Abstract
Study Objectives:
To investigate whether noninvasive application of recurrent airway obstructions induces early release of mesenchymal stem cells into the circulating blood in a rat model of obstructive sleep apnea.
Design:
Prospective controlled animal study.
Setting:
University laboratory.
Patients or Participants:
Twenty male Sprague-Dawley rats (250–300 g).
Interventions:
A specially designed nasal mask was applied to the anesthetized rats. Ten rats were subjected to a pattern of recurrent obstructive apneas (60 per hour, lasting 15 seconds each) for 5 hours. Ten anesthetized rats were used as controls.
Measurements and Results:
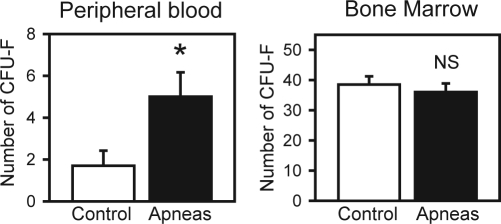
Mesenchymal stem cells from the blood and bone marrow samples were isolated and cultured to count the total number of colony-forming unit fibroblasts (CFU-F) of adherent cells after 9 days in culture. The number of CFU-F from circulating blood was significantly (P = 0.02) higher in the rats subjected to recurrent obstructive apneas (5.00 ± 1.16; mean ± SEM) than in controls (1.70 ± 0.72). No significant (P = 0.54) differences were observed in CFU-F from bone marrow.
Conclusions:
Application of a pattern of airway obstructions similar to those experienced by patients with sleep apnea induced an early mobilization of mesenchymal stem cells into circulating blood.
Citation:
Carreras A; Almendros I; Acerbi I; Montserrat JM; Navajas D; Farré R. Obstructive apneas induce early release of mesenchymal stem cells into circulating blood. SLEEP 2009;32(1):117-119.
Keywords: Obstructive sleep apnea, animal model, airway obstruction, circulating stem cells, bone marrow cells, mesenchymal stem cells
PATIENTS WITH OBSTRUCTIVE SLEEP APNEA (OSA) ARE SUBJECTED TO INCREASED SWINGS OF INTRATHORACIC PRESSURE, REPETITIVE EPISODES OF ARTERIAL blood oxygen desaturation and reoxygenation, sympathetic activation, and central nervous system arousals.1 The mid- and long-term consequences of the oxidative stress and systemic inflammation characterizing OSA include endothelial dysfunction and alteration in the vasoconstriction mechanisms of blood vessels, increasing the risk of cardiac and vascular diseases.1 Given that these consequences of OSA are associated with damage to various tissues, several physiologic repair mechanisms are activated. One of the potential responses for repairing the cellular injuries caused by OSA is the mobilization of adult stem cells from the injured tissues and, particularly, from the bone marrow. In fact, the mobilization of bone marrow hematopoietic and mesenchymal stem cells (MSC) and endothelial progenitor cells is expected. However, there are almost no data available on the activation of stem cells in patients with OSA.
Recent data suggest that the homeostatic response to oxidative and inflammatory challenges could include a protective mechanism that is still not completely understood: the release of MSC from the bone marrow into the peripheral blood. Indeed, it has been shown that there is an increase in circulating MSC in response to virus-induced acute inflammation2 or chronic hypoxia.3 In addition, recent evidence indicates that circulating MSC attenuate vasoconstriction4 and have a systemic anti-inflammatory effect5 through paracrine mechanisms. These data suggest that the primary stimuli characterizing OSA at the cellular level could induce the release of bone marrow MSC to peripheral blood. However, there is an absence of specific data from patients with OSA or from animal models mimicking this syndrome. The aim of the present work was, therefore, to test the hypothesis that noninvasive application of recurrent obstructive apneas with a pattern simulating the events experienced by patients with OSA induces an early mobilization of MSC into the peripheral blood.
