Abstract
To evaluate the usefulness of a real-time PCR for Leishmania DNA in the diagnosis and follow-up of patients with human immunodeficiency virus type 1 (HIV-1) and Leishmania coinfection, Leishmania DNA levels were measured in whole peripheral blood from 25 HIV-infected patients with clinical features suggestive of visceral leishmaniasis. Leishmania DNA was detected in 10 of 25 patients with microscopically confirmed visceral leishmaniasis and in none of those without this disease. Following treatment with liposomal amphotericin B, a clinical response was observed in 9 of 10 patients, in association with significantly decreased parasite loads. Seven patients relapsed clinically a median of 110 days after the end of treatment, in association with substantial increases in Leishmania DNA levels. Leishmania DNA levels correlated with the clinical course of visceral leishmaniasis, and their measurement at diagnosis and during and after treatment seems to be useful in the clinical management of HIV-infected patients with this disease.
Visceral leishmaniasis is a frequent and severe complication of advanced human immunodeficiency virus (HIV) infection in patients living in the Mediterranean basin. In this area, 25 to 75% of visceral leishmaniasis cases occur in HIV-infected patients, and 2 to 9% of HIV-infected patients acquire this disease (4, 11). Clinical manifestations include fever, hepatomegaly and/or splenomegaly, and pancytopenia. Bone marrow aspirate or biopsy followed by demonstration of Leishmania parasites by microscopic and/or cultural examination is the most common diagnostic procedure. Although microscopic examination of bone marrow aspirate has been reported to be 62 to 93% sensitive, it is an invasive procedure and the diagnostic yield may be low in HIV-infected patients, because of a hypoplastic bone marrow (7). Serology has limited diagnostic value in HIV-infected patients, because only 43 to 78% of leishmaniasis patients show detectable levels of anti-Leishmania antibodies (12, 14). Recently, PCR for Leishmania DNA in peripheral blood has been shown to be sensitive and specific for diagnosis and follow-up of patients with visceral leishmaniasis (2, 3, 8, 10, 13).
The response to antileishmanial treatment is lower in HIV-infected patients than in immunocompetent patients (10, 12, 14). HIV-infected patients show high rates of visceral leishmaniasis relapse (between 25 and 61%). Highly active antiretroviral therapy (HAART) has significantly reduced the incidence of visceral leishmaniasis (6, 9). However, both relapse and de novo cases of visceral leishmaniasis are commonly observed under HAART (5). Optimal treatment for visceral leishmaniasis should both target the pathogen by specific drugs and restore host immunity by the use of potent anti-HIV combinations. In order to monitor the response to this treatment strategy, markers of Leishmania infection are required, to be assessed in parallel with markers of HIV infection. In this study, a quantitative real-time PCR assay was used to measure Leishmania DNA levels in peripheral blood both at the time of diagnosis and during follow-up, in order to establish the value of this method for monitoring of HIV-Leishmania coinfection.
MATERIALS AND METHODS
Patients.
Twenty-five HIV-infected patients with clinical features suggestive of visceral leishmaniasis underwent bone marrow aspiration. Visceral leishmaniasis was diagnosed in 10 of 25 patients (40%) by demonstration of Leishmania amastigotes in Giemsa-stained bone marrow smears. A first diagnosis was made for six patients, while four patients had a relapse. Leukopenia was observed in all of the patients (median, 2,200 cells/mm3; range, 1,300 to 3,200); fever, splenomegaly or hepatomegaly, and anemia (8.9 g/dl; range, 7.8 to 10.9) each were observed in nine patients; and thrombocytopenia was observed in eight patients (64,000 cells/mm3). CD4+ cell counts, HIV type 1 (HIV-1) RNA levels, and anti-Leishmania antibody titers at the time of diagnosis are shown in Table 1. No other opportunistic disorders were identified in these patients. Of the 15 remaining patients, 3 had non-Hodgkin lymphoma, 1 had atypical mycobacteriosis, and 11 had no opportunistic and/or neoplastic diseases identified. None of these 15 patients had anti-Leishmania antibodies. Their mean CD4+ count and HIV RNA load were 107 cells/ml (range, 48 to 367) and 5.02 log copies/ml (range, 3.14 to 6.11), respectively.
