Transition metal-catalyzed reactions of alkynes with alkenes have proven to be reliable and important strategies for carbon-carbon bond formation.1 Previous efforts have focused primarily on non-oxidative reactions such as isomerizations of enynes to synthesize cyclic dienes and cyclopropyl derivatives,1a–g or cross-metathesis to synthesize acyclic dienes. 1e,h Extending alkyne-alkene reactivity to include oxidative couplings2 could provide convenient access to additional organic functionality. We recently used DNA-templated synthesis and in vitro selection to discover a Pd(II)-mediated alkyne-alkene cyclization reaction that generates a macrocyclic α,β-unsaturated ketone.3 Here we describe the development of an analogous intermolecular oxidative coupling reaction between alkynamides and terminal alkenes to generate acyclic α,β-unsaturated ketones4 (eq 1). Our results reveal that amides can mediate this mode of alkyne reactivity and provide efficient access to acyclic α,β-unsaturated ketones under very mild conditions.
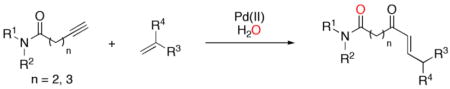 |
(1) |
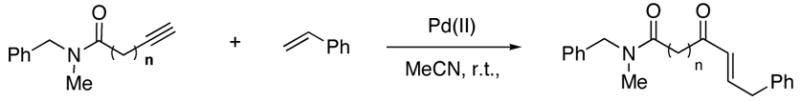
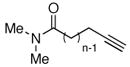
We began the development of this reaction by defining its basic requirements with respect to the alkyne substrate and the reaction conditions. Several alkynes were examined for their ability to react with styrene in the presence of various palladium salts. We discovered that alkynamides possessing a pentyn- or hexynamide backbone were required for efficient α,β-unsaturated ketone formation (Table 1). For example, slow addition of N-benzyl-N-methylpent-4-ynamide to a mixture of 1.5 equiv. of styrene and 1.0 equiv. of Na2PdCl4 in MeCN-H2O (3:2) at room temperature provided the E-α,β unsaturated ketone product in 53% isolated yield (Table 1, entry 4), whereas the analogous propyn-, butyn-and heptynamide substrate did not generate significant desired product under these conditions (Table 1, entries 2 and 8). Furthermore, no α,β-unsaturated ketone was observed when water was omitted from the reaction (Table 1, entry 3). The use of p-benzoquinone as a stoichiometric oxidant enabled multiple turnovers with 0.2 equiv. of Pd(II) to provide enone products in 54–58% yield (Table 1, entry 6 and 7).
Table 1.
Initial observations.a
 | ||||
|---|---|---|---|---|
| entry | n | Pd(II)/Oxidant | additive | yield (%)b |
| 1 | 0,1 | Na2PdCl4(1eq)/none | none | <1 |
| 2 | 0,1 | Na2PdCl4 (1eq)/none | H2O (1.6 mL) | <1 |
| 3 | 2 | Na2PdCl4 (1 eq)/none | none | <1 |
| 4 | 2 | Na2PdCl4 (1eq)/none | H2O (1.6 mL) | 53 |
| 5 | 2 | Na2PdCl4 (0.2 eq)/none | H2O (1.6 mL) | 8 |
| 6 | 2 | Na2PdCl4 (0.2 eq)/p-Benzoquinone (1.5 eq) | H2O (1.6 mL) | 58 |
| 7 | 3 | Na2PdCl4 (0.2 eq)/p-Benzoquinone (1.5 eq) | H2O (1.6 mL) | 54 |
| 8 | 4 | Na2PdCl4 (0.2 eq)/p-Benzoquinone (1.5 eq) | H2O (1.6 mL) | <1 |
Reactions were conducted at r.t. with 0.15 mmol of alkene in MeCN (2.4 mL) and 0.1 mmol of alkyne added dropwise over 5 h.
Isolated yield.