METHODS
Application of Recurrent Airway Obstructions
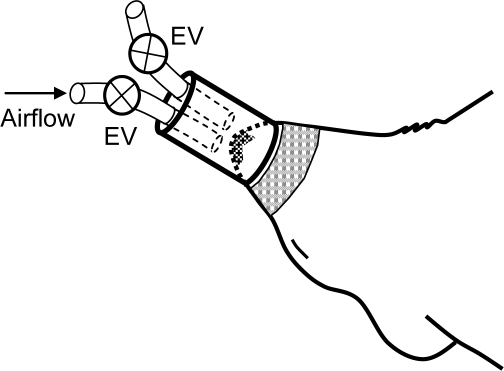
The study, which was approved by the Ethics Committee for Animal Research of the University of Barcelona, was carried out in 20 Sprague-Dawley male rats (250–300 g) intraperitoneally anesthetized with urethane (1 mg/kg). An experimental setting based on a nasal mask was specifically designed to noninvasively apply recurrent airway obstructions (Figure 1). The mask was connected to one tube open to the atmosphere and to another tube connected to an airflow source to prevent rebreathing. Two electronically synchronized electrovalves (L178B1, Sirai, Italy) allowed controlled application of airway obstruction (both valves closed) or spontaneous breathing (both valves open). Ten rats were subjected to recurrent obstructive apneas (60 per hour, lasting 15 seconds each) for 5 hours. These obstructions induced increased breathing efforts and hypoxia-reoxygenation events characterized by periodic arterial blood oxygen desaturations down to 74.1% ± 1.6% (mean ± SEM) as measured by pulse oxymetry (504; Critical Care Systems, Inc., Waukesha, WI). Ten anesthetized rats were used as controls. More details on the experimental setting for applying recurrent airway obstructions can be found in the on-line supplement.
Figure 1.
Diagram of the nasal mask designed to noninvasively apply recurrent obstructive apneas in rats. The mask was connected to one tube open to the atmosphere and to another tube connected to an air-flow source to prevent rebreathing. Two electronically synchronized electrovalves (EV) allowed the application of airway obstruction (both valves closed) or spontaneous breathing (both valves open). An adhesive tape around the mouth was used to avoid air leaks.
Extraction and Processing of Blood and Bone Marrow
After 5 hours of recurrent obstructive apneas (or control), 8 to 10 mL of blood from the carotid artery were collected in a tube with EDTA K3, and the rat was sacrificed by exsanguination. The bone marrow of both femurs was harvested by removing the epiphyses and flushing through the medullary cavity a solution of Dulbecco Modified Eagle Medium (DMEM) containing 10% inactivated fetal bovine serum (FBS), antibiotics, and antifungical solution (penicillin 50 U/mL, streptomycin 50 μg/mL, and amphotericin B 5 mg/mL). After blood and bone marrow extraction, the samples were processed to isolate MSC. On the one hand, the blood sample was mixed with a washing solution (phosphate buffered saline-1X [PBS] supplemented with 2% FBS solution [1:1 volume]) and centrifuged at 500 g, 20 °C, for 30 minutes following cell sorting by the Ficoll density gradient technique (Histopaque 1083, Sigma Chemical Co., St. Louis, MO). The second top white layer rendered by the Ficoll gradient (enriched in mononuclear cells) was collected and washed twice by centrifugation at 300 g, 20 °C, for 5 minutes with the supplemented PBS solution. The pellet was resuspended with 2 mL of the DMEM solution. On the other hand, the bone marrow cell suspension was disaggregated by subsequent passage through needles of decreasing sizes (19-gauge to 25-gauge) and centrifugation at 460 g for 10 minutes at room temperature using the Percoll gradient technique to isolate mononuclear cells. The second top white layer was collected and washed twice by centrifugation at 1000 g, 20 °C for 4 minutes with the supplemented PBS solution. The pellet was resuspended with the same medium used for the blood sample.
