Abstract
The agr specificity group distribution of persistent Staphylococcus aureus clones recovered from the airways of cystic fibrosis (CF) patients did not differ from that of isolates recovered from various clinical infections and healthy nasal carriers. The success of CF clones in terms of cocolonization and/or infection with S. aureus, prevalence of clones, or persistence appeared to be independent of agr group specificity.
In cystic fibrosis (CF), Staphylococcus aureus is highly prevalent and the course of colonization and infection is often persistent in spite of antistaphylococcal therapy (1, 2, 7, 15). The agr system of S. aureus represents a quorum sensing system. A secreted autoinducing peptide induces activation of the agr operon at a given threshold, thereby up-regulating extracellular protein production, while down-regulating the synthesis of cell wall-associated proteins, which serve as adhesins for the pathogen to host tissues (9). Sequence variability of the autoinducing peptide and its transmembrane receptor agrC allows the distinction of four agr specificity groups (4). While a member of a given agr group activates the agr response of a strain of the same group, strains of different agr groups cause the inhibition of the agr response. This type of bacterial interference has been suggested to influence colonization dynamics by enhancing or inhibiting the ability of S. aureus to colonize in the presence of resident strains (6). Furthermore, an association between agr groups and strains causing certain diseases has been described: agr group III has been associated with toxic shock syndrome (13), and agr group IV has been associated with staphylococcal scalded skin syndrome (4, 5). However, there is no knowledge about the association of a specific agr group and CF airway infection or about agr-dependent bacterial interference in persistent S. aureus colonization and/or infection in CF patients.
(Part of this work has been shown at the 42nd ICAAC, San Diego, 2002 [abstract no. B-800].)
The CF isolates used in the present study were collected during a 6-year prospective study from 50 CF patients (8). Molecular typing by pulsed-field gel electrophoresis (PFGE) of 685 S. aureus isolates allowed the distinction of 45 different S. aureus clones. Thirty-eight individual clones (isolates with distinct fragment patterns by PFGE analysis) were isolated from single patients, while six prevalent clonal lineages (38 isolates with indistinguishable fragment patterns or with fewer than 7 fragment differences) were cultured from more than two patients (17). An infection was considered persistent if isolation of S. aureus continued for more than 6 months. Thirty-three of 50 (66%) patients were persistently infected by a single S. aureus clone, while 17 (34%) patients were infected by several clones. For each patient, one isolate of every different clone was subjected to multiplex PCR for agr group determination (10).
Sixty-four S. aureus isolates were collected from patients with various S. aureus infections. Two hundred nineteen isolates from patients with bacteremia, collected during a German multicenter study, were included (18). As a control, 88 nasal carriage isolates were cultured from healthy volunteers from the same geographic area as the CF patients.
The multiplex PCR for agr group determination (10) revealed that the pattern of agr distribution did not differ between the three different clinical settings and the control group, with agr group I strains being the most prevalent strains (Table 1). These findings seem to reflect the natural distribution of S. aureus lineages in this geographical area according to agr groups and are similar to findings of other studies (3, 14, 16).
TABLE 1.
Prevalence of agr groups in different clinical settings
| agr group | No. (%) of isolates from:
|
Total | |||
|---|---|---|---|---|---|
| CF patients | Various infection sitesa | Multicenter studyb | Nasal carriage | ||
| I | 34 (44) | 29 (46) | 96 (44) | 41 (47) | 200 (45) |
| II | 22 (29) | 16 (24) | 63 (29) | 21 (24) | 122 (27) |
| III | 17 (22) | 12 (19) | 50 (23) | 25 (28) | 104 (23) |
| IV | 4 (5) | 7 (11) | 9 (4) | 1 (1) | 21 (5) |
| Total | 77 (100) | 64 (100) | 218 (100) | 88 (100) | 447 (100) |
Clinical isolates were recovered from patients with endocarditis (n = 7), osteomyelitis (n = 8), skin and soft tissue infections (impetigo, bursitis, staphylococcal scalded skin syndrome, finger pulp infection, cellulites, abscesses, and wound infection [n = 25]), device-related infection (n = 15), chronic otitis media (n = 7), pneumonia (n = 1), and cerebral nervous system infection (n = 1).
