Abstract
Normal immune responses stimulated by pathogenic and environmental antigens generate memory T cells that react with donor antigens and no currently used immunosuppressive drug completely inhibits memory T cell function. While donor-reactive memory T cells clearly compromise graft outcomes, mechanisms utilized by memory T cells to promote rejection are largely unknown. In the current study we investigated how early endogenous memory cells infiltrate and express effector function in cardiac allografts. Endogenous CD8 memory T cells in non-sensitized recipients distinguish syngeneic vs. allogeneic cardiac allografts within 24 hours of reperfusion. CD8-dependent production of IFN-γ and CXCL9/Mig was observed 24–72 hours post-transplant in allografts but not isografts. CXCL9 was produced by donor cells in response to IFN-γ made by recipient CD8 T cells reactive to donor class I MHC molecules. Activated CD8 T cells were detected in allografts at least three days before donor-specific effector T cells producing IFN-γ were detected in the recipient spleen. Early inflammation mediated by donor-reactive CD8 memory T cells greatly enhanced primed effector T cell infiltration into allografts. These results suggest that strategies for optimal inhibition of alloimmunity should include neutralization of infiltrating CD8 memory T cells within a very narrow window after transplantation.
Keywords: transplantation, T-cell immunity, memory CD8 T cells, allorecognition, trafficking, acute allograft rejection
Introduction
The therapeutic advantages of organ transplantation to treat end-stage organ disease are undermined by robust T cell reactivity to allogeneic MHC molecules (1, 2). Immunosuppressive drugs currently used to inhibit T cell activation are nephrotoxic and leave patients highly susceptible to infection and malignancy (3, 4). Importantly, current immunosuppression fails to completely abrogate acute rejection episodes as well as the development of transplant associated vasculopathy, so-called chronic rejection (5). Among T cell populations contributing to alloimmunity, memory T cells are now recognized as a formidable barrier to successful transplantation because currently used immunosuppressive drugs do not effectively neutralize these cells. This failure is explained by the unique phenotypic and functional properties of memory T cells including specialized trafficking patterns, reduced and alternative costimulatory requirements, and rapid expression of effector function (6, 7).
Memory T cells with reactivity to donor MHC molecules are present at varying frequencies in all transplant recipients. While direct exposures to non-self MHC molecules via blood transfusion, pregnancy, and prior transplantation are clearly sensitizing events (8), far more subtle mechanisms account for nearly universal allo-sensitization. Cross-reactivity between viral and allogeneic class I MHC molecules is well documented in rodents and humans (9–12). Termed ‘heterologous immunity,’ this phenomenon of T cell promiscuity enhances protective immunity against pathogens but is a major obstacle preventing successful transplantation (13, 14). Further, the generation of alloreactive memory T cells is not confined to pathogen-associated antigen exposure. Lymphopenia-driven homeostatic proliferation generates memory-phenotype T cells that compromise peritransplant T-cell depletion and tolerance induction strategies (15–18). Clinical data suggest that there is predictive value in quantifying alloreactive T cell memory pretransplant. The presence of high numbers of donor reactive memory T cells detected in peripheral blood pre-transplant correlates with increased risk of rejection episodes and poor post-transplant graft function in renal transplant recipients (19, 20).
CD8 memory T cells are of particular interest in transplantation because of the abundant expression of class I MHC molecules on the allograft vasculature and parenchymal cells. Several therapeutic regimens utilizing co-stimulatory blockade have successfully induced long-term allograft survival or tolerance in non-sensitized mice, but these strategies fail when donor-specific CD8 memory T cells are present pre-transplant (21, 22). Approaches to induce long-term allograft survival and/or tolerance often succeed in rodents but then prove far more difficult in larger animals which, relative to laboratory mice, have longer and more varied exposures to environmental antigens (14). Recently, efforts to induce mixed chimerism and allograft tolerance were applied in a preclinical model where donor bone marrow transplantation was delayed until four months after kidney transplantation in cynomolgus monkeys (23). This strategy was undermined by CD8 memory T cells that survived anti-thymocyte globulin conditioning and prevented long-term allograft survival unless depleting anti-CD8 antibody was administered at the time of bone marrow transplantation.
Although it is well documented that anti-donor CD8 memory T cell reactivity promotes graft rejection, when and where these memory T cells interact with donor antigens, as well as the mechanisms used by these CD8 memory T cells to promote rejection, are entirely unknown. While studying responses to murine cardiac iso- and allografts in naïve recipients, we observed CD8-dependent inflammatory events within 48 hours of transplantation (24, 25). Here, we have tested the hypothesis that donor-reactive CD8 memory T cells are present in naïve mice and can infiltrate allografts rapidly post-transplant and be activated to express effector function. We show that graft-infiltrating CD8 memory T cells produce IFN-γ as early as 24 hours post-transplant and create an inflammatory environment that optimizes the downstream recruitment of primed effector T cells. Our results demonstrate that inflammation induced by endogenous CD8 memory T cells within hours of reperfusion promotes allograft rejection.
Methods
Mice
The following mice were used: C57BL/6 (H-2b) and A/J (H-2a) mice (Charles River Laboratories, Wilmington, MA); B6.CD90.1, B6.IFNγ−/−, BALB/C (H-2d), DBA/1 (H-2q), and bq1 (H-2bq) mice (The Jackson Laboratory, Bar Harbor, ME); C3H (H-2k) mice (Taconic Farms, Germantown, NY); and, B6.CD4−/−, B6.CD8−/−, B6.Rag1−/−, B6.CXCL9−/−, B6.IFNγR−/−, A/J.CXCL9−/− and 2C TCR transgenic mice are maintained at our facility. B6.IFNγ−/− and 2C mice were interbred to generate B6.IFNγ−/− 2C TCR-transgenic mice. H-2(bxq) F1 mice were generated by crossing C57BL/10 and DBA/1, and these F1 recipients (Kb/q Db/q Lq I-Ab b/q I-Aa b/q I-Ebb I-Ea−) received class II disparate bq1 grafts (Kb Dq Lq I-Abk I-Aak I-Ebk I-Eak) or fully mismatched C3H grafts. All knockout and transgenic strains had been backcrossed onto the indicated backgrounds for ≥ 10 generations. In all experiments 8–12 week old male mice were used. All animal procedures were approved by the Cleveland Clinic Institutional Animal Care and Use Committee.