Based on the requirement for a pentynamide or hexynamide backbone, we hypothesized that α,β-unsaturated ketone formation proceeds through a cyclic oxypalladation intermediate.5 To test this proposal, we performed an 17O labeling experiment. When the reaction between N-benzyl-N-methylpent-4-ynamide (1) and styrene was conducted in MeCN-17OH2 (3:2), 17O NMR revealed the presence of broad peak at 318.5 ppm that is characteristic of an amide C=17O (eq 2) but inconsistent with an enone C=17O (eq 3).6 This result strongly suggests that the reaction proceeds through a cyclic oxypalladation intermediate (eq 2), rather than through an acyclic intermediate that would result from the direct hydration of a Pd(II)-alkyne complex (eq 3).7
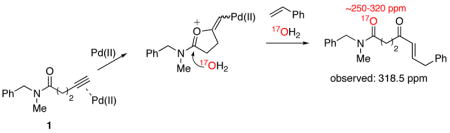 |
(2) |
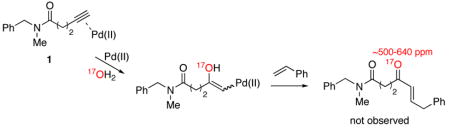 |
(3) |
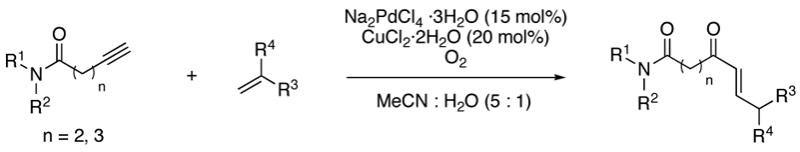
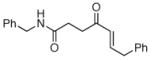
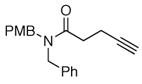
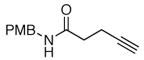
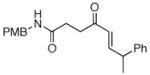
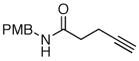
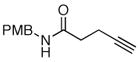
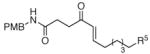
The scope of the reaction was further examined under optimized conditions using 0.15 equiv. of Na2PdCl4 0.2 equiv. of CuCl2, and molecular oxygen as the terminal oxidant in MeCN-H2O (5:1) (Table 2). Pentyn- or hexynamides containing both secondary and tertiary amides were reactive under these conditions. In addition to styrene and α-methylstyrene, a variety of terminal alkenes were effective substrates, including long-chain unactivated alkenes. Desired α,β-unsaturated ketone products in Table 2 were obtained with > 99:1 E/Z stereoselectivity and > 5:1 (for long-chain alkenes) to >20:1 (for styrenes) regioselectivity. The reaction is compatible with ester, carbamate, nitrile, acetate, and alkyl bromide functionalities. The high stereoselectivity in the reaction of long-chain unactivated terminal alkenes (Table 2, entries 10–16) is noteworthy in comparison with the intermolecular Heck reaction of unactivated alkenes, which typically exhibits lower selectivity (E/Z = ~2.5:1 to ~6:18).
Table 2.
Reaction scope of Pd(II)-catalyzed intermolecular reaction of alkynamides and alkenes.a
 | ||||
|---|---|---|---|---|
| entry | alkyne | alkene | product | yield (%)b |
| 1 |
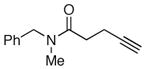
|
|
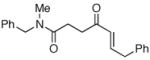
|
53c |
| 2 |
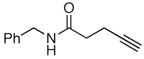
|
|
|
68c |
| 3 |
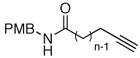
PMB:p-MeO(C6H4)H2- |
|
|
74c |
| 4 | 62c | |||
| 5 |
|
|
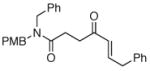
|
83c |
| 6 |
|
|
|
58c |
| 7 | 74c | |||
| 8 |
|
|
|
72c |
| 9 |
|
|
|
73 |
| 10 |
|
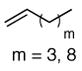
|
|
80d |
| 11 | 65d | |||
|
|
|
||
| 12 | R5 = CN | R5 = CN | 71d | |
| 13 | R5 = CO2Et | R5 = CO2Et | 54d | |
| 14 | R5 =OAc | R5 =OAc | 75d | |
| 15 | R5 =Br | R5 =Br | 76d | |
| 16 |
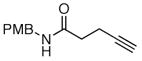
|
|
|
55d |
Reactions were conducted at r.t. or 40 °C with 15 mol% of Na2PdCl4·3H20,20 mol% of CuCl2·2H2O,1 atm O2,1.5 equiv of alkene in MeCN-H2O (5:1), and 1.0 equiv of alkyne added by dropwise addition for 8–12 h.