Bacteremic isolates.
The mean persistence durations of agr group I, II, and III were similar (28 months); they differed from the mean persistence of agr group IV clones (23.5 months), which were isolated from only four patients. Thus, the persistence of S. aureus for our study isolates did not vary significantly among agr groups, indicating that the clones of the different agr types were equally fit in persistence.
The prevalence of agr groups for patients infected by a single S. aureus clone (agr group I, 45.7%; agr group II, 25.7%; agr group III, 20%; agr group IV, 8.5%) did not differ from the prevalence for patients infected by several clones (agr group I, 44.2%; agr group II, 30.2%; agr group III, 23.2%; agr group IV, 2.3%), suggesting that no clone of a particular agr group was more competent than another in inhibiting colonization by other clones.
Seventeen CF patients (34%) were infected by several S. aureus clones. Most patients (15 of 17) harbored clones that belonged to different agr groups (Table 2). In clones with different agr groups, the agr signaling pathway may be inhibited, leading to down-regulation of secreted proteins and up-regulation of adhesive proteins. Such enhanced expression of adhesive proteins, e.g., fibronectin binding protein, would support the adhesion to host tissues, and in the case of CF, adhesion to the upper and lower airway epithelium (11, 12). Therefore, agr-related bacterial interference appears to affect early cocolonization of strains by supporting colonization of strains with different agr groups.
TABLE 2.
Analysis of bacterial interference according to agr groups of persistent S. aureus strains isolated from CF patients
| Patient no. | No. of clonesa | agr groupsb | Success of agr groupc |
|---|---|---|---|
| 1 | 2 | II and III | III > II |
| 2 | 2 | I and II | No competitiond |
| 3 | 3 | I, II, and III | No competition |
| 4 | 2 | I and II | I > II |
| 5 | 3 | I1, I2, and III | I1 > III, I2 > I1 |
| 6 | 2 | I and II | II > I |
| 7 | 3 | I1, I2, and III | I2 > I1, I2 > III |
| 8 | 2 | II and III | III > II |
| 9 | 3 | I1, I2, and I3 | No competition |
| 10 | 3 | II, III1, and III2 | II > III1, II > III2 |
| 11 | 3 | II1, II2, and III | III > II1, III or II2 |
| 12 | 2 | I1 and I2 | I2 > I1 |
| 13 | 2 | I and II | No competition |
| 14 | 3 | I1, I2, and II | I1 > I2, II > I2, I1 > II |
| 15 | 2 | I and III | III > I |
| 16 | 3 | II, III, and IV | No competition |
| 17 | 3 | I1, I2, and II | II > I1, II > I2 |
Number of different clones cultured from a single patient as determined by PFGE.
agr groups of the different clones from a single patient (with different or the same agr groups, as indicated by the superscript number).
agr group of a clone that replaced another clone.
The different persistent strains were isolated independently (after a time break of more than 1 year, during which no S. aureus strain was isolated or the different strains were cultured in parallel or consecutively without replacement of a strain).
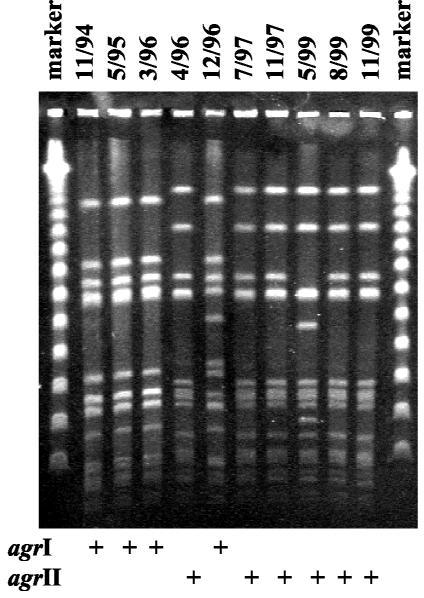
For 13 of 17 patients colonized and/or infected by several S. aureus clones, competition of clones for colonization was observed (Table 2). The success of a clone was suggested if a new clone was able to replace a resident clone and vice versa. Replacement of a clone was considered to have occurred if the respective clone was not isolated for 1 year or longer. In the case of cultures that were negative for at least 1 year before recovery of a new S. aureus clone, we assumed that no competition of clones occurred. If the clones were cultured in parallel or consecutively without loss of a clone, no success of a single clone was observed. Thus, in our study no clone of a particular agr group was more successful than another (Table 2). Interestingly, we observed that several clones of the same agr group as well as of different agr groups replaced another clone. Replacement of clones of the same agr group is consistent with the concept of agr-related bacterial interference (6). By triggering each other's agr response, the expression of adhesive proteins is inhibited, which presumably is deleterious for adhesion, and therefore for colonization. However, the finding that clones with different agr groups succeeded over other clones in terms of colonization for extended periods implies that mechanisms other than agr-related bacterial interference were more important for the long-term success of the clone in the host. An example for the replacement of a clone belonging to agr group I by a clone belonging to agr group II is given in Fig. 1.