Isolation and Culture of MSC
The MSC population was isolated based on different plastic-adherence properties. The mononuclear cells obtained from either circulating blood or bone marrow were plated at a density of 1.25×106 cells per well (3.66 cm2 growth area) in DMEM and incubated at 37°C in a humidified atmosphere containing 5% CO2. Three days after they were plated, nonadherent cells were removed, and the remaining adherent cells were cultured in supplemented MesenPro medium (Gibco™, Invitrogen, Carlsbad, CA) containing penicillin 50 U/mL, streptomycin 50 μg/mL, amphotericin B 5 mg/mL, and L-glutamine 1 mM. The cultures were maintained by changing the medium every 3 days. After the adherent cells were cultured for 9 days, the proliferation potential of the MSC was quantified by counting the total number of colony-forming unit fibroblasts (CFU-F) of adherent cells ( > 50 cells). The potential of the MSC obtained from peripheral blood for differentiation into adipocytes and osteocytes was assessed by using culture media to induce specific MSC differentiation (on-line supplement).
Statistical Analysis
Data are presented as mean ± SEM. Comparisons between different groups were carried out by the Student t-test or the Mann-Whitney test (for normal or nonnormal distributions, respectively). Statistical significance was assumed for P < 0.05.
RESULTS
The number of CFU-F obtained from circulating blood was 3-fold greater in the rats subjected to the recurrent obstructive apneas than in control rats (P = 0.02), as shown in Figure 2. This figure also shows that no significant difference was observed in the number of CFU-F obtained from bone marrow (P = 0.54). Differentiation into both adipocytes and osteocytes was observed (on-line supplement) in all the investigated MSC cultures obtained from peripheral blood of rats subjected to recurrent obstructive apneas (differentiation rates ranged 60% – 80%).
Figure 2.
Mesenchymal stem cells in peripheral blood and in bone marrow of the rats subjected to recurrent obstructive apneas and in controls. Number of colony-forming units (CFU-F) of mesenchymal stem cells obtained from 1.25×106 plated cells.
DISCUSSION
Application of repetitive obstructive apneas similar to the ones experienced by patients with OSA results in an increase in the circulating MSC in healthy rats. As expected, the amount of MSC in the bone marrow, which is the main pool of these stem cells, was not modified by their release into the bloodstream.3 The observed response was early, after a short stimulus (5 hours) roughly corresponding to 1 night of events, suggesting that the MSC mobilizing mechanism is particularly sensitive to the OSA stimulus. With the experimental model used, the rats were simultaneously subjected to two of the stimuli experienced by OSA patients: recurrent strenuous breathing against a closed airway and intermittent hypoxia/reoxygenation. Although it does not allow us to know the contribution of each of these two stimuli, the model employed in this work is a realistic approach to the actual challenge suffered by patients with OSA during sleep.
The experimental setting was specifically designed to apply the recurrent obstructive apneas in a noninvasive way to avoid the potential confounding effects caused by the surgical stimulus of invasive procedures.6 Moreover, the novel nasal mask built for this study had a small volume when occluded (to present a high impedance to inspiratory efforts, i.e., airway obstruction) and prevented rebreathing and positive pressure when open. To this end, the dimensions of the mask, the length and diameter of the tubing, and the magnitude of the bias flow (Figure 1) were adjusted. The effectiveness of the mask in simulating the obstructive events of OSA was verified by observing the pattern of periodic swings of arterial oxygen saturation ranging from approximately to 95% to 75%. The isolation of MSC from bone marrow and peripheral blood was carried out with the conventional method based on the different adherent properties of these cells on plates.3,7 The MSC were quantified by counting the number of CFU-F obtained after culturing in an adequate medium.3 The values of CFU-F obtained for both bone marrow and circulating blood in controls (Figure 2) were remarkably similar to the MSC baseline values recently reported in healthy rats,3 confirming the physiologic presence of MSC in circulating blood.