Cardiac transplantation and harvest
Heterotopic intra-abdominal cardiac transplantation was performed following the method of Corry and coworkers (26). Total operative times averaged 45 minutes and hearts resumed spontaneous contraction immediately upon reperfusion. At harvest the circulatory system was drained prior to removal of the heterotopic graft, which was immediately snap-frozen in liquid nitrogen or placed in media for digestion and purification of graft-infiltrating cells.
In-vivo antibody treatments
Allograft recipients were treated with control Ig (Sigma, St. Louis MO) or anti-CD40L mAb, MR1 (Bio Express, West Lebanon, NH) (0.4 mg i.p. on days 0, +1), CTLA4-Ig (purified in-house from hybridoma supernatants) (0.35 mg i.p. on days 0, +1, +2), or anti-IFN-γ mAb, XMG1.2 (Bio Express) (0.2 mg i.p. 8 hours before and after transplantation). CD8 T cell depletion was accomplished using a 1:1 cocktail of YTS169 and TIB105 mAbs (Bio Express) (0.2 mg given i.p. on days −3, −2, −1, +1 post-transplant). CD8 T cell depletion was ≥ 98% in peripheral blood.
Skin transplantation and adoptive transfer
Full-thickness donor trunk skin was transplanted onto the posteriolateral back. 8–10 weeks later splenic cell suspensions were passed through CD3 negative selection columns (R&D Systems, Minneapolis MN), stained, and flow sorted to greater than 98% purity using a FACSAria (BD Biosciences, San Jose CA). Purified cell populations were adoptively transferred to syngeneic recipients by tail vein injection.
RNA purification and qRT-PCR
Snap-frozen grafts were crushed, homogenized, and RNA was isolated using fibrous tissue kits (Qiagen, Valencia CA). Reverse transcription and real-time PCR were performed using commercially available reagents, probes, and a 7500 Fast Real-Time thermocycler, all from Applied Biosystems (Foster City, CA).
Protein purification and ELISA
Snap-frozen grafts were crushed and combined with 1 mL of 1.5% Triton-X in PBS and 0.5 mL of a proteinase inhibitor cocktail (Sigma, St. Louis MO). Following 30 minutes of incubation with shaking at 4°C and centrifugation at 12,000 × g for 10 minutes, total protein concentrations were measured in the resulting supernatants using the bicinchoninic acid assay (Pierce, Rockford IL). Total protein concentrations were equalized and samples were plated in duplicate on ELISA assay plates from R&D Systems (Minneapolis MN).
IFN-γ ELISPOT
Splenic responder cells and irradiated (3000 rad) self, donor, and third-party stimulator cell populations were co-cultured for 24 hours at 37°C in serum-free HL-1 media in 96-well plates coated with anti-IFN-γ capture Ab (R4-6A2, BD Biosciences, San Jose CA). After all cells were washed from the plate, biotinylated anti-IFN-γ detecting Ab (XMG1.2, BD Biosciences) was added, followed by anti-biotin alkaline phosphatase. Following substrate addition, the total number of spots per well was quantified using an ImmunoSpot Series 2 Analyzer (Cellular Technology Ltd., Shaker Heights OH) and compared to media and Concavalin A stimulated control cultures.
Flow cytometry
Flow cytometric detection of graft-infiltrating cells was performed using a modification of the method published by Afanasyev and colleagues (27). Briefly, harvested tissues were weighed prior to one hour incubation at 37°C in RPMI with Type II collagenase (Sigma, St. Louis MO) but without the addition of proteases. After incubation, samples were passed through 40 micron filters, washed twice in RPMI, counted using a hemocytometer, and stained for common phenotypic surface markers using commercially available antibodies (BD Bioscience, San Jose CA; eBioscience, San Diego CA).
Statistics
Statistical analyses were performed using Kruskal-Wallis and Mann-Whitney U testing. Error bars reflect SEM throughout.
Results
Naïve recipients distinguish allografts from isografts within the first 24 hours post-transplant
In order to determine the time point at which cardiac iso- and allografts are distinguished by recipients, intragraft mRNA levels of IFN-γ and the IFN-γ inducible T cell chemoattractant CXCL9 were compared in fully MHC mismatched A/J allografts or syngeneic grafts in C57BL/6 recipients at 24, 48, and 72 hours post-transplant (Figure 1A). IFN-γ mRNA levels in allografts were increased 3-fold relative to isografts as early as 24 hours post-transplant, and this difference increased thereafter. Coincident with IFN-γ, CXCL9 mRNA was also selectively elevated in allograft tissues. No significant differences were observed between isograft and allograft levels of the neutrophil chemoattractant CXCL1.