TABLE 1.
Clinical and laboratory parameters of HIV-infected patients with visceral leishmaniasis
| Patient | At time of diagnosis
|
After treatment
|
At time of clinical relapse
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sexa | Age (yr) | Risk factorb | No. of CD4+ cells/μlc | HIV RNA loadd (log copies/ml) | HAARTe | Anti-Leishmania ABf (titer) | Leishmania DNA (parasites/ml) | HAARTe | Leishmania DNA (parasites/ml)g | Leishmania prophylaxis (mo)h | No. of CD4+ cells/μl | HIV RNA loadd (log copies/ml) | HAARTe | Leishmania DNA (parasites/ml)i | |
| 1 | M | 30 | IDU | 8 | 4.38 | EFV, SQV, RTV | 1:640 | 110 | No | 7 (24) | ND | 4 | 5.17 | No | 477 (46) |
| 2 | M | 37 | IDU | 99 | 2.57 | AZT, 3TC, SQV | 1:40 | 362 | D4T, SQV, LPV-r | 2 (38) | ND | 79 | 3.32 | D4T, SQV, LPV-r | 102 (45) |
| 3 | F | 34 | IDU | 6 | 4.91 | No | 1:40 | 112 | No | 1 (38) | ND | 4 | 5.58 | No | 253 (298) |
| 4 | M | 42 | IDU | 43 | 5.77 | No | 1:640 | 41,000 | No | 2,677 (10) | ND | NA | NA | No | NA |
| 5 | M | 37 | IDU | 14 | 5.74 | No | 1:640 | 17,050 | D4T, 3TC, LPV-r | 6 (38) | ND | 20 | 2.30 | D4T, 3TC, LPV-r | 2,146 (110) |
| 6 | M | 38 | IDU | NA | NA | D4T, 3TC, EFV | NA | 3,210 | AZT, 3TV, LPV-r | 1 (17) | 10 | 162 | NA | AZT, 3TV, LPV-r | 1,466 (300) |
| 7 | F | 34 | Hetero | 106 | 3.11 | 3TC, EFV, RTV, IDV | 1:40 | 1,070 | 3TC, EFV, RTV, IDV | 3 (38) | 2 | 139 | 3.96 | 3TC, EFV, RTV, IDV | 364 (70) |
| 8 | M | 42 | IDU | 5 | 5.13 | No | 1:640 | 4,320 | AZT, 3TC, NFV | 5 (59) | 8 | 62 | <1.90 | AZT, 3TC, NFV | 259 (268) |
| 9 | M | 34 | Hetero | 246 | 4.55 | No | 1:80 | 819 | AZT, 3TC, NFV | 7 (66) | 1 | 239 | 4.17 | AZT, 3TC, NFV | No relapse |
| 10 | M | 42 | Homo | 42 | 6.11 | No | 1:5,120 | 2,150 | 3TC, RTV | <0.63 (66) | 3 | 755j | <1.90 | AZT, 3TC, IDV, RTV | No relapse |
M, Male; F, female.
IDU, intravenous drug user; Hetero, heterosexual; Homo, homosexual.
NA, not available.
Measured by a nucleic acid sequence-based amplification system (NASBA; BioMérieux, Marcy L'Etoile, France).
AZT, zidovudine; 3TC, lamivudine; D4T, stavudine; EFV, efavirenz; SQV, squinavir; RTV, ritonavir; IDV, indinavir; NFV, nelfinavir; LPV-r, lopinavir-ritonavir.
AB, antibodies. Anti-Leishmania antibodies were measured by immunofluorescence (Leishmania Spot IF; BioMérieux).
The last day of treatment is given in parentheses.
ND, not done.
The number of days from the end of treatment is given in parentheses.
One year after treatment withdrawal.
Treatment.
