Abstract
Purpose
The role of integrin/cell matrix interactions between the RPE and the basement membrane in retinal maintenance and function is not well characterized.In this study the functional importance of α1β1 integrin for RPE cell homeostasis and retinal health was assessed by comparing α1 integrin knockout mice with strain/age matched wild type mice.
Methods
Immunolocalization and western blot analysis of retinas and ARPE19 cells was performed to examine expression of α1β1 integrin in the RPE. Retinal pathology was assessed by funduscopy, histology, and transmission electron microscopy. Progressive retinal damage was quantified by direct counting of rod photoreceptors. Light-induced translocation of arrestin and α-transducin was documented by immunohistochemical analysis of retinal cryosections.
Results
Integrin α1β1 localizes to the basal aspect of retinal pigment epithelial cells co-localizing with the basal lamina of the RPE. Integrin α1 null mice have delayed onset progressive retinal degeneration associated with thickening of the basement membrane, dysmorphology of basal processes, synaptic malformations and funduscopic abnormalities. Integrin α1-null mice display marked delay in transducin translocation compared to wild type mice following exposure to light in dark-adapted animals.
Conclusions
Collectively, these data suggest an essential role for α1β1 integrin/basement membrane interactions in the RPE in basement membrane metabolism and translocation of transducin in photoreceptors. This is the first report describing evidence supporting an essential role for integrin/basement membrane interaction in the RPE. Further, this report demonstrates a direct link between integrin α1β1 function in RPE and molecular defects in photoreceptor cell function before retinal pathology is apparent.
Introduction
The retinal pigment epithelium (RPE) is comprised of a tight sheet-like monolayer of highly polarized epithelial cells. At the apical surface, the RPE interfaces with the photoreceptor outer segments adhering through the interphotoreceptor cell matrix (IPM).The RPE is critical for the maintenance, proper function, and health of photoreceptors. One of the well characterized functions of the RPE occurs at this apical interface, where shed disks are phagocytized as part of daily retinal maintenance.1 Disruption of this phagocytic process results in retinal dystrophy in both rodents and humans.2,3 The shed outer segment (OS) disks are bound by apically localized αvβ5 integrin, activating focal adhesion kinase.4,5 Internalization of the bound OS disks requires the scavenger receptor CD36 and phosphorylation of Mer tyrosine kinase.6,7
While much is known about the function of αvβ5 integrin in the apical RPE, very little is known about integrins that localize to the basal aspect of the RPE, where the basal processes contact the basal lamina of the RPE. It is clear from related studies in other organ systems that integrin binding to matrix proteins in basement membranes modulates cell signaling pathways that play key roles in cell behavior and gene expression programs.8 Recent studies suggest that RPE cells adhere to the basement membrane via interaction of integrin α3β1 and α6 β1 with laminins -111, -115, -511, and -521.9 In proliferative vitreoretinopathy, exposure of RPE to cytokines such as TNF-α may induce integrin α1 and α5 expression, altering the adhesion, migration, and proliferation of RPE cells.10 An essential role for integrin function at the basal aspect of the RPE in vivo has not been demonstrated.
Integrin α1β1 is a collagen binding integrin well characterized for its roles in regulating adhesion, migration, and proliferation.11 Integrin α1 null mice were produced over 10 years ago. The animals were reportedly without obvious phenotype, however fibroblasts from these mice did show deficits in adhesion to collagen matrix.12 Since then, integrin α1β1 has been shown to influence progression of a number of different diseases, including diabetes, Alport syndrome, inflammatory bowel disease, and arthritis.13–16 While the specific mechanisms whereby α1β1 integrin blockade influences disease progression in these models remains unclear, it is known that neutralization of α1β1 integrin influences cell signaling, matrix remodeling, and regulation of matrix metalloproteinases.
It is known that α1β1 integrin is expressed on cultured RPE cells.10 In RPE cell culture systems it has been demonstrated that α1β1 integrin mediated activation of MAPkinase can influence matrix remodeling in collagen gel contraction assays.17 We surmised that α1β1 integrin might play important functional roles in RPE cells in vivo. Here we explore this possibility through the structural and functional characterization of the neuroretina and RPE of integrin α1-null mice compared to wild type littermates.
Methods
Mice
Alpha1 integrin knockout mice were a gift from Humphrey Gardner (Novartis, Cambridge, MA). 12 All mice were on the 129 Sv background (129S4/SvJae, white bellied agouti) and bred in house. All animal experiments were conducted in compliance with an approved IACUC animal protocol and in agreement with the ARVO animal treatment statement.
Immunofluorescence microscopy for α1β1 integrin
Mouse retinas were frozen in aqueous embedding compound, sectioned at 4 microns, and fixed by immersion in cold acetone for 10 minutes. Slides were air-dried, rehydrated, and reacted with either a hamster monoclonal antibody specific for α1β1 integrin heterodimer (a gift from Biogen-Idec, Cambridge, MA), rabbit polyclonal antibodies against β1 integrin (Chemicon, International, Temecula, CA), or anti-CD31 antibodies (Cedar Lane Ltd., Hornby, Ontario).The antibodies were diluted at 1:100 into 7% non-fat dry milk in PBS. Following several washes, sections were reacted with a FITC-conjugated anti-rabbit or anti-hamster secondary antibodies (Vector Laboratories, Burlingame, CA) or Alexa-568 conjugated anti-hamster antibodies (for dual immunofluorescence), washed, and coverslipped. The fluorescent images were recorded using a Zeiss Axioplan 10 fluorescence microscope interfaced with a SPOTFLEX-CE digital camera supported by Image Pro-Plus software. Either pre-immune rabbit IgG or isotype-matched non-reactive hamster monoclonal antibodies were used as controls, and neither showed any immunoreactivity (data not shown).
Funduscopic analysis
Clinical retinal evaluation was performed as described previously.18 Pupils were dilated with atropine and evaluated by indirect opthalmoscopy using a 78 diopter lens. Photographs were taken using a Kowa Genesis small animal fundus camera (Torrance, CA).
Cell culture
ARPE-19 cells were purchased from the American Type Culture Collection and grown in Dulbecco’s Modified Eagle Medium/Hams F-12 (1:1) supplemented with 1% fetal calf serum, Penn/Step antibiotics (GibCo) and 1% bovine retinal extract. The retinal extracts were prepared from freshly isolated bovine retinas using the method described by Hu and Bok.19 Cells were differentiated by culturing for approximately 3 months under low serum (1% FCS) at confluency. Prolonged culture combined with the inclusion of retinal extract promoted tight junction formation with ZO-1 expression at cell junctions and a cobblestone morphology consistent with earlier reports.20
Western Blot analysis
The neural retinas were dissected from the RPE choroid. The lens was removed from the eye, and the optic nerve cut to release the neural retina. Retinal extracts prepared from each compartment using RIPA buffer. Protein was quantified using the Bradford microassay (Pierce). Ten µg of protein for beta1 and 40 µg of protein for alpha1 was analyzed. The samples were incubated for 40 min at 55°C in the presence of sample buffer without reducing agents and then loaded in a 7% acrylamide gel. The transfer was at 35 V, 4°C, overnight. Membranes were blocked by incubation for 4 hours at 4°C in blocking solution containing 10% non-fat dry milk in PBS. For integrin beta1 subunit; first antibody (Chemicon, rabbit anti-integrin beta-1 polyclonal antibody, AB1952) 1/100 in blocking solution overnight at 4°C; Second antibody (SIGMA anti-rabbit A9169) 1/20,000 in blocking solution, 1 hour at room temperature. For integrin alpha 1 subunit; first antibody (Biogen, hamster anti-mouse H318) 1/1000 in blocking solution overnight at 4°C; second antibody, biotinylated goat anti-hamster 1/500 in blocking solution for 1 hour room temperature.Blots were developed using streptavidin-HRP conjugate (BioRad) 1/500 in blocking solution for 1 hour at room temperature. For ARPE-19 cells, 10µg of protein for β1 integrin and 40 µg of protein for α1 integrin was analyzed from cells grown for 3 months after confluence in DMEM/F12 (1:1), 1% FCS and 1% of bovine retina extract. Samples were run on 7% PAGE gels under reducing conditions for β1 integrin and non-reducing conditions for α1 integrin. Blots were blocked overnight at 4°C using 10% non-fat dry milk in PBS. Incubation with a rabbit polyclonal anti-β1 integrin antibody (Chemicon, AB1952) in blocking solution at 1/100 overnight and developed with HRP conjugated anti-rabbit antibodies in blocking solution at 1/7,500 for 1 hour at room temperature. For anti-α1 integrin, blots were incubated with a mouse monoclonal anti-α1 integrin antibody (Chemicon, MAB1973) in 5% non-fat dry milk at 1/100 dilution overnight and developed using anti-mouse HRP conjugated antibodies diluted in 5% non-fat dry milk at 1/1000 for 1 hour at room temperature.