Release of adult progenitor stem cells from the bone marrow to peripheral blood is a well-known repair mechanism activated in response to a variety of injuries. For instance, hematopoietic stem cells are able to repopulate all hematopoietic cell lineages, endothelial progenitor cells contribute to vascular repair, and MSC can home to injured tissues and differentiate into different cell types: typically osteoblasts, adipocytes, and chondrocytes but also cardiac and vascular cells.7 The repairing capability of adult stem cells has therapeutic potential and, the effectiveness of their transplantation in treating different diseases is currently being investigated.7 Although research on MSC is focused on applications in the field of regenerative medicine, recent studies suggest that these adult stem cells could play other protective roles in response to challenges (hypoxia and inflammation) akin to those caused by obstructive apneas. Indeed, in vivo animal studies indicate that MSC are mobilized into peripheral blood by acute inflammation caused by virus-induced myocarditis2 or by 3-week chronic hypoxia.3 However, no data are available on the potential effect of the intermittent hypoxia characterizing OSA in mobilizing MSC into circulating blood. Moreover, an ex vivo study on human MSC cultured in serum obtained from athletes after 8 minutes of cycling has shown that short intensive exercise increases the migratory activity of MSC.8 Once recruited, the protective action of the MSC stimulated by inflammatory mediators or hypoxia is exerted via paracrine mechanisms4,5 by secretion of angiogenic growth factors, anti-apoptotic factors, or anti-inflammatory cytokines.9
This is, to our knowledge, the first direct evidence that the obstructive apneas typical of OSA are, per se, able to mobilize MSC. Although application of stem cells for OSA therapy is not currently considered, the elucidation of the mechanisms involved in the mobilization of MSC and in their anti-inflammatory and other repairing functions in OSA is of potential interest. Therefore, future studies on MSC in chronic animal models10 and in patients with OSA can provide useful information to better understand the pathophysiology of this syndrome and the evolution of its metabolic and cardiovascular consequences.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors wish to thank Rocío Nieto and Miguel A. Rodríguez for their technical assistance.
Sources of support: This work was supported in part by Ministerio de Ciencia e Innovaciòn (SAF2008-2991, FIS-PI08277).
SUPPLEMENTARY MATERIAL
METHODS
Experimental Setting to Apply Recurrent Obstructive Apneas
The nasal mask (Figure 1, printed publication) was a latex tube with an internal diameter (ID) of 5 mm and a length of 7 mm. The 2 tubes entering the nasal mask had different diameters. The tube connected to the airflow source had an ID of 1.2 mm, and the other tube had an ID of 3 mm. When the valves were open, the setting presented a low load to the rat's breathing: 0.125 cm H2O·mL−1·s. Accordingly, for a typical peak inspiratory flow of approximately 8 mL/s in rats,1-Suppl the experimental setting required an extra breathing effort of only approximately 1 cm H2O. This value of load to breathing is considered negligible, even for lung function measurements in patients. 2-Suppl
When the valves were closed, the setting connected to the rat nose had a compliance of 1.2 × 10−5 L/cm H2O which, at the typical rat breathing frequency (∼ 100 breath/min),3-Suppl corresponds to an impedance of approximately 1.0 × 104 cm H2O·L−1·s. This value is 2 orders of magnitude greater than rats' impedance at the breathing frequency (≈ 400 cm H2O·L−1·s).4-Suppl Accordingly, when the valves were closed, the rat was subjected to virtual airway closure.
The bias flow circulating through the mask when the valves were open was 4 mL per second, thereby ensuring air renewal taking into account rat ventilation (≈ 90 mL/min = 1.5 mL/s)3-Suppl.