Following diagnosis, all of the patients received liposomal amphotericin B (Ambisome; Gilead, San Diego, Calif.) on days 1 to 5 and once weekly thereafter, at a dosage of 3 mg/kg of body weight, except for one patient (patient 10 [Table 1]) who received 1.5 mg/kg because of renal failure. One patient (patient 4 [Table 1]) refused to continue the treatment after day 10. For the other patients, treatment duration was established by the physician caring for the patient, based on clinical response, and varied between 17 and 66 days. Following the end of therapy, five patients received secondary prophylaxis, consisting of liposomal amphotericin B at 3 mg/kg once a month. Clinical response was defined as remission of fever, improvement of hematological values, and regression in the size of the spleen and/or liver. Clinical relapse was defined as the recurrence of symptoms and signs of visceral leishmaniasis. Seven of 10 patients received HAART concomitantly with antileishmanial therapy (Table 1).
Samples.
Peripheral blood was collected at the time of diagnostic work-up from all of the 25 HIV-infected patients with clinical features suggestive of visceral leishmaniasis. For patients with visceral leishmaniasis, samples were also drawn weekly during treatment, monthly during secondary prophylaxis, and at the time of relapse. Informed consent was always obtained before samples were drawn.
Real-time PCR.
DNA was extracted from 350 μl of whole peripheral blood collected in EDTA by using the Easy-DNA kit (Invitrogen Ltd., Paisley, United Kingdom) according to the manufacturer's instructions. The extracted DNA was finally eluted in 100 μl of water. The real-time PCR primers and probe were selected in the SSU rRNA gene, which is repeated 160 times in the Leishmania genome and is highly conserved among Leishmania species (15). Primers and probe were chosen by using Primer Express software (Perkin-Elmer Applied Biosystems, Cheshire, United Kingdom) and consisted of 5′-AAGGTCAAAGAACAAGGCCAAG-3′ (LEIF-forward), 5′-GCATCGGAGTCGG-3′ (LEIR-reverse), and 5′-AGGAGCGTGTCCCCGTGGAGG-3′ (LEIP-probe). The fluorogenic probe was synthesized by using a FAM reporter molecule attached to the 5′ end and a TAMRA quencher linked to the 3′ end (Perkin-Elmer Applied Biosystems). A standard curve was constructed using DNA extracted from a Leishmania infantum culture (MON1), measured by spectrophotometer, and 10-fold serially diluted; 106 to 103 genomes/ml were used in the standard curve. Assuming that each parasite is harboring 160 copies of the SSU rRNA gene, these dilutions corresponded to 6,250 to 6.25 parasites/ml.
Amplification and detection were performed using an ABI Prism 5900 sequence detection system (Perkin-Elmer Applied Biosystems). Each standard, each sample, and one negative control were analyzed in triplicate for each run. A 5-μl volume of the sample and 20 μl of the PCR mixture, consisting of 12.5 μl of Universal Mastermix (Perkin-Elmer Applied Biosystems), 900 nM forward primer, 300 nM reverse primer, and 200 nM probe, were added to each well. Cycling parameters were 50°C for 2 min, 95°C for 10 min, and 50 cycles at 95°C for 15 s and 60°C for 1 min. A threshold cycle value (Ct) was calculated for each sample by determining the point at which the fluorescence exceeded the threshold limit. A standard curve was obtained by plotting the Ct values against each standard of known concentration. The number of parasites was calculated as the mean of the values obtained in the three sample aliquots. The analytical sensitivity of the method was estimated to be 0.625 parasites/ml.
Statistical analysis.
Data were analyzed using Spearman's correlation and the Mann-Whitney and paired sign tests.
RESULTS
Leishmania DNA load at the time of diagnosis of visceral leishmaniasis.
Leishmania DNA was found in all of the 10 patients with visceral leishmaniasis, with a median of 1,610 parasites/ml (range, 110 to 41,000) (Table 1), and in none of the 15 patients without this disease. Both the diagnostic sensitivity and the specificity of real-time PCR were 100%. Parasite loads at the time of diagnosis were not significantly different for patients with a first diagnosis and those with relapse. No significant correlation was found between the parasite load and CD4+ cell count, HIV RNA viral load, titer of anti-Leishmania antibodies, or HAART administration at the time of diagnosis.
Leishmania DNA load during treatment.