Light/Dark Adaptation
Procedures for handling of animals followed the guidelines of NIH. For dark adaptation, the animals were kept in cages in a lightproof darkroom without any detectable light. For light adaptation, the animals were first kept in darkroom for 8 hours dark adaptation, and then the animals were kept in transparent cages under various intensity of diffuse white fluorescent light (1500 lux intensity at cage level) for one hour. Four hours dark adaptation and 1 hour light adaptation at this light intensity is adequate for proteins to be translocated in rod photoreceptors in wild type mice.21
Immunohistochemistry for arrestin and transducin
Eyes were quickly removed from animals euthanized under deep anesthesia. After removal of the anterior segments, the posterior eyecups were fixed in 4% paraformaldehyde in 100 mM sodium phosphate buffer (PB, pH 7.3) at 4°C. The tissue was transferred sequentially into 5% and 30% sucrose in PB, each at 4°C overnight. Retinal sections were cut at 4 micron thickness using a Microm cryostat and mounted on gelatin-coated slides. Retinal sections were then incubated with 5% normal goat serum (Vector Laboratories) in PBS for 1 hour at room temperature, and then incubated with primary antibodies overnight at 4°C [antiarrestin antibody (Sigma, MO) 1:500; anti-transducin α (Cytosignal, CA) 1:1000], and followed by three washes in PBS of 15 min. each. The sections were then incubated with either Alexa 594-conjugated anti-mouse immunoglobulin antibody (Invitrogen, OR) 1:250 or Alexa 488-conjugated anti-rabbit immunoglobulin antibody (Invitrogen, OR) 1:250 for 2 hours at room temperature. Staining reactions were terminated by 3 washes with PBS (5 minutes each) and the slides were coverslipped with 50% glycerol in PBS for viewing under a Zeiss Axioplan 10 fluorescence microscope. Images were recorded as described above for integrin immunostaining. All incubation and wash buffers contained Triton X-100 (0.3%).
Electron microscopy
Eyecups were fixed in 3% glutaraldehyde and 4% paraformaldehyde in 0.1M cacodylate buffer (pH 7.3) at 4°C overnight, followed by post-fixation in 1% osmium tetroxide in 0.1M cacodylate buffer for one hour. After washing again in cacodylate buffer these sections were dehydrated in a graded series of alcohol and propylene oxide and stained with 1%uranyl acetate for 1 hour, infiltrated, and embedded in resin (EmBed 812, EMS, Fort Washington, PA). Ultrathin sections (70 nm) of the retinal cross sections were mounted on copper grids then stained with uranyl acetate and bismuth. The specimens were examined in a JEOL JEM-1010 microscope. Digitized images were acquired using an Orca CCD camera (Hamamatsu Photonics, Bridgewater, NJ) with AMT Advantage 12-HR software (version 5.4.2.239, Advanced Microscopy Techniques, Danvers, MA).
Counting total photoreceptor numbers
The number of nuclei in the ONL of retinal sections at different time points was calculated. At each time point, number was counted from retinal sections in central areas 2 mm eccentric from the optic nerve head site, which can be recognized under a light microscope. The central 1/3 of the entire length of retinal cross section traversing the entire retina width passing through the optic nerve head from superior Ora Serrata to inferior Ora Serrata was defined as the central region. Both vertical and horizontal sections across this site were examined. Serial retinal sections (4–5 µm each section for 12 sections) were taken. Mean number of nuclei in the ONL on these sections was counted. Only well-oriented sections with straight rod outer segments that did not have oblique orientation were used. Data obtained from integrin α1-null mice were compared with that from the wild type retina of the same eccentricity and ages. Three animals for each age/genotype were used, and analyzed statistically.
Statistical analysis
Results are expressed as means +/− SD. Statistical significance was determined using the student’s t-test with Bonferoni correction. P<0.05 is considered to reflect significance of difference.
Results
Immunolocalization of integrin α1β1 in wild type adult mouse retinas was performed using antibodies that recognize the heterodimer.22 Figure 1 (A and B) shows bright immunostaining in a linear pattern at the basal aspect of the RPE, consistent with the polarized distribution of integrin α1β1 previously described.23 As expected, β1 integrin-specific antibodies show positive immunostaining for the same structures, but are more widely distributed in the vessels of the choroid plexis (Figure 1C), since this antibody reacts to all β1-containing integrin heterodimers. Immunostaining for both α1β1 integrin and the integrin β1 subunit is also observed in the capillaries of the neural retina as shown by dual immunofluorescence immunostaining using both anti-α1β1 integrin and anti-CD31 antibodies (Figure 1E–G). No immunostaining for α1β1 integrin is observed in retinas from integrin α1-null mice (Figure 1D), demonstrating the specificity of the antibody.
Figure 1.