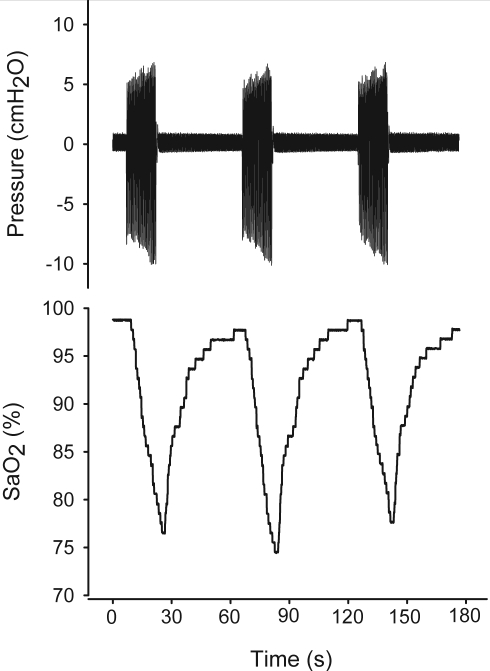
As shown in the example of Figure S1, with the experimental setting employed the rats were subjected to recurrent airway obstruction/reopening simulating OSA events. As indicated by the nasal pressure signal, during the periods of valve closure the rat exerted increased breathing efforts when trying to ventilate through the obstructed nasal mask. By contrast, when the valves were open the rat breathed normally, as indicated by the small pressure swings in the nasal mask. As a result of the recurrent obstruction/reopening imposed on the rat, arterial oxygen saturation exhibited a pattern similar to the one observed in patients with OSA.
Figure S1.
Example of nasal mask pressure arterial oxygen saturation (SaO2) in a rat subjected to recurrent obstructive apneas.
Assessment of the Differentiation Potential of MSC from Peripheral Blood
The differentiation potential of the MSC obtained from the peripheral blood was confirmed in five supplementary rats subjected to the challenge of recurrent obstructive apneas. To this end, MSC were obtained and processed as described in the printed publication, with additional cell passaging (P) to expand the culture. Conventional procedures for differentiation into adipocytes and osteocytes were applied5-Suppl.
Adipogenic induction:
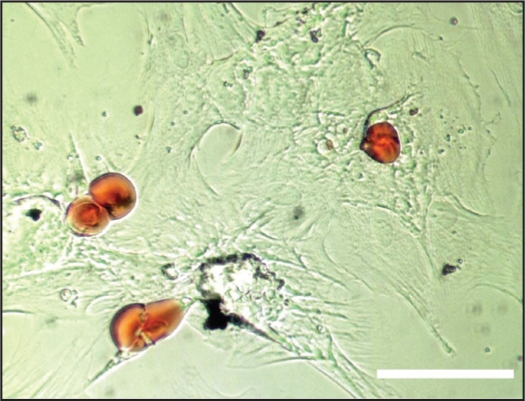
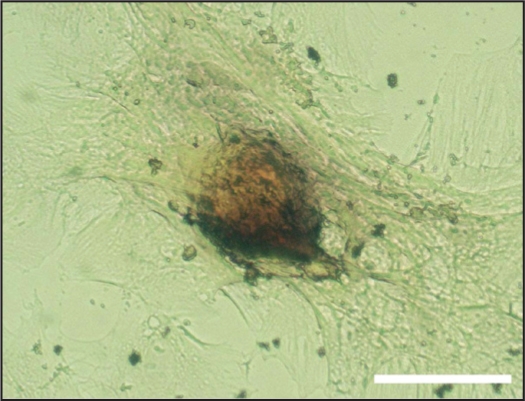
MSC from circulating blood at P1-P3 (when cells approached 90% confluence) were cultured in α-MEM (GIBCO, Gaithersburg, MD) containing 10% Fetal Bovine Serum (FBS) (GIBCO, Gaithersburg, MD), 100 μM isobutylmethilxanthine (SIGMA, St Louis, MO), 60 μM indomethacin (SIGMA, St Louis, MO), 1μg/ml insulin (SIGMA, St Louis, MO), 0,5 μM hydrocortisone (SIGMA, St Louis, MO) and 5 μg/ml D-glucose (Sigma, St Louis, MO). The culture medium was changed every 3 days. After one week, the cells were cultured for 12 more days in α-MEM (GIBCO, Gaithersburg, MD) containing 10% FBS (GIBCO, Gaithersburg, MD) and 5 μg/ml D-glucose (SIGMA, St Louis, MO). The culture medium was changed every 3 days. All the MSC samples exhibited adipogenic differentiation by observing accumulation of lipid vacuoles by staining cells with Oil Red O (SIGMA, St Louis, MO) (Figure S2).