Figure 1.
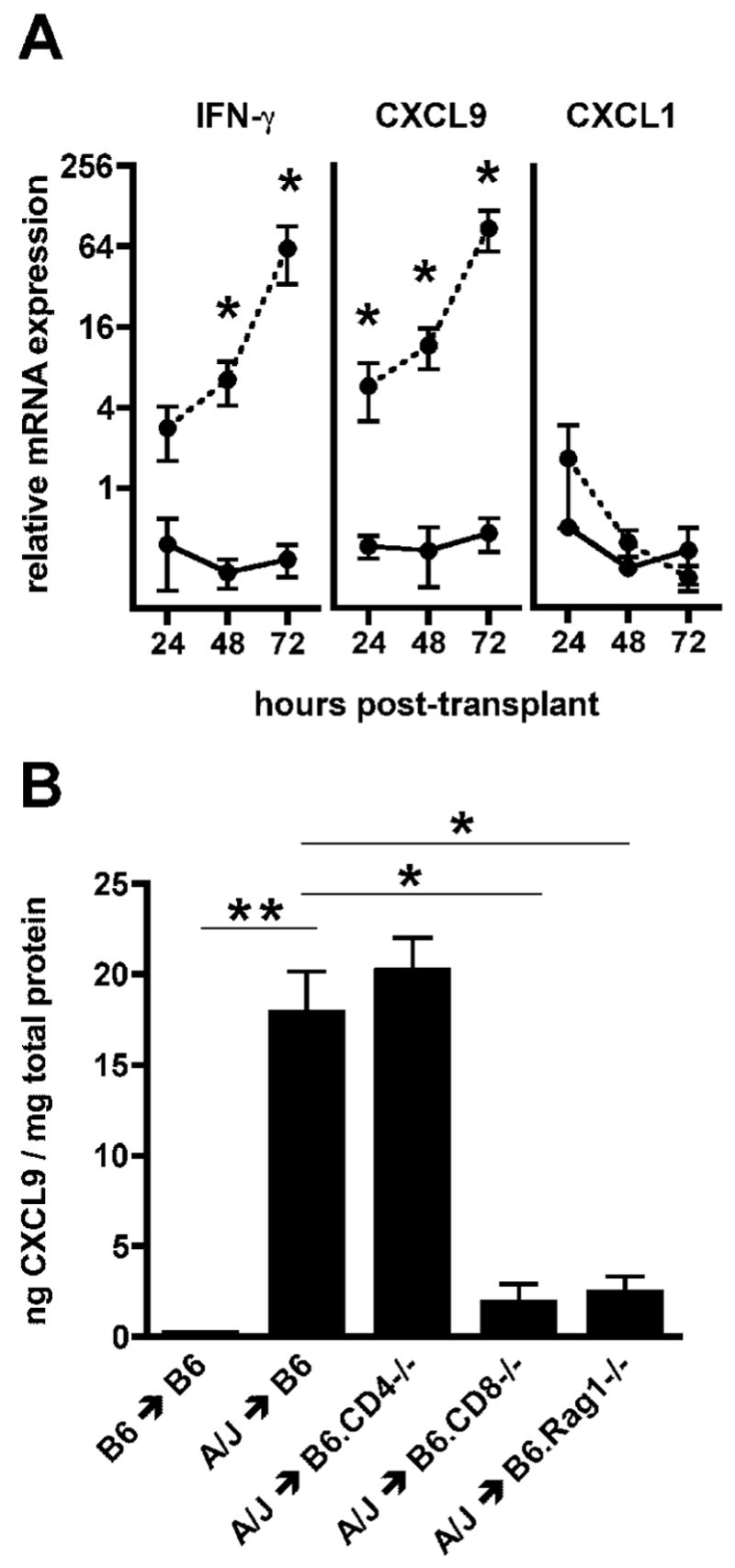
Early induction of IFN-γ and CXCL9 in allografts is CD8 T cell dependent. A. C57BL/6 mice received syngeneic or complete MHC mismatched A/J cardiac allografts. Groups of 4 iso- (—) and allografts ( - - ) were harvested 24, 48, and 72 hours post-transplant, RNA was isolated from total graft homogenates, and relative real-time PCR was used to measure cytokine and chemokine expression. Data are normalized relative to isograft expression levels at 24 hours post-transplant. (
 = p ≤ 0.05) B. Wild-type C57BL/6, B6.CD4−/−, B6.CD8−/− or B6.Rag1−/− mice received syngeneic or A/J cardiac allografts. Groups of 5 grafts were harvested 72 hours post-transplant and intragraft levels of CXCL9 were measured by ELISA. (
= p ≤ 0.05) B. Wild-type C57BL/6, B6.CD4−/−, B6.CD8−/− or B6.Rag1−/− mice received syngeneic or A/J cardiac allografts. Groups of 5 grafts were harvested 72 hours post-transplant and intragraft levels of CXCL9 were measured by ELISA. (
 = p ≤ 0.0001,
= p ≤ 0.0001,
 = p ≤ 0.002)
= p ≤ 0.002)
To begin to identify a source of the IFN-γ inducing CXCL9 transcription, CXCL9 protein was measured in iso- and allografts from wild-type recipients and CD4−/−, CD8−/− and T cell/B cell deficient Rag1−/− allograft recipients 72 hours post-transplant (Figure 1B). CXCL9 was produced at high levels in grafts from wild-type allogeneic recipients and was virtually undetectable in isografts. CXCL9 was reduced to near background levels in allografts from CD8−/− and Rag1−/− recipients, whereas allografts from CD4−/− recipients showed no decrease relative to wild-type allograft recipients, suggesting a role for CD8 but not CD4 T cells in CXCL9 induction. Hereafter, measurement of CXCL9 protein in cardiac grafts at 72 hours post-transplant was used as a functional indicator to monitor CD8 T cell dependent IFN-γ production.
Allograft cells produce CXCL9 in response to IFN-γ made by donor-reactive CD8 T cells
To test alloantigen specificity in this early response and to determine if CD8 T cells are the critical source of IFN-γ stimulating CXCL9 production, T cell receptor transgenic 2C (reactive to Ld) or IFN-γ−/− 2C CD8 T cells were adoptively transferred into Rag1−/− mice and these animals then received Ld-expressing BALB/C or third-party (non-Ld-expressing) DBA/1 heart allografts (Figure 2A). Allografts from BALB/C but not third party DBA/1 donors contained high levels of CXCL9 72 hours after transplantation. CXCL9 was undetectable in BALB/C grafts transplanted into Rag1−/− mice reconstituted with IFN-γ−/− 2C CD8 T cells, although these CD8 T cells did infiltrate the allografts (data not shown).
Figure 2.

Early CXCL9 production requires IFN-γ produced by class I MHC alloreactive CD8 T cells. A. Ld-reactive 2C TCR-transgenic CD8 cells or IFN-γ−/− 2C cells were adoptively transferred into Rag1−/− mice. After 10 weeks BALB/C (Ld-expressing) or DBA/1 (Ld null) hearts were transplanted into groups (n = 4) of reconstituted Rag1−/− recipients and graft CXCL9 proteins levels were measured by ELISA 72 hours post-transplant. (
 = p ≤ 0.05 vs. BALB/C ≤ Rag1−/− + 2C) B. (C57BL/10xDBA/1)F1 mice received syngeneic, class II MHC-disparate bq1, or complete MHC-disparate C3H cardiac allografts. Protein levels of CXCL9 were measured in groups of 5 grafts harvested 72 hours after transplantation. (
= p ≤ 0.05 vs. BALB/C ≤ Rag1−/− + 2C) B. (C57BL/10xDBA/1)F1 mice received syngeneic, class II MHC-disparate bq1, or complete MHC-disparate C3H cardiac allografts. Protein levels of CXCL9 were measured in groups of 5 grafts harvested 72 hours after transplantation. (
 = p ≤ 0.005 vs. complete MHC disparity)
= p ≤ 0.005 vs. complete MHC disparity)
To confirm that donor class I MHC is the critical allogeneic determinant in this response, CXCL9 protein levels were quantified in class I MHC syngeneic/class II MHC-disparate allografts (Figure 2B). (C57BL/10xDBA/1)F1 mice (Kb/q Db/q Lq I-Abb/q I-Aab/q I-Ebb I-Ea−) received fully allogeneic C3H (H-2k) cardiac allografts or class II MHC-disparate bq1 (Kb Dq Lq I-Abk I-Aak I-Ebk I-Eak) allografts. Grafts mismatched for only class II MHC molecules expressed nearly isograft levels of CXCL9 whereas complete MHC-disparate grafts developed high expression of CXCL9 in these F1 recipients similar to that observed in the A/J to C57BL/6 strain combination.