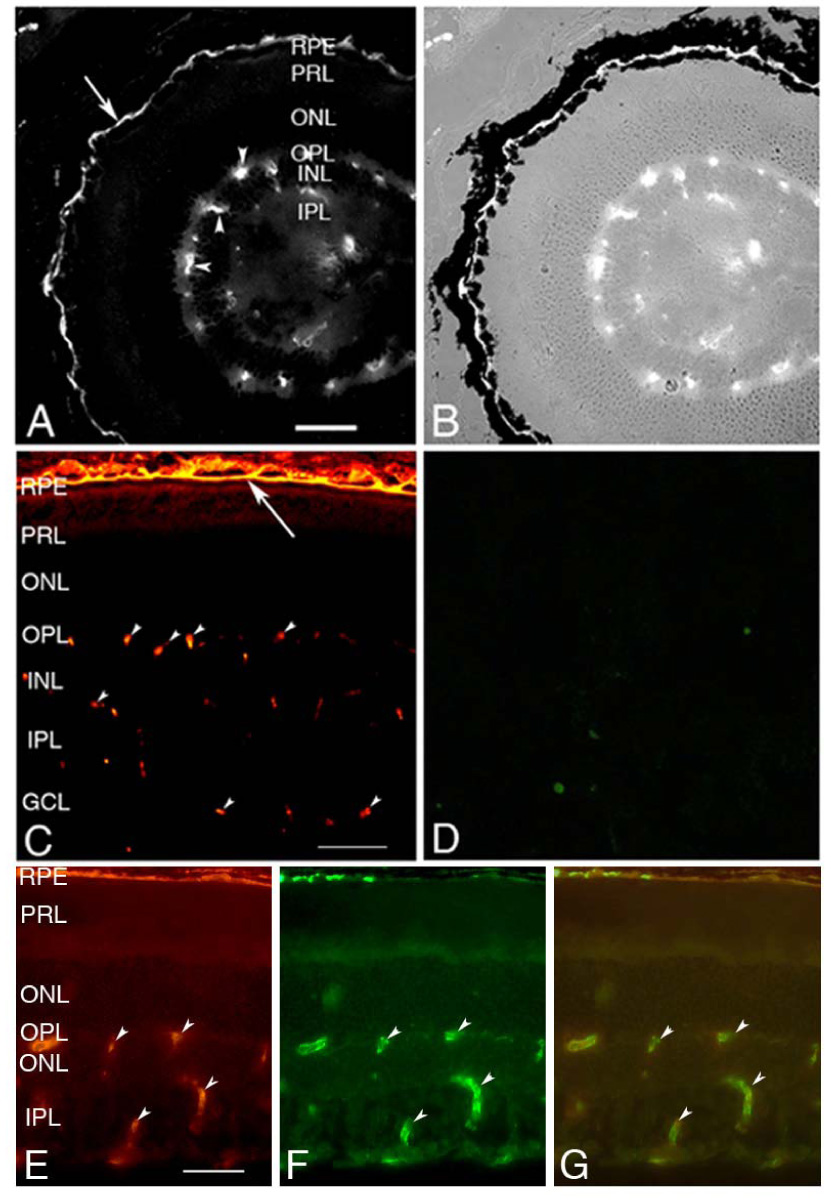
Immunofluorescence localization of integrin α1β1 at the basolateral aspect of the RPE. An α1β1 integrin-specific antibody shows bright immunostaining at the basal aspect of the RPE (arrow) in the mouse retina (A). A Bright field image of this same stained section (B) clearly identifies the signal just basal to the pigmented epithelium. Panel C shows immunostaining of wild type mouse retina for integrin β1 subunit, which also localizes to the basal aspect of the RPE (arrow). Panel D shows absence of staining for integrin α1in retinal sections from the integrin α1-null mouse, demonstrating the specificity of the antibody. Specific immunostaining is also observed in the capillaries of the neuroretina (arrow heads), as confirmed by dual immunofluorescence immunostaining with anti-CD31 antibodies (E, anti-α1β1 integrin; F, anti-CD31; G, merged images). RPE=Retinal Pigment Epithelium; PRL=Photoreceptor Layer; ONL=Outer Nuclear Layer; OPL=Outer Plexiform Layer; INL=Inner Nuclear Layer; IPL=Inner Plexiform Layer; GCL=Ganglion Cell Layer; scale bars are 50 µm.
To confirm the presence of α1β1 integrin in mouse retinas biochemically, western blots were performed. Protein extracts were produced from the neural retina and the remaining RPE choroid, which contains the retinal pigment epithelium and the choroid. Extracts were fractionated on PAGE gels, blotted onto nylon membranes, and duplicate blots probed using antibodies specific for either α1 or β1 integrin subunits. The results in Figure 2 show that wild type mice express α1 and β1 integrin subunits predominantly in the RPE choroid, which is consistent with our immunolocalization studies. Expression in the neural retina was expected based on immunostaining data (Figure 1) but not observed, presumably due to the limited sensitivity of the western blot analysis. For integrin α1 null mice, there is no α1 band, demonstrating the specificity of the antibody on western blots. The β1 subunit is still present, which is expected since it can form heterodimers with other α chains. Lane 5 on the two blots in Figure 2 shows that differentiated ARPE-19 cells express both the α1 and β1 integrin subunits, demonstrating that RPE cells express α1β1 integrin heterodimers.
Figure 2.
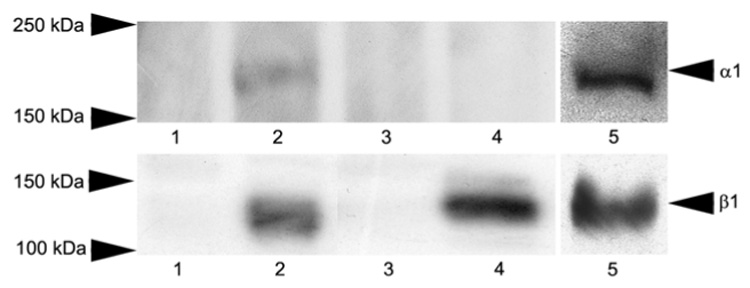
Western blot analysis of α1β1 integrin in mouse retinas and ARPE 19 cells. Detergent extracts of neuroretina (lanes 1 and 3) and RPE choroid (lanes 2 and 4) following removal of the neuroretina from wild type mice (lanes 1 and 2) and integrin α1-null mice (lanes 3 and 4) were analyzed by western blots probed with antibodies specific for either integrin α1 (upper panel) or β1 (lower panel) subunits. Lane 5 shows western blots of ARPE19 cell extracts probed with antibodies against either human α1 integrin subunit (upper panel) or human β1 integrin subunit (lower panel)
Integrin α1-null mice exhibit clinical, structural, and ultrastructural defects indicative of retinal pathogenesis
Since integrin α1β1 appears to localize to the basal aspect of the RPE, we presumed it may be important for retinal function. The gene knockout mouse for integrin α1 does not exhibit any life threatening phenotypic characteristics,12 so we employed this mouse model to address this question. Several integrin α1 knockout mice as well as age and strain-matched controls were examined by funduscopic photography. Spots consistent with retinal degenerative disease were observed in retinas of knockout mice, but never in strain/age matched controls. Spots were small and infrequent in animals younger than 7 to 8 months of age. Shown in Figure 3A are representative funduscopic photos of 10-month-old wild type and integrin α1-null (α1 −/−) mice. The spots were prevalent in the peripheral retina (shown) but more difficult to find in the central retina, and appeared to worsen with age. Figure 3B shows a typical cross section of the retina from a 15 month old integrin α1-null mouse and a strain/age matched wild type mouse. The integrin α1-null mouse exhibits thinning of the outer nuclear layer in the retina relative to wild type mice. Shortening of the outer segments of the photoreceptors is also evident in the retinas of the integrin α1-null mice relative to wild type mice. This is often associated with retinal degeneration.24,25 Since integrin α1β1 is expressed at the basal aspect of the retinal pigment epithelium (Figure 1), and since this collagen binding integrin is known to influence matrix remodeling in other experimental systems,11,15 we surmised that we may observe defects in the basal lamina of the RPE in diseased retinas. Several 12 month old integrin α1 null mice and age and strain-matched wild type mice were analyzed. From each mouse, one eye was prepared for histology and the other for transmission electron microscopy. Once pathology was confirmed, by histological staining and counting of the outer nuclear layer, the other eye was processed for thin sectioning. The results in Figure 3C show typical ultrastructure of the basement membrane just beneath the basal processes of the RPE in wild type mice and integrin α1-null (α1−/−) mice. We chose an image from a strain matched 24 month old wild type mouse to demonstrate that the normal architecture of the basement membrane and basal processes of the RPE are maintained even in much older mice, showing a well defined and uniform lamina densa flanked by lamina rara interna and externa, associated with a confluence of RPE basal processes. In contrast, the basement membrane of the integrin α1-null mouse was markedly thickened and the basal processes are retracted and disorganized (arrow). As noted in Figure 1 (E–G) the capillaries of the neuroretina are also immunopositive for α1β1 integrin. We did not observe any significant differences in capillary basement membrane ultrastructure when comparing wild type and integrin α1-null mice (data not shown).
Figure 3.
