado, F. Torrico, R. Ugarte-Fernandez, F. Gomez, R. Correa-Oliveira, A. C. Chaves, A. J. Romanha, and T. C. Araujo-Jorge. 1999. Early, immediate, and late acute stages in Chagas' disease: a study combining anti-galactose IgG, specific serodiagnosis, and polymerase chain reaction analysis. Am. J. Trop. Med. Hyg. 61:308-314. [DOI] [PubMed] [Google Scholar]
- 3.Ávila, H. A., A. M. Gonçalves, and N. S. Nehme. 1990. Schizodeme analysis of Trypanosoma cruzi stocks from South and Central America by analysis of PCR-amplified minicircle variable region sequences. Mol. Biochem. Parasitol. 42:175-188. [DOI] [PubMed] [Google Scholar]
- 4.Ávila, H. A., D. S. Sigman, L. M. Cohen, R. C. Millikan, and L. Simpson. 1991. Polymerase chain reaction amplification of Trypanosoma cruzi kinetoplast minicircle DNA isolated from whole blood lysates: diagnosis of chronic Chagas' disease. Mol. Biochem. Parasitol. 48:211-222. [DOI] [PubMed] [Google Scholar]
- 5.Ávila, H. A., J. Borges-Pereira, O. Thiemann, E. de Paiva, W. Degrave, C. M. Morel, and L. Simpson. 1993. Detection of Trypanosoma cruzi in blood specimens of chronic chagasic patients by polymerase chain reaction amplification of kinetoplast minicircle DNA: comparison with serology and xenodiagnosis. J. Clin. Microbiol. 31:2421-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenière, S. F., M. F. Bosseno, F. Noireau, N. Yacsik, P. Liegeard, C. Aznar, and M. Hontebeyrie. 2002. Integrate study of a Bolivian population infected by Trypanosoma cruzi, the agent of Chagas disease. Mem. Inst. Oswaldo Cruz 97:289-295. [DOI] [PubMed] [Google Scholar]
- 7.Britto, C., M. A. Cardoso, P. Wincker, and C. M. Morel. 1993. A simple protocol for the physical cleavage of Trypanosoma cruzi kinetoplast DNA present in blood samples and its use in polymerase chain reaction (PCR)-based diagnosis of chronic Chagas' disease. Mem. Inst. Oswaldo Cruz 88:171-172. [DOI] [PubMed] [Google Scholar]
- 8.Britto, C., M. A. Cardoso, C. Ravel, A. Santoro, J. Borges-Pereira, J. R. Coura, C. M. Morel, and P. Wincker. 1995. Trypanosoma cruzi: parasite detection and strain discrimination in chronic chagasic patients from northeastern Brasil using amplification of kinetoplast DNA and nonradioactive hybridization. Exp. Parasitol. 81:462-471. [DOI] [PubMed] [Google Scholar]
- 9.Britto, C., M. A. Cardoso, C. M. M. Vanni, A. Hasslocher-Moreno, S. Xavier, W. Oeleman, A. Santoro, C. Pimez, C. M. Morel, and P. Wincker. 1995. Polymerase chain reaction detection of Trypanosoma cruzi in blood samples as a tool for diagnosis and treatment evolution. Parasitology 110:241-247. [DOI] [PubMed] [Google Scholar]
- 10.Britto, C., C. Silveira, M. A. Cardoso, P. Marques, A. Luquetti, V. Macedo, and O. Fernandes. 2001. Parasite persistence in treated chagasic patients revealed by xenodiagnosis and polymerase chain reaction. Mem. Inst. Oswaldo Cruz 96:823-826. [DOI] [PubMed] [Google Scholar]
- 11.Bronfen, B., F. S. A. Rocha, G. B. N. Machado, M. M. Perillo, A. J. Romanha, and E. Chiari. 1989. Isolamento de amostras do Trypanosoma cruzi por xenodiagnóstico e hemocultura de pacientes na fase crônica da doença de Chagas. Mem. Inst. Oswaldo Cruz 84:237-240. [DOI] [PubMed] [Google Scholar]
- 12.Castro, A. M., A. O. Luquetti, A. Rassi, G. G. Rassi, E. Chiari, and L. M. C. Galvão. 2002. Blood culture and polymerase chain reaction for the diagnosis of the chronic phase of human infection with Trypanosoma cruzi. Parasitol. Res. 88:894-900. [DOI] [PubMed] [Google Scholar]
- 13.Chiaramonte, M. G., F. M. Frank, G. M. Furer, N. J. Taranto, R. A. Margni, and E. L. Malchiodi. 1999. Polymerase chain reaction reveals Trypanosoma cruzi infection suspected by serology in cutaneous and mucocutaneous leishmaniasis patients. Acta Trop. 72:295-308. [DOI] [PubMed] [Google Scholar]
- 14.Chiari, E., J. C. P. Dias, M. Lana, and C. A. Chiari. 1989. Hemocultures for the parasitological diagnosis of human chronic Chagas' disease. Rev. Soc. Bras. Med. Trop. 22:19-23. [DOI] [PubMed] [Google Scholar]
- 15.Coura, J. R., L. L. Abreu, H. P. F. Wilcox, N. Anunziato, and W. Petana. 1991. Evaluation of the xenodiagnosis of chronic Chagas patients infected ten years or over in an area where transmission has been interrupted—Iguatama and Pains, west Minas Gerais State, Brazil. Mem. Inst. Oswaldo Cruz 86:395-398. [DOI] [PubMed] [Google Scholar]