To test whether donor-derived graft cells or recipient graft-infiltrating cells produce CXCL9 in response to CD8-derived IFN-γ, A/J.CXCL9−/− grafts were transplanted into wild-type C57BL/6 recipients, and wild-type A/J grafts were transplanted into B6.CXCL9−/− recipients and B6.IFN-γR−/− recipients (Figure 3). CXCL9 levels were significantly reduced when allograft cells were unable to produce CXCL9, and unaffected in recipients that cannot produce CXCL9. Allografts from recipient mice unable to respond to IFN-γ contained the highest levels of CXCL9, confirming that intragraft CXCL9 is primarily of donor-origin and suggesting that CXCL9 production is both induced and limited by IFN-γ signaling.
Figure 3.

Donor cells are the major source of CXCL9 produced in the graft early post-transplant. Wild-type, B6.CXCL9−/−, or B6.IFN-γR−/− mice received wild-type syngeneic, wild-type A/J, or A/J.CXCL9−/− cardiac allografts. Protein levels of CXCL9 were measured in groups of 5 grafts harvested 72 hours after transplantation. (
 = p ≤ 0.01)
= p ≤ 0.01)
CD8 T cells infiltrate allografts prior to the priming of donor-specific IFN-γ producing T cells in the spleen
To directly test the infiltration of CD8 T cells into allografts early post-transplant, A/J allografts were harvested from C57BL/6 recipients 24, 48 and 72 hours post-transplant and analyzed by flow cytometry (Figure 4A). A population of CD8 T cells was clearly identified within allografts as early as 24 hours post-transplant and this T cell infiltration continued to increase thereafter. A small population of CD8 T cells was also observed infiltrating cardiac isografts at 24 hours post-transplant (data not shown) but because this population did not increase thereafter or correlate with CD8-dependent CXCL9 production the reactivity of these cells was not investigated further.
Figure 4.

Activated CD8 T cells infiltrate allografts before donor-specific T cell priming is detected in the spleen. A. Flow cytometry was used to detect C57BL/6 CD8 T cells infiltrating A/J cardiac allografts 24, 48, and 72 hours post-transplant. Representative data from 3 independent experiments are shown. B. Cardiac grafts and spleens were removed from C57BL/6 recipients of A/J allografts 48 hours after transplantation. Equal numbers of splenic and graft-infiltrating CD8 cells were isolated by flow sorting and stained for further analysis. Representative data are shown. C. Spleens were harvested from non-transplanted mice or C57BL/6 recipients of A/J cardiac allografts on days 1–5 post-transplant. Splenocytes were mixed with A/J (■) or third party DBA/1 (□) stimulator cells in 24-hour IFN-γ ELISPOT assays. (n = 3/group,
 = p ≤ 0.05,
= p ≤ 0.05,
 = p ≤ 0.005)
= p ≤ 0.005)
At 48 hours post-transplant, graft-infiltrating CD8 T cells were purified and compared with an equal number of CD8+ splenocytes taken from the same animals (Figure 4B). Relative to their splenic counterparts, graft infiltrating CD8 T cells expressed cell surface markers associated with an activated phenotype including increased CD44, reduced CD62L, and downregulated IL-7 receptor. Allograft-infiltrating CD8+ cells were CCR7-negative and distinct from the splenic IL-15Rhi population which most likely represents CD8+ NKT cells (28).
In all preceding experiments, donor-specific alloimmunity in the graft appeared faster than a donor-stimulated primary immune response is expected to develop from naïve precursors. To directly test whether early CXCL9 production is induced by alloreactive CD8 T cells that undergo priming in the spleen, IFN-γ ELISPOT assays were used to detect the presence of donor-specific priming during days 1–5 post-transplant (Figure 4C). Donor-specific T cells producing IFN-γ were not evident until the fourth or fifth post-transplant day in the spleen, indicating that the CD8 T cells infiltrating cardiac allografts within 24 hours of reperfusion express effector function without activation in the spleen.
Early intragraft CXCL9 production is restored in CD8−/− recipients by transferred donor-specific CD44hi memory CD8 T cells
To test the capacity of different CD8 populations to induce early CXCL9 production in cardiac allografts, an adoptive transfer model was used. Six weeks after wild-type C57BL/6 mice rejected A/J skin grafts, CD44lo and CD44hi CD8 cells were purified by cell sorting from the spleens of these sensitized mice and adoptively transferred into non-sensitized CD8−/− mice that received A/J or third-party DBA/1 cardiac allografts (Figure 5A and 5B). CD44hi but not CD44lo cells infiltrated A/J allografts 72 hours post-transplant. Infiltration of these CD8 memory T cells into third party DBA/1 allografts was markedly decreased. Primed CD8 effector T cells purified from the spleens of wild-type C57BL/6 recipients of A/J cardiac allografts seven days after transplantation were also capable of infiltrating A/J cardiac allografts in CD8−/− recipients. More CD8 memory T cells trafficked to the A/J allografts than CD8 effector T cells, but the efficiency of transfer may have differed between these two populations as suggested by the numbers of CD8 T cells detected in the CD8−/− recipient spleens. Cellular infiltration into cardiac allografts and reactivity to the allograft correlated with intragraft expression levels of IFN-γ and CXCL9 mRNA (Figure 5C). When equal numbers of central (CD62Lhi) and effector (CD62Llo) memory T cells were sorted and transferred, effector memory CD8 T cells infiltrated the allografts more efficiently than central memory cells (Figure 5D).
Figure 5.

Adoptive transfer of CD8+CD44hi memory cells restores early CXCL9 production in CD8−/− allograft recipients. A/J skin grafts were placed on C57BL/6 mice and 8–10 weeks later naïve (CD8+CD44lo) and memory (CD8+CD44hi) cell populations were flow-sort purified from spleens and 5 × 106 cells were adoptively transferred to B6.CD8−/− mice which then received either A/J or DBA/1 cardiac allografts. For adoptive transfer of primed T cells, CD8 T cells were isolated from spleens of C57BL/6 mice actively rejecting A/J cardiac allografts at day 7 post-transplant. A. Flow cytometric detection of adoptively transferred CD8 T cells infiltrating allografts in CD8−/− recipients 72 hours post-transplant. Representative data are shown. B. Quantification of the adoptively transferred CD8 T cell populations within spleens and allografts 72 hours post-transplant (n = 4/group,
 = p ≤ 0.05). C. Quantification of IFN-γ and CXCL9 mRNA within allografts 72 hours post-transplant and normalized to expression levels in the no-transfer control group (n = 4/group,
= p ≤ 0.05). C. Quantification of IFN-γ and CXCL9 mRNA within allografts 72 hours post-transplant and normalized to expression levels in the no-transfer control group (n = 4/group,
 = p ≤ 0.05). D. Using the experimental design described above, 2 × 105 central memory (CD8+CD44hiCD62Lhi) or effector memory (CD8+CD44hiCD62Llo) cells were flow-sort purified and adoptively transferred into CD8−/− recipients of A/J cardiac allografts. Quantification of graft-infiltration by the adoptively transferred CD8 T cells 72 hours after transplantation is shown (n = 4/group).