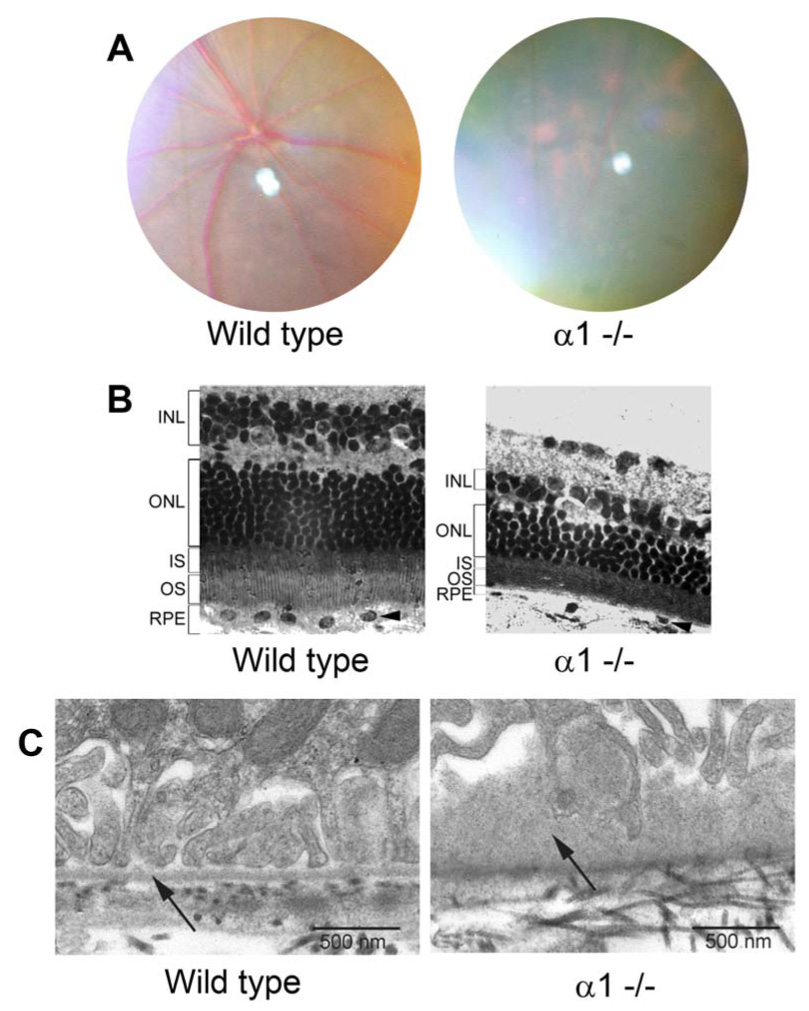
Integriṇα1-knockout mice show clinical pathology of the retina associated with thinning of the outer nuclear layer and thickening of the basal lamina of the RPE. Panel A. typical funduscopic images from 10 month old wild type mice and integrin α1-null (α1KO) mice reveals spots in the peripheral retina of ạ α1-null mouse characteristic of retinal degeneration. Panel B. Histological examination of 15-month-old wild type and integrin α1-null mice. The retina of integrin α1-null mice showed significantly reduced number of nuclei in the outer nuclear layer (ONL) when compared with the wild type retina of the same age. The outer segments of photoreceptors (OS) appear shortened in integrin α1-null mice relative to wild type mice. Panel C. Ultrastructural analysis of the basal lamina of the RPE in 24-month-old wild type mouse and 12-month-old integrin α1-null mouse. The basement membranes in the basal lamina of the RPE shows a retraction of the basal processes of the RPE associated with irregular thickening (arrow) of the basal lamina of the RPE for the integrin α1 null mice. This basement membrane thickening is not evident in integrin α1-null mice prior to observed reductions in ONL thickness (Between 8 and 10 months).
To better characterize the progressive retinal degeneration in the α1 null mice, we analyzed retinal sections encompassing the full length of the retina. The total number of rods was quantified as described in the methods. The process was repeated for 3 wild type mice and 3 integrin α1-null mice at 3, 6, 9, 12, and 15 months of age, and the data statistically analyzed. The results in Figure 4 show that loss of rod photoreceptors is first evident in the integrin α1-null retinas compared to wild type mice when the animals are 12 months of age, and progressively worsens through 15 months of age. At earlier timepoints analyzed, there was no significant difference in rod numbers.
Figure 4.
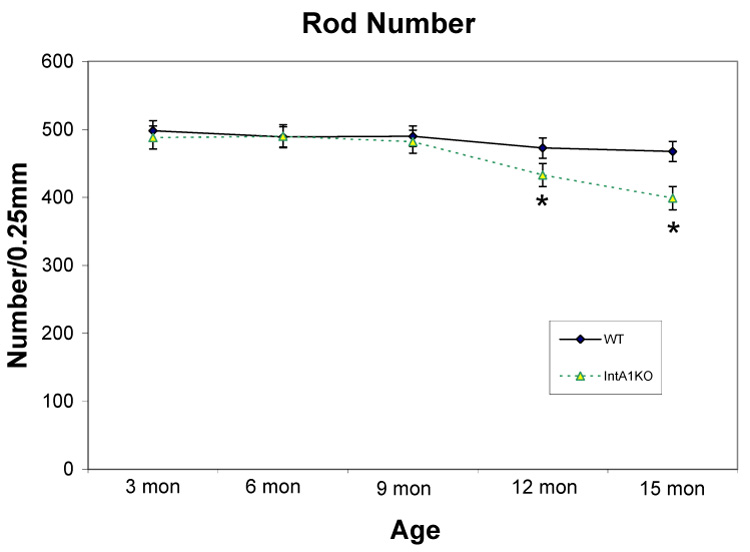
Kinetics of rod loss as a function of age in integrin α1-null mouse retinas relative to wild type mouse retinas. Data points represent quantitative measures of outer nuclei across the entire retina for wild type mice compared to α1 integrin null mice at the indicated ages. Statistically significant differences between wild type and integrin α1-null groups are marked by asterisks (P<0.05).
Synaptic defects are often observed in photoreceptor synaptic terminals during photoreceptor degeneration.26–29 We compared the synaptic ultrastructure of integrin α1-null mice with that of age/strain matched wild type mice to determine if synaptic abnormalities were present. Figure 5 shows that synaptic defects suggested the missing of bipolar cell dendrites from photoreceptor synaptic terminals are observed for both rod and cone photoreceptors in integrin α 1-null mice >12 months of age. In 12-month-old wild type mouse retina, invaginating synaptic complexes with both invaginating horizontal processes and bipolar cell dendrites can be easily and frequently detected under EM (>85%, Figure 5A and C). In α 1-null mice, however, there are very few intact invaginating ribbon synapses with characteristic synaptic bipolar cell dendrites in both rod and cone terminals. More than 75% cone synaptic terminals have only floating ribbons (arrows) without any post-synaptic component (Figure 5B).The flat contact synapses (small arrows) on the basal side, however, appear intact, suggesting the synaptic contacts between cones and OFF-bipolar cells remained normal. For each calculated cone terminal, the orientation of cone terminal was determined by the clear visible basal side with flat contact synapses to minimize the possibility of problem in sectioning angle. Likewise, more than 80% of rod spherules in the integrin α1-null mice display only a dyad structure or double ribbon without invaginating bipolar cell dendrites (Figure 5D). All these results suggest the photoreceptors in integrin α1-null mice may lose the invaginating bipolar cell dendrites.
Figure 5.