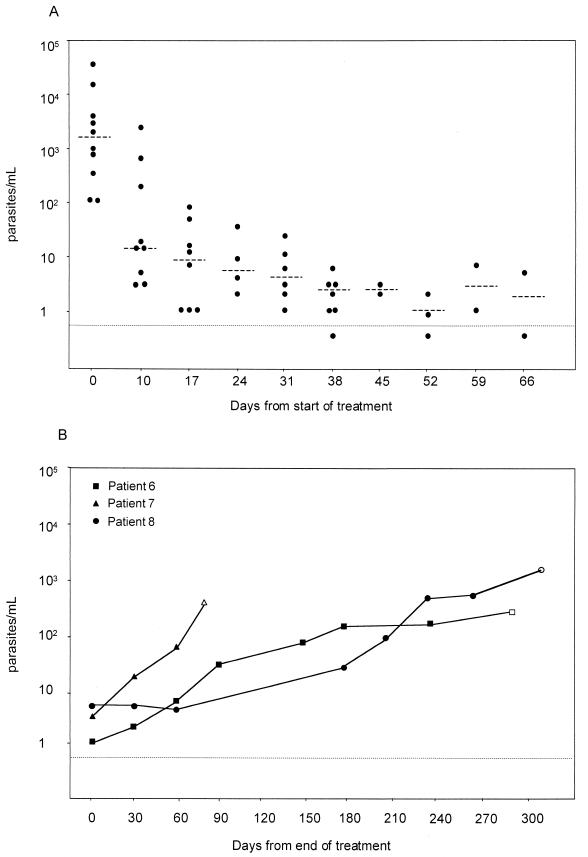
Figure 1A shows Leishmania DNA levels during treatment. Following liposomal amphotericin B administration on days 1 to 5, parasite levels decreased significantly from the baseline (median, 14 versus 1,680 parasites/ml; P = 0,004). During subsequent cycles of treatment, Leishmania loads decreased further in all the patients, except for patient 4, who stopped treatment at day 10. At the end of therapy, Leishmania DNA was undetectable (<0.63 parasites/ml) in one patient (patient 10), whereas the median DNA load in the other eight patients was 4 parasites/ml (Table 1). A clinical response was observed in these patients concomitantly with the decrease in parasite concentrations in blood.
FIG. 1.
(A) Leishmania DNA levels in peripheral blood of patients with visceral leishmaniasis in the course of treatment. Blood samples were drawn prior to weekly drug administration. The horizontal line indicates the detection limit of the PCR assay. Dashed horizontal bars indicate median parasite levels. (B) Leishmania DNA levels in three patients (patient 6, patient 7, and patient 8 according to the numbering in Table 1) with visceral leishmaniasis receiving secondary prophylaxis. Blood samples were drawn prior to monthly drug administration. Open symbols indicate the time of clinical relapse.
Leishmania DNA load during posttreatment follow-up and clinical relapse.
Following treatment, five patients received secondary prophylaxis (Table 1). The patient with undetectable Leishmania DNA in blood after therapy (patient 10) discontinued prophylaxis after 3 months, never relapsed, and had no detectable Leishmania DNA for 5 years of follow-up. One patient (patient 9) was clinically stable at the last follow-up after 2 months of prophylaxis, with decreased Leishmania DNA levels (median, 5 parasites/ml). Three patients had a clinical relapse of visceral leishmaniasis a median of 268 days after the end of treatment. Their Leishmania DNA levels were low at the end of treatment (median, 3 parasites/ml) but increased progressively during prophylaxis, reaching a median of 364 parasites/ml at the time of clinical relapse (Table 1; Fig. 1B). In these patients, an increase in Leishmania load above 10 parasites/ml preceded clinical relapse by 50 to 120 days (Fig. 1B).
Four patients did not receive secondary prophylaxis. All of them relapsed clinically a median of 78 days after the end of the treatment, with a median of 365 parasites/ml (Table 1). Blood samples from the time between the end of treatment and relapse were not available. Patient 4, who refused to continue treatment after day 10, died 250 days after the diagnosis of leishmaniasis.
Upon HAART, two of seven patients showed a virological response (Table 1). For one patient (patient 10), the CD4 count increased to >500 cells/μl, Leishmania DNA remained undetectable, and there was no relapse. The CD4 cell count for the other patient (patient 8) remained below 100 cells/μl, the Leishmania DNA load increased progressively, and the patient relapsed. No significant response to HAART was observed in the six remaining patients.