Isolated yield.
>99:1 linear:branched regioselectivity.
Linear:branched regioselectivity >5:1.
Although a cyclic oxypalladation intermediate has been proposed in the Pd(II)-catalyzed hydration of alkynyl ketones,5 pentynyl ketones such as hex-5-yn-2-one and 2-prop-2-ynylcyclopentanone were not reactive under the above conditions, further demonstrating the necessity of the amide group. We also note that the Wacker oxidation product9 derived from styrene, acetophenone, was not observed under these conditions.
Based on the above observations, a possible mechanism for the reaction is shown in Scheme 1. We propose that the initial step involves the activation of the alkynamide with Pd(II) to provide the cyclic oxypalladation intermediate A. This intermediate is then hydrated to generate acyclic oxypalladation intermediate B in which the oxygen from water is incorporated into the amide carbonyl. Intermediate B reacts with an alkene substrate through a Heck-like process10 resulting in Pd-alkyl species C. β-Hydride elimination generates a Pd-alkene complex such as D and sequential olefin insertion-β-hydride elimination steps result in migration of the olefin to the α,β position in E.11 Release of α,β-unsaturated ketone product followed by reductive elimination results in Pd(0), which is oxidized to regenerate Pd(II).
Scheme 1.
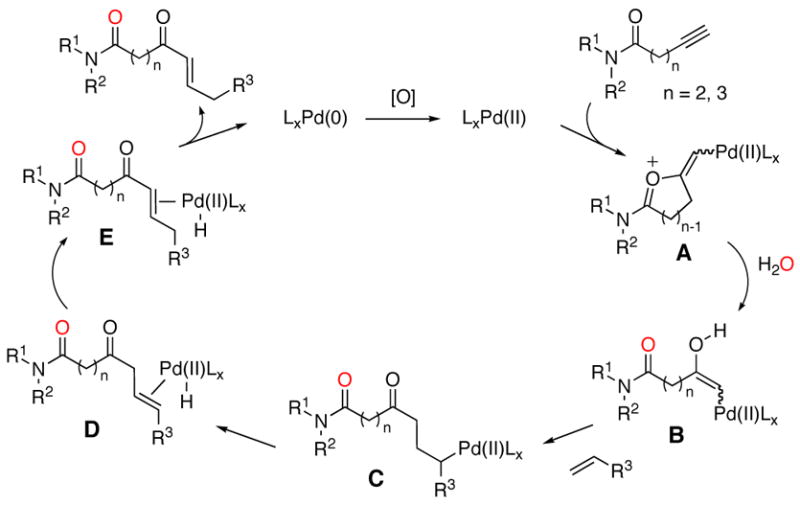
Proposed Mechanism.
In summary, we have demonstrated the Pd(II)-catalyzed intermolecular oxidative coupling of alkynamides and alkenes to provide α,β-unsaturated ketones with high stereo- and regioselectivity under very mild conditions. These findings identify alkynamides as efficient oxypalladation precursors that undergo hydration followed by a Heck-type process. Further studies to explore the reactivity of intermediates proposed in Scheme 1 are underway.
Supplementary Material
Supporting Information Available: Experimental procedures and spectral data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We thank Prof. Andrew G. Myers for helpful discussions and Dr. Shaw Huang for assistance with 17O NMR. This research was supported by NIH R01GM065865 and by the Howard Hughes Medical Institute. N.M. acknowledges a Damon Runyon Cancer Research Foundation Postdoctoral Fellowship (DRG: 1869-05).