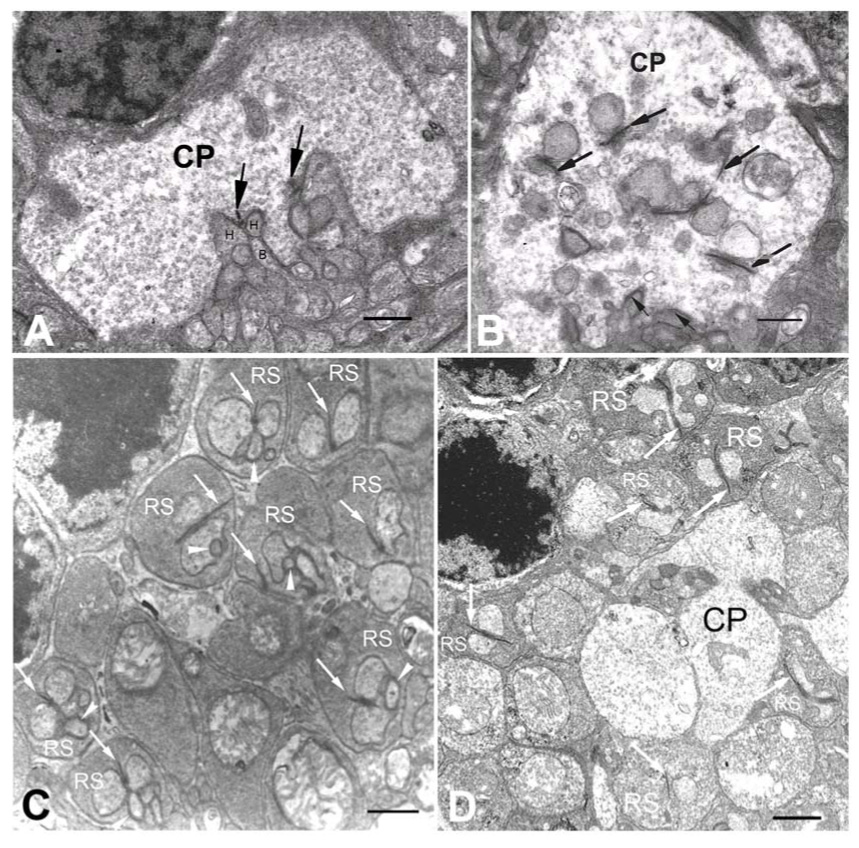
Synaptic malformations are observed in both rod and cone synaptic terminals in integrin α1-null mice. A. Retinal section from a 12-month-old wild type under TEM showing the cone synaptic terminal (CP = Cone Pedicle). In this normal cone photoreceptor synaptic terminal, invaginating synapses (recognized by the synaptic ribbons, as indicated by the arrows) with post-synaptic horizontal cell processes (H) and ON-cone bipolar cell dendrites (B) are located superficially on the basal side of the terminal. B. A retinal section of 15-month-old integrin α1-null mouse under TEM showing the cone synaptic terminal (CP). Most invaginating synapses (recognized by the synaptic ribbons, as indicated by large arrows) do not have post-synaptic bipolar cell dendrites, and are located deeply inside the cone synaptic terminal. The basal side can be recognized by conventional flat contact synapses (small arrows).C. A retinal section from a 12 month-old wild type mouse under TEM illustrating the outer plexiform layer. The rod synaptic termini (RS) display the normal triad structure. D. A retinal section of 15-month-old integrin α1-null mouse under TEM showing the outer plexiform layer. Most rod photoreceptor synaptic terminals (RS) do not have normal triad structure, instead, they have double ribbons (arrows) or dyads. The cone terminal (CP) does not have any invaginating synapses. Scale bar = 500 nm.
Integrin α1-null mice show defects in light induced translocation of transducin a-subunit, but not arrestin
Following exposure to light in dark adapted animals, transducin α-subunit rapidly translocates from the outer segments to the inner segments of rod photoreceptors, and arrestin translocates from the inner segments to the outer segments. This light-driven massive protein translocation is believed to be an important aspect of tuning photoreceptor sensitivity to various light intensities.30 Recent studies have suggested that RPE may play important roles in this translocation. In RPE65 knockout mice, which lack the retinoid isomerase needed to convert all trans retinyl ester to 11-cis retinol for rhodopsin activation, translocation of both transducin α-subunit and arrestin are blocked.31 We surmised defects in α1β1 integrin function in RPE cells might also influence protein translocation. We compared protein translocation in integrin α1-null mice with age matched wild type mice. Four month old mice (before any signs of retinal degeneration are observed) were dark adapted overnight, exposed to 1500 lux illumination for 1 hour, and the eyes immediately fixed. Retinal sections were immunostained using antibodies specific for either arrestin or transducin α-subunit. The results in Figure 6 show that light-induced translocation of arrestin is apparently normal in the integrin α1-null mice (Figure 6C, D, G and H). Conversely, translocation of transducin α-subunit is markedly delayed in integrin α1-null mice relative to wild type mice (Figure A, B, E and F). In integrin α1-null mice, after dark adaptation for 8 hours, transducin α is also found mostly in the rod outer segments (Figure 6E) as that in the wild type mouse (Figure 6A). After one hour light adaptation (1500 lux), transducin α in the wild type mouse is translocated to the inner segments (Figure 6B). In integrin α1-null mice, however, after one hour light adaptation under the same light intensity as that used in the wild type, the strongest immunostaining of transducin α is still in the outer segments of the rod photoreceptors (Figure 6F), indicating the light-induced translocation of transducin α is delayed. The defect in transducin α–.subunit translocation is apparent before photoreceptor cell degeneration is observed. Interestingly, the delay in transducin translocation is more pronounced in the peripheral retina than in the central retina (data not shown). Delayed translocation of transducin α-subunit was observed in integrin α1-null mice at 2, 6, 8, 12, and 15 months of age, and the degree of this delay compared to age/strain-matched wild type mice was not qualitatively different across these age groups (not shown). This suggests that the protein translocation defect in these animals does not worsen as a function of progressive retinal degeneration. It should be noted that transducin translocation in the rod photoreceptors of integrin α-null mice is not completely blocked. After longer light adaptation (> 4 hours), most of the transducin is translocated to the inner parts of rod photoreceptors as in wild-type mice (data not shown).
Figure 6.
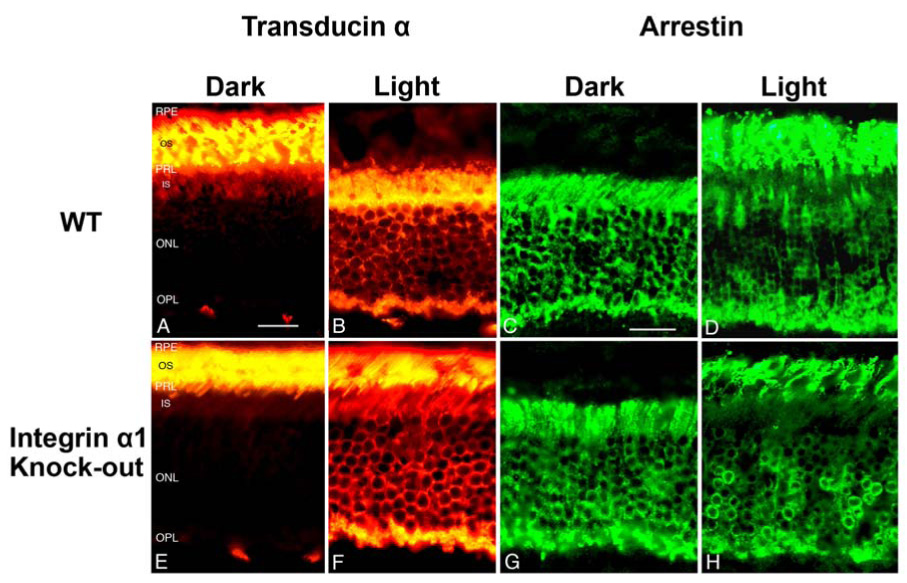
Integrin α1 null mice show defects in light-induced protein translocation of transducin α-subunit, but not arrestin. Immunostaining of transducin α subunit on wild type (A and B) and integrin α1-null (E and F) retinas under dark adaptation for 8 hours (A and E) and light adaptation for 1 hour (B and F). Note the failure of transcudin-α subunit to completely translocate from the outer segments into the inner segments in light adapted retinas from α1-null mice (compare panel B with panel F). Om the other hand, arrestin translocation to the outer segments following 1 hour light adaptation appeared qualitatively similar to wild type mice (compare panel H with panel D). OS=Outer Segments; IS=Inner Segments; other labels are the same as in Figure 1. scale bar=25 µm.