DISCUSSION
Leishmania DNA was found in all the patients with leishmaniasis confirmed by microscopic examination of bone marrow aspirate and in none of the patients without this disease. Despite the low number of patients examined in this study, this quantitative PCR proved to be a reliable and noninvasive tool for diagnosis of visceral leishmaniasis in HIV-infected patients. Because of its high diagnostic sensitivity, this assay is also likely to aid in the diagnosis of visceral leishmaniasis in patients without HIV infection, in whom the yield of parasites may be low. Our findings confirm those obtained in previous studies with regard to the diagnostic value of PCR (2, 3, 8, 10, 13). In addition, the potential ability of the real-time PCR to accurately measure the circulating parasite load provided a valuable tool for disease control after diagnosis.
Leishmania DNA loads differed widely among patients. However, they were not correlated with clinical outcome or with other parameters of HIV or Leishmania infection.
Following treatment with liposomal amphotericin B, a clinical response was observed in all of the patients. However, Leishmania DNA was cleared from peripheral blood in only one case. In the remaining patients, including those who received a long treatment course, PCR still revealed the presence of low levels of circulating parasite DNA. Actually, clinical relapse was observed in seven out of eight patients harboring Leishmania DNA in blood posttreatment, but not in the patient whose blood became PCR negative. These observations suggest that the persistence of parasites in the blood after treatment is associated with a high risk of relapse, irrespective of clinical response.
Secondary prophylaxis seemed to protect only partially against relapse. Although no difference in parasite burden was found at the time of relapse between patients who had received prophylaxis and those who had not, the latter relapsed after a shorter time than the former. However, two of four patients who did not receive prophylaxis were not on HAART, and this might have affected the clinical outcome.
It has been suggested that visceral leishmaniasis accelerates HIV replication and impairs the patient condition's by further immunosuppression (1). In our study, HAART was administered together with specific antileishmanial therapy to seven patients. However, most of the patients showed no significant virological and/or immunological response. This might have resulted from poor adherence to HAART, but also from a synergistic immunosuppressive effect of Leishmania and HIV. On the other hand, the only patient who did not relapse and in whom no parasite DNA was detected showed an optimal response to HAART, in terms of both a decrease in the viral load and an increase in the number of CD4+ cells. Another patient relapsed despite a virological response to HAART, but his CD4 cell count never increased above 100 per μl. These observations support the idea that a sustained immunological response rather than a virological response is essential to cure visceral leishmaniasis in HIV-infected patients.
In conclusion, real-time PCR seems to be a reliable, rapid, and noninvasive method for the diagnosis of visceral leishmaniasis. Treatment with liposomal amphotericin B was associated with clearance or decrease of Leishmania DNA levels. Following the end of treatment, parasite DNA remained detectable in all patients who relapsed, including patients receiving both secondary prophylaxis and HAART. Furthermore, clinical relapse was preceded by a substantial elevation of parasite levels. Therefore, real-time PCR might also be of value in monitoring the response to antileishmanial treatment and as an early, preclinical, prognostic marker of relapse.