References
- 1.(a) Trost BM. Ace Chem Res. 1990;23:34–42. [Google Scholar]; (b) Trost BM, Krische M. J Synlett. 1998:1–16. [Google Scholar]; (c) Tsuji J. Transition Metal Reagents and Catalysts: innovations in organic synthesis. Wiley; Chichester: 2000. pp. 227–277. [Google Scholar]; (d) Thiel OR. In: Transition Metals for Organic Synthesis. Seller M, Bolm C, editors. Vol. 1. Wiley-VCH; Weinheim: 2004. pp. 321–333. [Google Scholar]; (e) Diver ST, Giessert A. J Chem Rev. 2004;104:1317–1382. doi: 10.1021/cr020009e. [DOI] [PubMed] [Google Scholar]; (f) Braneau C. Angew Chem Int Ed. 2005;44:2328–2334. doi: 10.1002/anie.200462568. [DOI] [PubMed] [Google Scholar]; (g) Zhang L, Sun J, Kozmin SA. Adv Synth Catal. 2006;348:2271–2296. [Google Scholar]; (h) Connon SJ, Blechert S. Angew Chem Int Ed. 2003;42:1900–1923. doi: 10.1002/anie.200200556. [DOI] [PubMed] [Google Scholar]; (i) Gibson SE, Stevenazzi A. Angew Chem Int Ed. 2003;42:1800–1810. doi: 10.1002/anie.200200547. [DOI] [PubMed] [Google Scholar]
- 2.For recent representative examples of oxidative coupling reactions catalyzed by Pd(II) see Yip KT, Yang M, Law KL, Zhu NY, Yang D. J Am Chem Soc. 2006;128:3130–3131. doi: 10.1021/ja060291x.Lee JM, Ahn DS, Jung DY, Lee J, Do Y, Kim SK, Chang S. J Am Chem Soc. 2006;128:12954–12962. doi: 10.1021/ja0639315.Silva F, Reiter M, Mills-Webb R, Sawicki M, Klär D, Bensel N, Wagner A, Gouverneur V. J Org Chem. 2006;71:8390–8394. doi: 10.1021/jo061292a.Hull KL, Lanni EL, Sanford MS. J Am Chem Soc. 2006;128:14047–14049. doi: 10.1021/ja065718e.
- 3.Kanan MW, Rozenman MM, Sakurai K, Snyder TM, Liu DR. Nature. 2004;431:545–549. doi: 10.1038/nature02920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.For representative examples of α,β-unsaturated ketone synthesis via oxidative reaction catalyzed by Pd(II) see Ito Y, Hirao T, Saegusa T. J Org Chem. 1978;43:1011–1013.Ito Y, Aoyama H, Hirao T, Mochizuki A, Saegusa T. J Am Chem Soc. 1979;101:494–496.
- 5.Imi K, Imai K, Utimoto K. Tetrahedron Lett. 1987;28:3127–3130. [Google Scholar]
- 6.Delseth C, Nguyên TT, Kintzinger IP. Helv Chem Act. 1980;63:498–503.(b) Reference guide provided by Bruker, published in 1994.
- 7.A Pd-alkynl intermediate may be considered as another possibility; we disfavor such an intermediate as a major contributor to product formation, however, because it is inconsistent with the requirement for a pentynamide or hexynamide backbone.
- 8.For a representative example and additional references see Littke AF, Fu GC. J Am Chem Soc. 2001;123:6989–7000. doi: 10.1021/ja010988c.
- 9.For representative reviews of the Wacker oxidation see Smidt I. Chem Ind. 1962:54–62.Tsuji I. Synthesis. 1984;5:369–384.Feringa BL. Transition Metals for Organic Synthesis. 1998;2:307–315.Takacs IM, Jiang X-T. Curr Org Chem. 2003;7:369–396.
- 10.For reviews of the Heck reaction see Heck RF. Org React. 1982;27:345–390.Cabri W, Candiani I. Ace Chem Res. 1995;28:2–7.
- 11.For representative examples of olefin migration mediated by PdH see Ozawa F, Kubo A, Matsumoto Y, Hayashi T. Organometallics. 1993;12:4188–4196.Loiseleur O, Meier P, Pfaltz A. Angew Chem Int Ed. 1996;35:200–202.Qian H, Widenhoefer RA. J Am Chem Soc. 2003;125:2056–2057. doi: 10.1021/ja0293002.Hatano M, Mikami K. J Am Chem Soc. 2003;125:4704–4705. doi: 10.1021/ja0292748.Trend RM, Ramtohul YK, Stoltz BM. J Am Chem Soc. 2005;127:17778–17788. doi: 10.1021/ja055534k.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Available: Experimental procedures and spectral data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.