Discussion
Epithelial cells adhere to basement membranes through cell matrix integrin interactions, and in a broad array of cell culture and organ systems, these interactions have been shown to influence both cytoskeletal organization and cell signaling.32 These cell matrix integrin interactions play pivotal roles in both the development and maintenance of a highly differentiated state, and disruption of these interactions have been shown to have profound effects on tissue development and function. Despite the proliferation of this evidence in other systems, no studies have addressed whether integrin interactions with basement membrane proteins in the retinal pigment epithelial compartment influence RPE function or retinal health. In this study we show that α1β1 integrin localizes to the basal aspect of the RPE where it likely binds to matrix molecules in the basal lamina of the RPE. Expression of α1β1 integrin in cultured ARPE19 cells confirms RPE expression of this integrin. Alpha1beta1 integrin is a collagen binding integrin that displays distinct recognition for various collagen subtypes.33 One well established function of α1β1 integrin/collagen interaction is to activate signaling cascades that influence MMP regulation.34–37 Basement membrane thickening in the basal lamina of the RPE in integrin α1 null mice suggests an imbalance in synthesis and/or turnover of basement membrane proteins. This basement membrane thickening results in an apparent loss of focal contacts between the basal processes of the RPE and the basement membrane. These morphological changes are progressive, becoming apparent when the animals approach 10 months of age, which coincides with the onset of our observed reduction in the rod number. This accumulation of electron dense material is reminiscent of basal laminar deposits (BLD) associated with the aging macula in humans and with certain mouse models.38,39 BLD, which are largely comprised of extracellular matrix39 accumulate between the RPE and a structurally identifiable basement membrane. In our mice the basement membrane is not identifiable separately from the thickened material, suggesting it may be distinct from BLD.
Synaptic malformations were observed at synaptic termini for both rods and cones, suggesting both photoreceptor cell types are influenced by α1β1 integrin signaling in the RPE. Recent studies on different RP animal models indicate that the missing of invaginating bipolar cell dendrites from photoreceptor terminals may be an early sign of photoreceptor death.28,29 Thus, both cones and rods show synaptic malformations in integrin α1-null mice, suggesting that degeneration involves both photoreceptor cell types.
Collectively, these data strongly suggest that basement membrane/integrin interactions at the RPE interface with the basal lamina of the RPE play an important role in regulating RPE cell function. Absence of α1β1 integrin/cell matrix interactions at this interface may influence RPE cell morphology, basement membrane homeostasis, and photoreceptor health, and culminates in photoreceptor degeneration. It should be noted that α1β1 integrin also localizes to the capillary endothelium, and thus some of the pathological changes in α1-null retinas may emanate from this compartment. We feel it is unlikely, however, since we observe no structural of ultrastructural abnormalities the capillaries of α1-null mice compared to wild type mice.
There are 18 integrin alpha subunits and 8 beta subunits that can heterodimerize in various combinations to produce 24 known integrin heterodimers.8 Integrins bind a broad spectrum of ligands with the common feature that the integrin binding site within the ligand generally contains the arginine-glycine-aspartic acid (RGD) sequence. The study of integrin function in the RPE has been focused largely on the role of αvβ5 integrin in phagocytosis of shed disks at the apical surface of the cells,4,40 a process that interestingly appears regulated through activation of focal adhesion kinase signaling.5
Early work on integrins at the basal aspect of RPE cells showed that β1 integrins promoted attachment of cultured cells to provisional matrices including the basal lamina of the RPE.10,41 While early studies aiming to define polarized expression of different integrins on RPE cells were inconclusive,42 more recently, a specific subset of basally localized integrins has been described that may function in RPE attachment to matrix.23 One study defined integrin α3 and α6 containing heterodimers bind to laminin 511,9 suggesting that these integrin/cell matrix may also be important for RPE cell function.
The potential role for α1β1 integrin in collagen matrix remodeling has been explored for a variety of tissues and cell types, including RPE cells. The experimental platform most widely employed involves culturing cells in a collagen gel and assessing the function of specific integrins in facilitating contraction of these gels, which is considered a measure of collagen matrix remodeling. Using this approach, integrin α1β1 was first implicated in fibroblast matrix remodeling using neutralizing antibodies against the α1 integrin subunit.34 These observations correlate well with later studies showing collagen I dysregulation, matrix accumulation, and MMP-13 up-regulation in the skin of integrin α1 null mice.43 Similarly, integrin α1β1 influenced collagen gel contraction in cultured mesangial cells from the renal glomerulus via activation of the MAPkinase signaling pathway.44,45 Recent in vivo data bear out these observations, showing that integrin α1β1 does influence MAPkinase-mediated regulation of MMP-2, 9, and 14, and that dysregulation of this pathway can influence progressive glomerular pathogenesis in various disease models.37
Interestingly, recent studies have shown that the collagen gel contraction of ARPE-19 cells is inhibited by neutralizing antibodies against α1 integrin,17 and that this effect is mediated through focal adhesion kinase, and downstream activation of MAPkinase.46 In light of these findings and the work discussed above, it is likely that α1β1 integrin in RPE cells influences basement membrane homeostasis, at least in part, by altering turnover of extracellular matrix via influences on MMP expression. These influences may account, at least in part, for the basement membrane thickening and dysmorphology of RPE basal processes observed in our study.
Light driven massive protein translocation is a prominent feature in photoreceptors. It was discovered more than twenty years ago and reconfirmed more recently.21,47–49 Its functional roles in photoreceptors, however, remain unclear and the mechanism of such massive protein translocation is being investigated.28,50 In healthy eyes, when light stimulation reaches certain threshold intensity,51 transducin is moved out of the outer segments to reduce the activation of the phototransduction pathway and arrestin is transported into the outer segments to inactivate rhodopsin.52 One of the functions of protein translocation, therefore, may be to prevent constant activation of saturated rods under strong light intensity.53 Diseases with defects in photoreceptor protein translocation show retinal degeneration.54–56 It is not known how aberrant protein translocation leads to photoreceptor degeneration. Defects in protein translocation may delay the inactivation of the pathway under light adaptation. Under these circumstances, long periods of high intensity light adaptation may induce degeneration of photoreceptors.54,57
The influence of the RPE on protein translocation in photoreceptors is unclear. RPE65 knock-out mice show defects in translocation of both transducin and arrestin,58 suggesting the RPE may have a dominant influence on the translocation mechanism. Current interpretation is that the absence of retinoid replacement in RPE65 knock-out retinas blocks activation of rhodopsin, which is critical for triggering protein translocation. Our results reported here suggest that the RPE may play more sophisticated roles in affecting photoreceptor protein translocation. One interpretation is that integrin α1β1 signaling regulates the velocity of transducin translocation in rods. Translocation defects in integrin α1β1-null mice can not be explained by absence of rhodopsin activation, since arrestin translocation is not affected. Our results, therefore, also suggest that translocation of arrestin and transducin may be regulated by different mechanisms.
In summary, here we provide the first example suggesting an essential role for integrin/matrix interaction at the basal aspect of the RPE in retinal health. Given the complexity of laminins in the basal lamina of the RPE and the presence of additional integrins at the basal aspect of the RPE, it appears likely that integrin adhesion and signaling at the basement membrane interface plays important roles in maintaining RPE architecture and function. While this is not surprising considering what we know from related studies in other tissues/organs, it underscores the need for further research aimed at understanding this niche in the RPE.
Acknowledgements
We thank John (Skip) Kennedy for help in figure preparation and Charlotte Lieser for secretarial help. This work was supported by R01 DC04844 and R01DK55000 to DC, by P20 RR018788 (COBRE) to Y-WP, and the tobacco settlement fund from the State of Nebraska.
Contributor Information
You-Wei Peng, Boys Town National Research Hospital, Omaha, NE.
Marisa Zallocchi, Boys Town National Research Hospital, Omaha, NE.
Daniel T. Meehan, Boys Town National Research Hospital, Omaha, NE
Duane Delimont, Boys Town National Research Hospital, Omaha, NE.
Bo Chang, The Jackson Laboratories, Bar Harbor, ME.