= p ≤ 0.05). D. Using the experimental design described above, 2 × 105 central memory (CD8+CD44hiCD62Lhi) or effector memory (CD8+CD44hiCD62Llo) cells were flow-sort purified and adoptively transferred into CD8−/− recipients of A/J cardiac allografts. Quantification of graft-infiltration by the adoptively transferred CD8 T cells 72 hours after transplantation is shown (n = 4/group).
Co-stimulatory blockade fails to reduce early intragraft CXCL9 production
Because memory T cells have decreased costimulatory requirements for activation and often utilize alternative costimulatory molecules (21, 22, 29), the ability of anti-CD40L and CTLA4-Ig to inhibit early CXCL9 production was tested as an indicator of early CD8 T cell mediated IFN-γ production (Figure 6). Neither reagent blocked early CXCL9 production. In contrast, anti-IFN-γ antibody effectively reduced early CXCL9 production in the cardiac allografts.
Figure 6.

Early CD8 T cell alloreactivity is not inhibited by α-CD40L mAb or CTLA4-Ig. C57BL/6 recipients of A/J cardiac allografts were treated with control Ig or α-CD40L mAb (0.4 mg on day 0, +1), CTLA4-Ig (0.35 mg on day 0, +1, +2), or α-IFN-γ (0.2 mg 8 hours before and after transplantation). Protein levels of CXCL9 were measured in grafts harvested 72 hours after transplantation (n ≥ 3 group and the experiment was performed twice with identical results,
 = p ≤ 0.05 vs. IgG).
= p ≤ 0.05 vs. IgG).
Early CD8 memory T cell responses promote subsequent infiltration of primed effector T cells
During the normal course of acute cardiac allograft rejection, naive T cells undergo priming in the spleen and subsequently migrate to the allograft and express effector functions that mediate rejection. To test the hypothesis that early CD8 memory T cell mediated graft inflammation promotes recruitment of primed effector T cells to the graft following effector T cell priming in the spleen, the endogenous CD8 memory T cell response in groups of wild-type recipients was preserved or eliminated by treatment with control Ig or depleting anti-CD8 antibodies prior to transplantation of A/J cardiac allografts. At day 2 post-transplant, CD90.1 congenic CD4 effector T cells primed against A/J alloantigens were adoptively transferred and their ability to infiltrate allografts in control and CD8 T cell depleted recipients was compared after 48 hours. A greater than 50% reduction was observed in the percentage (Figure 7A) and total number (Figure 7B) of primed CD90.1 CD4 T cells infiltrating allografts in the absence of an early endogenous CD8 memory T cell response. Associated with this reduced infiltration of transferred effector CD4 T cells, intragraft IFN-γ and CXCL9 levels were decreased by CD8 T cell depletion and reduced endogenous T cell infiltration (Figure 7C and 7D).
Figure 7.
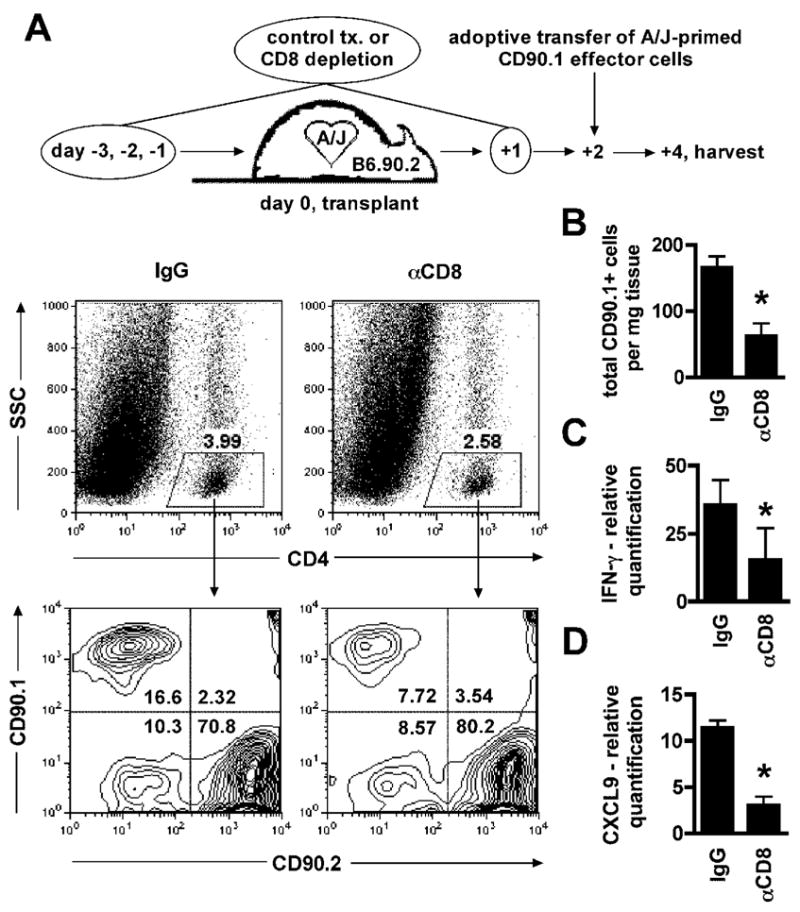
Early CD8 memory T cell alloreactivity enhances recruitment of primed effector T cells to the allograft. Wild-type C57BL/6 mice were treated with control Ig or CD8 depleting antibodies (0.2 mg on days −3, −2, −1, +1). On day 0 mice received A/J cardiac allografts. On day +2, 8 × 106 purified CD90.1 CD4+ cells from spleens of wild-type CD90.1 C57BL/6 mice rejecting A/J cardiac allografts on day 7 post-transplant were adoptively transferred and 48 hours later allografts were harvested and graft-infiltrating CD90.1 CD4+ T cells were detected using flow cytometry. A. Representative data illustrating the gating strategy used to identify the graft-infiltrating CD4+ CD90.1 test population. B. Quantification of the total number of graft-infiltrating CD4+ CD90.1 cells (n = 6/group,
 = p ≤ 0.05). C–D. Relative mRNA quantification of IFN-γ and CXCL9 in grafts harvested from IgG and γ-CD8 treated recipients 4 days after transplantation and 48 hours after adoptive transfer. Data are normalized to a sample randomly chosen from the γ-CD8 treated group (n = 6/group,
= p ≤ 0.05). C–D. Relative mRNA quantification of IFN-γ and CXCL9 in grafts harvested from IgG and γ-CD8 treated recipients 4 days after transplantation and 48 hours after adoptive transfer. Data are normalized to a sample randomly chosen from the γ-CD8 treated group (n = 6/group,
 = p ≤ 0.05).