- 16.Degrave, W., S. P. Fragoso, C. Britto, H. Van-Heuverswyn, G. Z. Kidane, M. A. Cardoso, R. U. Mueller, L. Simpson, and C. M. Morel. 1988. Peculiar sequence organization of kinetoplast DNA minicircles from Trypanosoma cruzi. Mol. Biochem. Parasitol. 27:63-70. [DOI] [PubMed] [Google Scholar]
- 17.Espinoza, A. G., A. Taibi, O. Billaut-Mulot, and A. Ouaissi. 1996. PCR-based detection of Trypanosoma cruzi useful for specific diagnosis of human Chagas' disease. J. Clin. Microbiol. 34:485-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galvão, L. M. C., J. R. Cançado, D. F. Rezende, and A. U. Krettli. 1989. Hemocultures from chronic chagasic patients using EDTA or heparin as anticoagulants. Braz. J. Med. Biol. Res. 22:841-843. [PubMed] [Google Scholar]
- 19.Gomes, M. L., A. M. Macedo, A. R. Vago, S. D. J. Pena, L. M. C. Galvão, and E. Chiari. 1998. Trypanosoma cruzi: optimization of polymerase chain reaction for detection in human blood. Exp. Parasitol. 88:28-33. [DOI] [PubMed] [Google Scholar]
- 20.Gomes, M. L., L. M. C. Galvão, A. M. Macedo, S. D. J. Pena, and E. Chiari. 1999. Chagas' disease diagnosis: comparative analysis of parasitologic, molecular, and serological methods. Am. J. Trop. Med. Hyg. 60:205-210. [DOI] [PubMed] [Google Scholar]
- 21.Hanley, J. A., and B. J. McNeil. 1982. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143:29-36. [DOI] [PubMed] [Google Scholar]
- 22.Junqueira, A. C. V., E. Chiari, and P. Wincker. 1996. Comparison of polymerase chain reaction with two classical parasitological methods for diagnosis of Chagas disease patients in a north-eastern endemic region of Brazil. Trans. R. Soc. Trop. Med. Hyg. 90:129-132. [DOI] [PubMed] [Google Scholar]
- 23.Lages-Silva, E., E. Crema, L. E. Ramirez, A. M. Macedo, S. D. Pena, and E. Chiari. 2001. Relationship between Trypanosoma cruzi and human chagasic megaesophagus: blood and tissue parasitism. Am. J. Trop. Med. Hyg. 65:435-441. [DOI] [PubMed] [Google Scholar]
- 24.Meira, W. S. F., L. M. C. Galvão, E. D. Gontijo, G. L. L. Machado-Coelho, K. A. Norris, and E. Chiari. 2002. Trypanosoma cruzi recombinant complement regulatory protein: a novel antigen for use in an enzyme-linked immunosorbent assay for diagnosis of Chagas' disease. J. Clin. Microbiol. 40:3735-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rassi, A., and A. O. Luquetti. 2003. Specific treatment for Trypanosoma cruzi infection (Chagas disease), p. 117-125. In K. M. Tyler and M. A. Miles (ed.), American trypanosomiais. Kluwer Academic Publishers, Boston, Mass.
- 26.Santos, F. R., S. D. J. Pena, and J. T. Epplen. 1993. Genetic and population study of a Y-linked tetranucleotide repeat DNA polymorphism with a simple non-isotopic technique. Hum. Genet. 90:655-656. [DOI] [PubMed] [Google Scholar]
- 27.Sosa Estani, S., E. L. Segura, A. M. Ruiz, E. Velazquez, B. M. Porcel, and C. Yampotis. 1998. Efficacy of chemotherapy with benznidazole in children in the indeterminate phase of Chagas' disease. Am. J. Trop. Med. Hyg. 59:526-529. [DOI] [PubMed] [Google Scholar]
- 28.Sturm, N. R., W. Degrave, C. M. Morel, and L. Simpson. 1989. Sensitive detection and schizodeme classification of Trypanosoma cruzi cells by amplification of kinetoplast minicircle DNA sequences: use in diagnosis of Chagas' disease. Mol. Biochem. Parasitol. 33:205-214. [DOI] [PubMed] [Google Scholar]
- 29.Tarleton, R. L., and L. Zhang. 1999. Chagas' disease etiology: autoimmunity or parasite persistence? Parasitol. Today 15:94-99. [DOI] [PubMed] [Google Scholar]
- 30.Wincker, P., M. F. Bosseno, C. Britto, N. Yaksic, M. A. Cardoso, C. M. Morel, and S. F. Brenière. 1994. High correlation between Chagas' disease serology and PCR-based detection of Trypanosoma cruzi kinetoplast DNA in Bolivian children living in an endemic area. FEMS Microbiol. Lett. 124:419-424. [DOI] [PubMed] [Google Scholar]
- 31.Wincker, P., J. Telleria, M. F. Bosseno, M. A. Cardoso, P. Marques, N. Yaksic, C. Aznar, P. Liegeard, M. Hontebeyrie, F. Noireau, C. M. Morel, and S. F. Brenière. 1997. PCR-based diagnosis for Chagas' disease in Bolivian children living in an active transmission area: comparison with conventional serology and parasitological diagnosis. Parasitology 114:367-373. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. 2002. Control of Chagas disease. Report of a WHO expert committee. WHO Tech. Rep. Ser. 905:1-109. [PubMed] [Google Scholar]