Norman Hawes, The Jackson Laboratories, Bar Harbor, ME.
Weimin Wang, Boys Town National Research Hospital, Omaha, NE.
Dominic Cosgrove, Boys Town National Research Hospital, Omaha, NE.
References
- 1.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 2.Edwards RB, Szamier RB. Defective phagocytosis of isolated rod outer segments by RCS rat retinal pigment epithelium in culture. Science. 1977;197:1001–1003. doi: 10.1126/science.560718. [DOI] [PubMed] [Google Scholar]
- 3.Gal A, Thompson DA, Weir J, Orth U, Jackobson SG, Apfelstedt-Sylla E, Vollrath D. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat Genet. 2003;26:270–271. doi: 10.1038/81555. [DOI] [PubMed] [Google Scholar]
- 4.Finnemann SC, Bonilha VL, Marmorstein AD, Rodriguez-Boulan E. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires alpha(v)beta5 integrin for binding but not for internalization. Proc Natl Acad Sci U S A. 1997;94:12932–12937. doi: 10.1073/pnas.94.24.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finnemann SC. Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J. 2003;22:4143–4154. doi: 10.1093/emboj/cdg416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finnemann SC, Silverstein RL. Differential roles of CD36 and alphavbeta5 integrin in photoreceptor phagocytosis by the retinal pigment epithelium. J Exp Med. 2001;194:1289–1298. doi: 10.1084/jem.194.9.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng W, Yasumura D, Matthes MT, LaVail MM, Vollrath D. Mertk triggers uptake of photoreceptor outer segments during phagocytosis by cultured retinal pigment epithelial cells. J Biol Chem. 2002;277:17016–17022. doi: 10.1074/jbc.M107876200. [DOI] [PubMed] [Google Scholar]
- 8.Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aisenbrey S, Zhang M, Bacher D, Yee J, Brunken WJ, Hunter DD. Retinal pigment epithelial cells synthesize laminins, including laminin 5, and adhere to them through alpha3- and alpha6-containing integrins. Invest Ophthalmol Vis Sci. 2006;47:5537–5544. doi: 10.1167/iovs.05-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin M, He S, Worpel V, Ryan SJ, Hinton DR. Promotion of adhesion and migration of RPE cells to provisional extracellular matrices by TNF-alpha. Invest Ophthalmol Vis Sci. 2000;41:4324–4332. [PubMed] [Google Scholar]
- 11.Pozzi A, Zent R. Integrins: sensors of extracellular matrix and modulators of cell function. Nephron Exp Nephrol. 2003;94:e77–e84. doi: 10.1159/000072025. [DOI] [PubMed] [Google Scholar]
- 12.Gardner H, Kreidberg J, Kotelianski V, Jaenisch R. Deletion of integrin alpha 1 by homologous recombination permits normal murine development but gives rise to a specific deficit in cell adhesion. Dev Biol. 1996;175:301–313. doi: 10.1006/dbio.1996.0116. [DOI] [PubMed] [Google Scholar]
- 13.Cosgrove D, Rodgers K, Meehan D, Miller C, Bovard K, Gilroy A, Gardner H, Kotelianski V, Gotwals P, Amatucci A, Kalluri R. Integrin alpha1beta1 and transforming growth factor-beta1 play distinct roles in alport glomerular pathogenesis and serve as dual targets for metabolic therapy. Am J Pathol. 2000;157:1649–1659. doi: 10.1016/s0002-9440(10)64802-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Fougerolles AR, Sprague AG, Nickerson-Nutter CL, Chi-Rosso G, Rennert PD, Gardner H, Gotwals PJ, Lobb RR, Koteliansky VE. Regulation of inflammation by collagen-binding integrins α1β1 and α1β1 in models of hypersensitivity and arthritis. J Clin Invest. 2000;105:721–729. doi: 10.1172/JCI7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krieglstein CF, Cerwinka WH, Sprague AG. Collagen-binding integrin alpha 1 beta 1 regulates intestinal inflammation in experimental colitis. J Clin Invest. 2002;110:773–1782. doi: 10.1172/JCI200215256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zent R, Yan X, Su Y, Hudson BG, Borza DB, Moeckel GW, Qi Z, Sado Y, Breyer MD, Voziyan P, Pozzi A. Gomerular injury is exacerbated in diabetic integrin alpha1-null mice. Kidney Int. 2006;70:460–470. doi: 10.1038/sj.ki.5000359. [DOI] [PubMed] [Google Scholar]
- 17.Bando H, Ikuno Y, Hori Y, Sayanagi K, Tano Y. Mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3 kinase (PI3K) pathways differently regulate retinal pigment epithelial cell-mediated collagen gel contraction. Exp Eye Res. 2006;82:529–537. doi: 10.1016/j.exer.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Hawes NL, Smith RS, Chang B, Davisson M, Heckenlively JR, John SW. Mouse fundus photography and angiography: a catalogue of normal and mutant phenotypes. Mol Vis. 1999;5:22. [PubMed] [Google Scholar]
- 19.Hu J, Bok D. Technical Brief: A cell culture medium that supports the differentiation of human retinal pigment epithelium into functionally polarized monolayers. Molecular Vision. 2000;7:14–19. [PubMed] [Google Scholar]
- 20.Luo Y, Zhuo Y, Fukuhara M, Rizzolo LJ. The effects of culture conditions on heterogeneity and the apical junctional complex of the ARPE-19 cell line. Invest Ophthalmol Vis Sci. 2006;47:3644–3655. doi: 10.1167/iovs.06-0166. [DOI] [PubMed] [Google Scholar]
- 21.Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, Govardovskii VI, Pugh EN, Jr, Arshavsky VY. Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron. 2002;34:95–106. doi: 10.1016/s0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- 22.Mendrick DL, Kelly DM, duMont SS, Sandstrom DJ. Glomerular epithelial and mesangial cells differentially modulate the binding specificities of VLA-1 and VLA-2. Lab Invest. 1995;72:367–375. [PubMed] [Google Scholar]
- 23.Zarbin MA. Analysis of retinal pigment epithelium integrin expression and adhesion to aged submacular human Bruch's membrane. Trans Am Ophthalmol Soc. 2003;101:499–520. [PMC free article] [PubMed] [Google Scholar]
- 24.Kedzierski W, Lloyd M, Birch DG, Bok D, Travis GH. Generation and analysis of transgenic mice expressing P216L-substituted rds/peripherin in rod photoreceptors. Invest Ophthalmol Vis Sci. 1997;38:498–509. [PubMed] [Google Scholar]
- 25.Machida S, Kondo M, Jamison JA, et al. P23H rhodopsin transgenic rat: correlation of retinal function with histopathology. Invest Ophthalmol Vis Sci. 2000;41:3200–3209. [PubMed] [Google Scholar]
- 26.Jansen HG, Sanyal S. Development and degeneration of retina in rds mutant mice: electron microscopy. J Comp Neurol. 1984;224:71–84. doi: 10.1002/cne.902240107. [DOI] [PubMed] [Google Scholar]
- 27.Libby RT, Lavallee CR, Balkema GW, Brunken WJ, Hunter DD. Disruption of laminin beta2 chain production causes alterations in morphology and function in the CNS. J Neurosci. 1999;19:9399–9411. doi: 10.1523/JNEUROSCI.19-21-09399.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng YW, Hao Y, Petters RM, Wong F. Ectopic synaptogenesis in the mammalian retina caused by rod photoreceptor-specific mutations. Nat Neurosci. 2000;3:1121–1127. doi: 10.1038/80639. [DOI] [PubMed] [Google Scholar]
- 29.Peng YW, Senda T, Hao Y, Matsuno K, Wong F. Ectopic synaptogenesis during retinal degeneration in the royal college of surgeons rat. Neuroscience. 2003;119:813–820. doi: 10.1016/s0306-4522(03)00153-2. [DOI] [PubMed] [Google Scholar]