= p ≤ 0.05).
Discussion
Outcome-based studies have clearly established a harmful role for CD8 memory T cells in organ transplantation. This study was initiated to address kinetic and mechanistic aspects of CD8 memory T cell reactivity in cardiac allografts. In wild-type allograft recipients, we observed CD8 T cells within allograft parenchyma as early as 24 hours post-transplant. These CD8 T cells displayed an activated phenotype, reacted to allogeneic class I MHC, and were activated to produce IFN-γ before donor-specific IFN-γ producing effector T cells were primed in the spleen. Adoptive transfer of donor-reactive CD8 memory T cells restored intragraft IFN-γ production in CD8−/− allograft recipients at this early timepoint. IFN-γ increased the intensity of post-transplant inflammation, in part by inducing CXCL9 production in allograft cells. We focused upon CXCL9 among IFN-γ-induced genes because of studies demonstrating roles for graft-derived CXCR3 ligands in recruiting T cells into cardiac allografts (30, 31). In this report, depletion of the endogenous CD8 T cell response in wild-type mice reduced intragraft IFN-γ and CXCL9 levels early post-transplant and attenuated subsequent graft infiltration by primed T cells. These results demonstrate that inflammatory events promoting allograft rejection are initiated within hours of reperfusion by graft infiltrating CD8 memory T cells.
Heterologous CD8 T cell immunity may have evolved to amplify local inflammation necessary to facilitate recruitment of primed effector T cells from lymphoid priming sites to virally infected tissues. A unique feature of solid organ transplantation is the intense tissue inflammation induced immediately following the combined insults of surgical trauma, ischemia, and reperfusion. We suggest that this inflammation directs CD8 memory T cells into allograft tissues and promotes their activation. CD8 memory T cells then, in turn, are activated by donor alloantigens and optimize rejection by sustaining and enhancing inflammation during the 4–6 days required for donor-specific priming to peak in the spleen. When conventional immunosuppression blocks naïve T cell priming, graft-infiltrating CD8 memory T cells are likely to perpetuate an inflammatory cycle that is destructive in and of itself. The finding that CD8 T cells can contribute to transplant arteriosclerosis despite CD154 blockade (32) is consistent with this hypothesis.
Many therapies which successfully prolong allograft survival in murine models have failed in non-human primate trials, and the greater numbers of memory T cells found in larger animals are thought to contribute substantially to these treatment failures (14). Laboratory mice are deliberately housed in relatively clean cages to protect them from pathogenic exposure. Although often referred to as naïve, these mice do have populations of ‘memory’ T cells which can be generated by homeostasis or limited environmental exposures and are capable of functioning as alloreactive effectors. We have observed early allo-specific CXCL9 production in three different strain combinations tested to date and these results likely reflect that rapid graft infiltration by CD8 memory T cells is common across strains and species. This rapid CD8 memory T cell reactivity is apt to be highly increased in human populations with lifelong exposure to diverse pathogens and other environmental antigens.
Surprisingly, we found that early CD8 memory T cell reactivity appeared completely intact in CD4−/− animals (Figure 1B). The role of CD4 T cell help in the development of robust CD8 memory remains poorly understood (33). Among transplantation studies, conflicting findings of competent and defective CD8 memory T cell formation in response to alloantigen priming have been reported (34, 35). In our experiments, CD4−/− animals had no prior exposure to alloantigens and the observed memory response must derive from TCR cross-reactivity. Therefore, studies which report completely functional CD8 T cell memory development in the absence of CD4 help following some viral infections best support our model (36).
CD4 memory T cells are also known to facilitate allograft rejection. Whereas alloreactive CD4 memory T cells function within secondary lymphoid organs providing help for CD8 T cell and B cell responses (37), this report shows that CD8, and not CD4, memory T cells function within the allograft parenchyma early post-transplant. We did detect a small population of CD4 cells infiltrating allografts early, but this population did not increase in size with time post-transplant and as yet we have not identified inflammatory markers expressed coincident with their presence in the allograft. These cells are most likely CD4 memory T cells but the low levels of class II MHC on murine endothelial cells may be insufficient for their activation.
If an immune response generating effector T cells that cross-react with donor antigens is ongoing at the time of transplantation, either overtly or subclinically, these primed T cells may add to the insult rendered by alloreactive CD8 T cell memory (3, 38). In our adoptive transfer model donor-primed CD8 effector T cells were compared with donor-reactive CD8 memory cells. Effector cells also infiltrated allografts rapidly post-transplant and promoted inflammation. These data underscore the importance of addressing ongoing causes of immune activation prior to transplantation.
High expression of class I MHC on allograft vasculature and activation of effector CD8 T cells via direct pathway recognition of alloendothelial cells has been reported in other model systems (39–41). Our current finding that CD8 memory cells enhance effector cell infiltration parallels our previous observation of CD8-dependent neutrophil infiltration and tissue damage within 12–24 hours post-transplant in cardiac allo- but not iso- grafts (25). Taken together, these studies suggest that CD8 memory T cells are activated early and play an important role in regulating leukocyte interactions with the endothelial barrier and infiltration into the graft parenchymal tissue.
Though perhaps vital for host-defense, memory T cell crossreactivity severely compromises outcomes in transplantation and there is an urgent need for deeper understanding of mechanisms used by memory cells to destroy allografts. CD8 memory T cells consistently lie beyond the reach of currently used therapies and are a major obstacle for clinicians and patients. This report stresses the rapidity with which CD8 memory T cells infiltrate allografts post-transplant and are activated to promote an inflammatory environment that optimizes leukocyte recruitment. Our demonstration that adaptive alloimmunity can be immediate implies that clinical intervention to neutralize CD8 memory T cell function should accompany allograft reperfusion.