FIG. 1.
Replacement of an agr group I clone by an agr group II clone, as shown by PFGE of consecutive isolates (from the sputum of a CF patient, collected from November 1994 to November 1999) after SmaI digestion of chromosomal DNA. The agr group I clone was isolated for 17 months before the agr group II clone was isolated for the first time in April 1996. Then, the agr group I clone was isolated only once again (December 1996), with some differences in fragment patterns, after it was replaced by the agr group II clone.
Thirty-nine patients were colonized and/or infected by one of six prevalent clonal lineages (Table 3). A significant association of prevalent clonal lineage and agr group was found for clone B, agr group I (χ2 = 6.42; P = 0.0104), isolated from 10 patients, and for clone A, agr group III (χ2 = 7.12; P = 0.008), recovered from 12 patients. However, because these two prevalent clones with statistically significant association to a particular agr group belonged to different agr groups, the prevalence of clones appears to be not affected by agr group specificity and agr group-associated genes.
TABLE 3.
Prevalence of prevalent clonal lineages versus individual clones within a particular agr group
| agr group | No. of patients with:
|
Pa | Total no. of patients (no. of clones) | |
|---|---|---|---|---|
| Individual clones | Prevalent clonal lineagesb | |||
| I | 17 | 10 (B) | 0.012 | 34 (20) |
| 4 (D) | 0.204 | |||
| 3 (E) | 0.338 | |||
| II | 12 | 7 (C) | 0.052 | 22 (14) |
| 3 (G) | 0.341 | |||
| III | 5 | 12 (A) | 0.008 | 17 (6) |
| IV | 4 | 0 | ND | 4 (4) |
| Total | 38 | 39 (6) | 77 (44) | |
P value comparing the number of individual or prevalent clones of a particular agr group to the number of all clones of this specific agr group. Boldface values indicate statistical significance.
Capital letters in parentheses indicate distinct clonal lineages. A number in parentheses indicates the number of different prevalent clonal lineages.
In summary, CF-related persistent airway infection was not associated with a distinct agr specificity group. While agr-related bacterial interference appears to have an impact in early cocolonization of clones, yet-unknown factors other than agr-related interference were important for the late success of clones in terms of cocolonization, occurrence of prevalent clones, and persistence in CF airway infection.
Acknowledgments
This work was supported by a grant from the Innovative Medical Research Foundation (IMF) of the Medical Faculty, University of Muenster (Project KA 11 01 23), and in part by the Interdisciplinary Clinical Research Foundation (IZKF C20).
We thank R. P. Novick for providing the agr specificity group type strains and S. Deiwick, B. Schuhen, and M. Schulte for expert technical assistance.