- 30.Calvert PD, Strissel KJ, Schiesser WE, Pugh EN, Jr, Archavsky VY. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol. 2006:560–568. doi: 10.1016/j.tcb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122:449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berrier AL, Yamada KM. Cell-matrix adhesion. J Cell Physiol. 2007;213:565–573. doi: 10.1002/jcp.21237. [DOI] [PubMed] [Google Scholar]
- 33.Nykvist P, Hongmin T, Ivaska J, Kapayla J, Pihlajaniemi T, Heino J. Distinct recognition of collagen subtypes by a1b1 and a2b1 integrins: α1β1 mediates cell adhesion to type XIII collagen. J. Biol. Chem. 2000;275:8255–8261. doi: 10.1074/jbc.275.11.8255. [DOI] [PubMed] [Google Scholar]
- 34.Langholz O, Rockel D, Mauch C, et al. Collagen and collagenase gene expression in three-dimensional collagen lattices are differentially regulated by alpha 1 beta 1 and alpha 2 beta 1 integrins. J Cell Biol. 1995;131:1903–1915. doi: 10.1083/jcb.131.6.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pozzi A, Moberg PE, Miles LA, Wagner S, Soloway P, Gardner HA. Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc Natl Acad Sci U S A. 2000;97:2202–2207. doi: 10.1073/pnas.040378497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang C, Zeisberg M, Lively JC, Nyberg P, Afdhal N, Kalluri R. Integrin alpha1beta1 and alpha2beta1 are the key regulators of hepatocarcinoma cell invasion across the fibrotic matrix microenvironment. Cancer Res. 2003;63:8312–8317. [PubMed] [Google Scholar]
- 37.Cosgrove D, Meehan DT, Delimont D, et al. Integrin {alpha}1{beta}1 Regulates Matrix metalloproteinases via P38 mitogen-activated protein kinase in mesangial cells: Implications for Alport syndrome. Am J Pathol. 2008;172:761–773. doi: 10.2353/ajpath.2008.070473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Espinosa-Heidmann DG, Sall J, Hernandez EP, Cousins SW. Basal laminar deposit formation in APO B100 transgenic mice: complex interactyions between dietary fat, blue light, and vitamin E. Invest Ophthalmol Vis Sci. 2004;45:260266. doi: 10.1167/iovs.03-0910. [DOI] [PubMed] [Google Scholar]
- 39.Van der Schaft TL, Mooy CM, deBruijn WC, Bosman FT, deJong PT. Immunohistochemical light and electron microscopy of basal laminar deposit. Graefes Arch Clin Exp Ophthalmol. 1994;232:40–46. doi: 10.1007/BF00176436. [DOI] [PubMed] [Google Scholar]
- 40.Nandrot EF, Kim Y, Brodie SE, Huang X, Sheppard D, Finnemann SC. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alphavbeta5 integrin. J Exp Med. 2004;200:1539–1545. doi: 10.1084/jem.20041447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho TC, Del Priore LV. Reattachment of cultured human retinal pigment epithelium to extracellular matrix and human Bruch's membrane. Invest Ophthalmol Vis Sci. 1997;38:1110–1118. [PubMed] [Google Scholar]
- 42.Brem RB, Robbins SG, Wilson DJ, et al. Immunolocalization of integrins in the human retina. Invest Ophthalmol Vis Sci. 1994;35:3466–3474. [PubMed] [Google Scholar]
- 43.Gardner H, Broberg A, Pozzi A, Laato M, Heino J. Absence of integrin alpha1beta1 in the mouse causes loss of feedback regulation of collagen synthesis in normal and wounded dermis. J Cell Sci. 1999;112:263–272. doi: 10.1242/jcs.112.3.263. [DOI] [PubMed] [Google Scholar]
- 44.Kagami S, Kondo S, Loster K, et al. Alpha1beta1 integrin-mediated collagen matrix remodeling by rat mesangial cells is differentially regulated by transforming growth factor-beta and platelet-derived growth factor-BB. J Am Soc Nephrol. 1999;10:779–789. doi: 10.1681/ASN.V104779. [DOI] [PubMed] [Google Scholar]
- 45.Kagami S, Urushihara M, Kitamura A, et al. PDGF-BB enhances alpha1beta1 integrin-mediated activation of the ERK/AP-1 pathway involved in collagen matrix remodeling by rat mesangial cells. J Cell Physiol. 2004;198:470–478. doi: 10.1002/jcp.10433. [DOI] [PubMed] [Google Scholar]
- 46.Morales SA, Mareninov S, Prasad P, Wadehra M, Braun J, Gordon LK. Collagen gel contraction by ARPE-19 cells is mediated by a FAK-Src dependent pathway. Exp Eye Res. 2007;85:790–798. doi: 10.1016/j.exer.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 47.Brann MR, Cohen LV. Diurnal expression of transducin mRNA and translocation of transducin in rods of rat retina. Science. 1987;235:585–587. doi: 10.1126/science.3101175. [DOI] [PubMed] [Google Scholar]
- 48.Philp NJ, Chang W, Long K. Light-stimulated protein movement in rod photoreceptor cells of the rat retina. FEBS Lett. 1987;225:127–132. doi: 10.1016/0014-5793(87)81144-4. [DOI] [PubMed] [Google Scholar]
- 49.Whelan JP, McGinnis JF. Light-dependent subcellular movement of photoreceptor proteins. J. Neurosci. Res. 1988;20:263–270. doi: 10.1002/jnr.490200216. [DOI] [PubMed] [Google Scholar]
- 50.Kalra D, Elsaesser R, Gu Y, Venkatachalam K. Transducin in rod photoreceptors: translocated when not terminated. J Neurosci. 2007;27:6349–6351. doi: 10.1523/JNEUROSCI.1399-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, Govardovskii VI, Pugh EN, Jr, Arshavsky VY. Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron. 2002;34:95–106. doi: 10.1016/s0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- 52.Calvert PD, Strissel KJ, Schiesser WE, Pugh EN, Arshavsky VY. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol. 2006;11:560–568. doi: 10.1016/j.tcb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Kalra D, Elsaesser R, Gu Y, Venkatachalam K. Transducin in rod photoreceptors: translocated when not terminated. J Neurosci. 2007;27:6349–6351. doi: 10.1523/JNEUROSCI.1399-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kong L, Feng L, Soleman CE, Sheng L, Rajesh VE, Xiaohong Z, Lewis DA, McGinnis JF, Cao W. Bright cyclic light accelerates photoreceptor cell degeneration in tubby mice. Neurobiol. Dis. 2006;21:468–477. doi: 10.1016/j.nbd.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 55.Mendez A, Lem J, Simon M, Chen J. Light-dependent translocation of arrestin in the absence of rhodopsin phosphorylation and transducin signaling. J Neurosci. 2003;23:3124–3129. doi: 10.1523/JNEUROSCI.23-08-03124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abd-El-Barr MM, Sykoudis K, Andrabi S, Eichers ER, Pennesi ME, Tan PL, Wilson JH, Katsanis N, Lupski JR, Wu SM. Impaired photoreceptor protein transport and synaptic transmission in a mouse model of Bardet-Biedl syndrome. Vision Res. 2007;47:3394–3407. doi: 10.1016/j.visres.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen J, Simon MI, Matthes MT, Yasumura D, LaVail MM. Increased Susceptibility to Light Damage in an Arrestin Knockout Mouse Model of Oguchi Disease (Stationary Night Blindness) Invest Ophthalmol Vis Sci. 1999;40:2978–2982. [PubMed] [Google Scholar]
- 58.Mendez A, Lem J, Simon M, Chen J. Light-dependent translocation of arrestin in the absence of rhodopsin phosphorylation and transducin signaling. J Neurosci. 2003;23:3124–3129. doi: 10.1523/JNEUROSCI.23-08-03124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