Acknowledgments
The authors wish to thank Dr. Peter Heeger for his valuable insights. We also thank Jennifer Powers and Alexander Rodriguez for excellent flow sorting.
This work was supported by NIH AI40459 (R.F.), NIH AI58088 (A.V.) and by the Roche Organ Transplant Research Foundation grant 60495086 (R.F.). A.S. was supported in part by NIH T32 GM07250, an AHA Predoctoral Fellowship, and the CWRU MSTP.
Funding Sources: NIH AI40459 (R.F.) NIH AI58088 (A.V.), Roche Organ Transplant Research Foundation grant 60495086 (R.F.)., NIH T32 GM07250 (A.S.), American Heart Association Predoctoral Fellowship (A.S.), Case Medical Scientist Training Program (A.S.)
References
- 1.Lechler RI, Sykes M, Thomson AW, Turka LA. Organ transplantation--how much of the promise has been realized? Nat Med. 2005;11(6):605–613. doi: 10.1038/nm1251. [DOI] [PubMed] [Google Scholar]
- 2.Lindahl KF, Wilson DB. Histocompatibility antigen-activated cytotoxic T lymphocytes. II Estimates of the frequency and specificity of precursors. J Exp Med. 1977;145(3):508–522. doi: 10.1084/jem.145.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357(25):2601–2614. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 4.Ajithkumar TV, Parkinson CA, Butler A, Hatcher HM. Management of solid tumours in organ-transplant recipients. Lancet Oncol. 2007;8(10):921–932. doi: 10.1016/S1470-2045(07)70315-7. [DOI] [PubMed] [Google Scholar]
- 5.Sayegh MH, Remuzzi G. Clinical update: immunosuppression minimisation. Lancet. 2007;369(9574):1676–1678. doi: 10.1016/S0140-6736(07)60762-4. [DOI] [PubMed] [Google Scholar]
- 6.Brook MO, Wood KJ, Jones ND. The impact of memory T cells on rejection and the induction of tolerance. Transplantation. 2006;82(1):1–9. doi: 10.1097/01.tp.0000226082.17507.da. [DOI] [PubMed] [Google Scholar]
- 7.Rocha B, Tanchot C. The Tower of Babel of CD8+ T-cell memory: known facts, deserted roads, muddy waters, and possible dead ends. Immunol Rev. 2006;211:182–196. doi: 10.1111/j.0105-2896.2006.00378.x. [DOI] [PubMed] [Google Scholar]
- 8.Stadlbauer TH, Kupiec-Weglinski JW. Immunobiology of sensitization in transplant recipients. Am J Med Sci. 1997;313(5):268–274. doi: 10.1097/00000441-199705000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111(12):1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brehm MA, Markees TG, Daniels KA, Greiner DL, Rossini AA, Welsh RM. Direct visualization of cross-reactive effector and memory allo-specific CD8 T cells generated in response to viral infections. J Immunol. 2003;170(8):4077–4086. doi: 10.4049/jimmunol.170.8.4077. [DOI] [PubMed] [Google Scholar]
- 11.Burrows SR, Khanna R, Burrows JM, Moss DJ. An alloresponse in humans is dominated by cytotoxic T lymphocytes (CTL) cross-reactive with a single Epstein-Barr virus CTL epitope: implications for graft-versus-host disease. J Exp Med. 1994;179(4):1155–1161. doi: 10.1084/jem.179.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gamadia LE, Remmerswaal EB, Surachno S, Lardy NM, Wertheim-van Dillen PM, van Lier RA, et al. Cross-reactivity of cytomegalovirus-specific CD8+ T cells to allo-major histocompatibility complex class I molecules. Transplantation. 2004;77(12):1879–1885. doi: 10.1097/01.tp.0000131158.81346.64. [DOI] [PubMed] [Google Scholar]
- 13.Selin LK, Brehm MA, Naumov YN, Cornberg M, Kim SK, Clute SC, et al. Memory of mice and men: CD8+ T-cell cross-reactivity and heterologous immunity. Immunol Rev. 2006;211:164–181. doi: 10.1111/j.0105-2896.2006.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams AB, Pearson TC, Larsen CP. Heterologous immunity: an overlooked barrier to tolerance. Immunol Rev. 2003;196:147–160. doi: 10.1046/j.1600-065x.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 15.Kieper WC, Jameson SC. Homeostatic expansion and phenotypic conversion of naive T cells in response to self peptide/MHC ligands. Proc Natl Acad Sci U S A. 1999;96(23):13306–13311. doi: 10.1073/pnas.96.23.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murali-Krishna K, Ahmed R. Cutting edge: naive T cells masquerading as memory cells. J Immunol. 2000;165(4):1733–1737. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 17.Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004;10(1):87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neujahr DC, Chen C, Huang X, Markmann JF, Cobbold S, Waldmann H, et al. Accelerated memory cell homeostasis during T cell depletion and approaches to overcome it. J Immunol. 2006;176(8):4632–4639. doi: 10.4049/jimmunol.176.8.4632. [DOI] [PubMed] [Google Scholar]
- 19.Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, et al. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163(4):2267–2275. [PubMed] [Google Scholar]
- 20.Augustine JJ, Siu DS, Clemente MJ, Schulak JA, Heeger PS, Hricik DE. Pre-transplant IFN-gamma ELISPOTs are associated with post-transplant renal function in African American renal transplant recipients. Am J Transplant. 2005;5(8):1971–1975. doi: 10.1111/j.1600-6143.2005.00958.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhai Y, Meng L, Gao F, Busuttil RW, Kupiec-Weglinski JW. Allograft rejection by primed/memory CD8+ T cells is CD154 blockade resistant: therapeutic implications for sensitized transplant recipients. J Immunol. 2002;169(8):4667–4673. doi: 10.4049/jimmunol.169.8.4667. [DOI] [PubMed] [Google Scholar]
- 22.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2(6):501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 23.Koyama I, Nadazdin O, Boskovic S, Ochiai T, Smith RN, Sykes M, et al. Depletion of CD8 memory T cells for induction of tolerance of a previously transplanted kidney allograft. Am J Transplant. 2007;7(5):1055–1061. doi: 10.1111/j.1600-6143.2006.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapoor A, Morita K, Engeman TM, Koga S, Vapnek EM, Hobart MG, et al. Early expression of interferon-gamma inducible protein 10 and monokine induced by interferon-gamma in cardiac allografts is mediated by CD8+ T cells. Transplantation. 2000;69(6):1147–1155. doi: 10.1097/00007890-200003270-00020. [DOI] [PubMed] [Google Scholar]