REFERENCES
- 1.Branger, C., C. Gardye, and N. Lambert-Zechovsky. 1996. Persistence of Staphylococcus aureus strains among cystic fibrosis patients over extended periods of time. J. Med. Microbiol. 45:294-301. [DOI] [PubMed] [Google Scholar]
- 2.Burns, J. L., J. Emerson, J. R. Stapp, D. L. Yim, J. Krzewinski, L. Louden, B. W. Ramsey, and C. R. Clausen. 1998. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin. Infect. Dis. 27:158-163. [DOI] [PubMed] [Google Scholar]
- 3.Goerke, C., M. Kümmel, K. Dietz, and C. Wolz. 2003. Evaluation of intraspecies interference due to agr polymorphism in Staphylococcus aureus during infection and colonization. J. Infect. Dis. 188:250-256. [DOI] [PubMed] [Google Scholar]
- 4.Jarraud, S., G. J. Lyon, S. Figueiredo, G. Lina, F. Vandenesch, J. Etienne, T. W. Muir, and R. P. Novick. 2000. Exfoliatin-producing strains define a fourth agr specificity group in Staphylococcus aureus. J. Bacteriol. 182:6517-6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarraud, S., C. Mougel, J. Thioulouse, G. Lina, H. Meugnier, F. Forey, X. Nesme, J. Etienne, and F. Vandenesch. 2002. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect. Immun. 70:631-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji, G., R. C. Beavis, and R. P. Novick. 1997. Bacterial interference caused by autoinducing peptide variants. Science 276:2027-2030. [DOI] [PubMed] [Google Scholar]
- 7.Kahl, B., M. Herrmann, A. Schulze Everding, H. G. Koch, K. Becker, E. Harms, R. A. Proctor, and G. Peters. 1998. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J. Infect. Dis. 177:1023-1029. [DOI] [PubMed] [Google Scholar]
- 8.Kahl, B. C., A. Duebbers, G. Lubritz, J. Haeberle, H. G. Koch, B. Ritzerfeld, M. Reilly, E. Harms, R. A. Proctor, M. Herrmann, and G. Peters. 2003. Population dynamics of persistent Staphylococcus aureus isolated from the airways of cystic fibrosis patients during a 6-year prospective study. J. Clin. Microbiol. 41:4424-4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kornblum, J., B. N. Kreiswirth, S. J. Projan, H. Ross, and R. P. Novick. 1990. Agr: a polycistronic locus regulating exoprotein synthesis in Staphylococcus aureus, p. 373-402. In R. P. Novick (ed.), Molecular biology of the staphylococci. VCH Publishers, Inc., New York, N.Y.
- 10.Lina, G., F. Boutite, A. Tristan, M. Bes, J. Etienne, and F. Vandenesch. 2003. Bacterial competition for human nasal cavity colonization: role of staphylococcal agr alleles. Appl. Environment. Microbiol. 69:18-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mongodin, E., O. Bajolet, J. Cutrona, N. Bonnet, F. Dupuit, E. Puchelle, and S. De Bentzmann. 2002. Fibronectin-binding proteins of Staphylococcus aureus are involved in adherence to human airway epithelium. Infect. Immun. 70:620-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mongodin, E., O. Bajolet, J. Hinnrasky, E. Puchelle, and S. de Bentzmann. 2000. Cell wall-associated protein A as a tool for immunolocalization of Staphylococcus aureus in infected human airway epithelium. J. Histochem. Cytochem. 48:523-533. [DOI] [PubMed] [Google Scholar]
- 13.Musser, J. M., P. M. Schlievert, A. W. Chow, P. Ewan, B. N. Kreiswirth, V. T. Rosdahl, A. S. Naidu, W. Witte, and R. K. Selander. 1990. A single clone of Staphylococcus aureus causes the majority of cases of toxic shock syndrome. Proc. Natl. Acad. Sci. USA 87:225-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peacock, S. J., C. E. Moore, A. Justice, M. Kantzanou, L. Story, K. Mackie, G. O'Neill, and N. P. J. Day. 2002. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect. Immun. 70:4987-4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renders, N. H. M., A. van Belkum, S. E. Overbeek, J. W. Mouton, and H. A. Verbrugh. 1997. Molecular epidemiology of Staphylococcus aureus strains colonizing the lungs of related and unrelated cystic fibrosis patients. Clin. Microbiol. Infect. 3:216-221. [DOI] [PubMed] [Google Scholar]
- 16.Shopsin, B., B. Mathema, P. Alcabes, B. Said-Salim, G. Lina, A. Matsuka, J. Martinez, and B. N. Kreiswirth. 2003. Prevalence of agr specificity groups among Staphylococcus aureus strains colonizing children and their guardians. J. Clin. Microbiol. 41:456-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Eiff, C., K. Becker, K. Machka, H. Stammer, and G. Peters. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. N. Engl. J. Med. 344:11-16. [DOI] [PubMed] [Google Scholar]