REFERENCES
- 1.Cacopardo, B., L. Nigro, W. Preiser, A. Fama, M. I. Satariano, J. Braner, B. M. Celesia, B. Weber, R. Russo, and H. W. Doerr. 1996. Prolonged Th2 cell activation and increased viral replication in HIV-Leishmania co-infected patients despite treatment. Trans. R. Soc. Trop. Med. Hyg. 90:434-435. [DOI] [PubMed] [Google Scholar]
- 2.Cascio, A., S. Calattini, C. Colomba, C. Scalamogna, M. Galazzi, M. Pizzuto, R. Camilli, M. Gramiccia, L. Titone, M. Corbellino, and S. Antinori. 2002. Polymerase chain reaction in the diagnosis and prognosis of Mediterranean visceral leishmaniasis in immunocompetent children. Pediatrics 109:E27. [DOI] [PubMed] [Google Scholar]
- 3.Fisa, R., C. Riera, E. Ribero, M. Gallego, and M. Portus. 2002. A nested polymerase chain reaction for diagnosis and follow-up of human visceral leishmaniasis patients using blood samples. Trans. R. Soc. Trop. Med. Hyg. 96(Suppl. 1):S191-S194. [DOI] [PubMed] [Google Scholar]
- 4.Gradoni, L., A. Scalone, M. Gramiccia, and M. Troiani. 1996. Epidemiological surveillance of leishmaniasis in HIV-1 infected individuals in Italy. AIDS 10:785-791. [DOI] [PubMed] [Google Scholar]
- 5.Jimenez-Exposito, M. J., C. Alonso-Villaverde, P. Sarda, and L. Masana. 1999. Visceral leishmaniasis in HIV-infected patients with non-detectable HIV-1 viral load after highly active antiretroviral therapy. AIDS 13:152-153. [PubMed] [Google Scholar]
- 6.Kaplan, J. E., D. Hanson, M. S. Dworkin, T. Frederick, J. Bertolli, M. L. Lindegren, S. Holmberg, and J. L. Jones. 2000. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin. Infect. Dis. 30(Suppl. 1):S5-S14. [DOI] [PubMed] [Google Scholar]
- 7.Kubar, J., P. Marty, A. Lelievre, J. F. Quaranta, P. Staccini, C. Caroli-Bosc, and Y. Le Fichoux. 1998. Visceral leishmaniasis in HIV-positive patients: primary infection, reactivation and latent infection. Impact of the CD4+ T-lymphocyte counts. AIDS 12:2419-2422. [DOI] [PubMed] [Google Scholar]
- 8.Lachaud, L., J. Dereure, E. Chabbert, J. Reynes, J. M. Mauboussin, E. Oziol, J. P. Dedet, and P. Bastien. 2000. Optimized PCR using patient blood samples for diagnosis and follow-up of visceral leishmaniasis, with special reference to AIDS patients. J. Clin. Microbiol. 38:236-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palella, F. J., Jr., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, S. D. Holmberg, et al. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338:853-860. [DOI] [PubMed] [Google Scholar]
- 10.Piarroux, R., F. Gambarelli, H. Dumon, M. Fontes, S. Dunan, C. Mary, B. Toga, and M. Quilici. 1994. Comparison of PCR with direct examination of bone marrow aspiration, myeloculture and serology for diagnosis of visceral leishmaniasis in immunocompromised patients. J. Clin. Microbiol. 32:746-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pintado, V., and R. Lopez-Velez. 2001. HIV-associated visceral leishmaniasis. Clin. Microbiol. Infect. Dis. 7:291-300. [DOI] [PubMed] [Google Scholar]
- 12.Pintado, V., P. Martin-Rabadan, M. L. Rivera, S. Moreno, and E. Bouza. 2001. Visceral leishmaniasis in HIV-infected and non-HIV-infected patients: a comparative study. Medicine (Baltimore) 80:54-73. [DOI] [PubMed] [Google Scholar]
- 13.Pizzuto, M., M. Piazza, D. Senese, C. Scalamogna, S. Calattini, L. Corsico, T. Persico, B. Adriani, C. Magni, G. Guaraldi, G. Gaiera, A. Ludovisi, M. Gramiccia, M. Galli, M. Moroni, M. Corbellino, and S. Antinori. 2001. Role of PCR in diagnosis and prognosis of visceral leishmaniasis in patients coinfected with human immunodeficiency virus type 1. J. Clin. Microbiol. 39:357-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenthal, E., P. Marty, Y. le Fichoux, and J. Cassuto. 2000. Clinical manifestations of visceral leishmaniasis associated with HIV infection: a retrospective study of 91 French cases. Ann. Trop. Med. Parasitol. 94:159-162. [DOI] [PubMed] [Google Scholar]
- 15.van Eys, G. J., G. J. Schoone, N. C. Kroon, and S. B. Ebeling. 1992. Sequence analysis of small subunit ribosomal RNA genes and its use for detection and identification of Leishmania parasites. Mol. Biochem. Parasitol. 51:133-142. [DOI] [PubMed] [Google Scholar]