- 25.El-Sawy T, Miura M, Fairchild R. Early T cell response to allografts occurring prior to alloantigen priming up-regulates innate-mediated inflammation and graft necrosis. Am J Pathol. 2004;165(1):147–157. doi: 10.1016/s0002-9440(10)63283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16(4):343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Afanasyeva M, Georgakopoulos D, Belardi DF, Ramsundar AC, Barin JG, Kass DA, et al. Quantitative analysis of myocardial inflammation by flow cytometry in murine autoimmune myocarditis: correlation with cardiac function. Am J Pathol. 2004;164(3):807–815. doi: 10.1016/S0002-9440(10)63169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubois S, Waldmann TA, Muller JR. ITK and IL-15 support two distinct subsets of CD8+ T cells. Proc Natl Acad Sci U S A. 2006;103(32):12075–12080. doi: 10.1073/pnas.0605212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valujskikh A. The challenge of inhibiting alloreactive T-cell memory. Am J Transplant. 2006;6(4):647–651. doi: 10.1111/j.1600-6143.2005.01215.x. [DOI] [PubMed] [Google Scholar]
- 30.Miura M, Morita K, Kobayashi H, Hamilton TA, Burdick MD, Strieter RM, et al. Monokine induced by IFN-gamma is a dominant factor directing T cells into murine cardiac allografts during acute rejection. J Immunol. 2001;167(6):3494–3504. doi: 10.4049/jimmunol.167.6.3494. [DOI] [PubMed] [Google Scholar]
- 31.Hancock WW, Gao W, Csizmadia V, Faia KL, Shemmeri N, Luster AD. Donor-derived IP-10 initiates development of acute allograft rejection. J Exp Med. 2001;193(8):975–980. doi: 10.1084/jem.193.8.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ensminger SM, Witzke O, Spriewald BM, Morrison K, Morris PJ, Rose ML, et al. CD8+ T cells contribute to the development of transplant arteriosclerosis despite CD154 blockade. Transplantation. 2000;69(12):2609–2612. doi: 10.1097/00007890-200006270-00022. [DOI] [PubMed] [Google Scholar]
- 33.Khanolkar A, Badovinac VP, Harty JT. CD8 T cell memory development: CD4 T cell help is appreciated. Immunol Res. 2007;39(1–3):94–104. doi: 10.1007/s12026-007-0081-4. [DOI] [PubMed] [Google Scholar]
- 34.Jones ND, Carvalho-Gaspar M, Luo S, Brook MO, Martin L, Wood KJ. Effector and memory CD8+ T cells can be generated in response to alloantigen independently of CD4+ T cell help. J Immunol. 2006;176(4):2316–2323. doi: 10.4049/jimmunol.176.4.2316. [DOI] [PubMed] [Google Scholar]
- 35.Zhai Y, Wang Y, Wu Z, Kupiec-Weglinski JW. Defective alloreactive CD8 T cell function and memory response in allograft recipients in the absence of CD4 help. J Immunol. 2007;179(7):4529–4534. doi: 10.4049/jimmunol.179.7.4529. [DOI] [PubMed] [Google Scholar]
- 36.Marzo AL, Vezys V, Klonowski KD, Lee SJ, Muralimohan G, Moore M, et al. Fully functional memory CD8 T cells in the absence of CD4 T cells. J Immunol. 2004;173(2):969–975. doi: 10.4049/jimmunol.173.2.969. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Heeger PS, Valujskikh A. In vivo helper functions of alloreactive memory CD4+ T cells remain intact despite donor-specific transfusion and anti-CD40 ligand therapy. J Immunol. 2004;172(9):5456–5466. doi: 10.4049/jimmunol.172.9.5456. [DOI] [PubMed] [Google Scholar]
- 38.Cainelli F, Vento S. Infections and solid organ transplant rejection: a cause-and-effect relationship? Lancet Infect Dis. 2002;2(9):539–549. doi: 10.1016/s1473-3099(02)00370-5. [DOI] [PubMed] [Google Scholar]
- 39.Kreisel D, Krupnick AS, Balsara KR, Riha M, Gelman AE, Popma SH, et al. Mouse vascular endothelium activates CD8+ T lymphocytes in a B7-dependent fashion. J Immunol. 2002;169(11):6154–6161. doi: 10.4049/jimmunol.169.11.6154. [DOI] [PubMed] [Google Scholar]
- 40.Kreisel D, Krupnick AS, Gelman AE, Engels FH, Popma SH, Krasinskas AM, et al. Non-hematopoietic allograft cells directly activate CD8+ T cells and trigger acute rejection: an alternative mechanism of allorecognition. Nat Med. 2002;8(3):233–239. doi: 10.1038/nm0302-233. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi H, Koga S, Novick AC, Toma H, Fairchild RL. T-cell mediated induction of allogeneic endothelial cell chemokine expression. Transplantation. 2003;75(4):529–536. doi: 10.1097/01.TP.0000048377.59350.E4. [DOI] [PubMed] [Google Scholar]
- 42.Larsen CP, Knechtle SJ, Adams A, Pearson T, Kirk AD. A new look at blockade of T-cell costimulation: a therapeutic strategy for long-term maintenance immunosuppression. Am J Transplant. 2006;6(5 Pt 1):876–883. doi: 10.1111/j.1600-6143.2006.01259.x. [DOI] [PubMed] [Google Scholar]
- 43.St Clair EW, Turka LA, Saxon A, Matthews JB, Sayegh MH, Eisenbarth GS, et al. New reagents on the horizon for immune tolerance. Annu Rev Med. 2007;58:329–346. doi: 10.1146/annurev.med.58.061705.145449. [DOI] [PubMed] [Google Scholar]
- 44.Dai Z, Li Q, Wang Y, Gao G, Diggs LS, Tellides G, et al. CD4+CD25+ regulatory T cells suppress allograft rejection mediated by memory CD8+ T cells via a CD30-dependent mechanism. J Clin Invest. 2004;113(2):310–317. doi: 10.1172/JCI19727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang J, Brook MO, Carvalho-Gaspar M, Zhang J, Ramon HE, Sayegh MH, et al. Allograft rejection mediated by memory T cells is resistant to regulation. Proc Natl Acad Sci U S A. 2007;104(50):19954–19959. doi: 10.1073/pnas.0704397104. [DOI] [PMC free article] [PubMed] [Google Scholar]