Figure S2.
Example of adipogenic differentiation of the MSC obtained from the peripheral blood of a rat subjected to recurrent obstructive apneas. Lipid vacuoles are stained in red. Bar = 100 μm.
Osteogenic induction:
MSC from circulating blood at P1-P3 (when cells approached 60%–80% confluence) were cultured using STEMPRO Osteogenesis Differentiation Kit (GIBCO, Gaithersburg, MD) following the manufacturer's instructions. After 21 days, cells were stained with Alizarin Red S (ALFA AESAR, Karlsruhe, Germany) to detect mineralization areas within cells (Figure S3).
Figure S3.
Example of osteogenic differentiation of the MSC obtained from the peripheral blood of a rat subjected to recurrent obstructive apneas. The mineralization areas are stained in red. Bar = 100 μm.
REFERENCES (SUPPLEMENT)
- 1-suppl:Farré R, Rotger M, Montserrat JM, Calero G, Navajas D. Collapsible upper airway segment to study the obstructive sleep apnea/hypopnea syndrome in rats. Respir Physiol Neurobiol. 2003;136:199–209. doi: 10.1016/s1569-9048(03)00082-x. [DOI] [PubMed] [Google Scholar]
- 2-suppl:ERS Task Force on Respiratory Impedance Measurements. Oostveen E, MacLeod D, Lorino H, Farré R, Hantos Z, Desager K, Marchal F. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. 2003;22:1026–41. doi: 10.1183/09031936.03.00089403. [DOI] [PubMed] [Google Scholar]
- 3-suppl:Strohl KP, Thomas AJ, St Jean P, Schlenker EH, Koletsky RJ, Schork NJ. Ventilation and metabolism among rat strains. J Appl Physiol. 1997;82:317–323. doi: 10.1152/jappl.1997.82.1.317. [DOI] [PubMed] [Google Scholar]
- 4-suppl:Gomes RF, Shen X, Ramchandani R, Tepper RS, Bates JH. Comparative respiratory system mechanics in rodents. J Appl Physiol. 2000;89:908–16. doi: 10.1152/jappl.2000.89.3.908. [DOI] [PubMed] [Google Scholar]
- 5-suppl:Beyer Nardi N, da Silva Meirelles L. Mesenchymal stem cells: isolation, in vitro expansion and characterization. Handb Exp Pharmacol. 2006;174:249–82. [PubMed] [Google Scholar]
REFERENCES
- 1.Caples SM, Garcia-Touchard A, Somers VK. Sleep-disordered breathing and cardiovascular risk. Sleep. 2007;30:291–303. doi: 10.1093/sleep/30.3.291. [DOI] [PubMed] [Google Scholar]
- 2.Zhao L, Li S, Ge J, et al. Temporal changes in stem cells in the circulation and myocardium of mice with Coxsackie virus B3-induced myocarditis. Microvasc Res. 2008;75:358–66. doi: 10.1016/j.mvr.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Rochefort GY, Delorme B, Lopez A, et al. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006;24:2202–8. doi: 10.1634/stemcells.2006-0164. [DOI] [PubMed] [Google Scholar]
- 4.Patel KM, Crisostomo P, Lahm T, et al. Mesenchymal stem cells attenuate hypoxic pulmonary vasoconstriction by a paracrine mechanism. J Surg Res. 2007;143:281–5. doi: 10.1016/j.jss.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Xu J, Woods CR, Mora AL, et al. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L131–41. doi: 10.1152/ajplung.00431.2006. [DOI] [PubMed] [Google Scholar]
- 6.Nacher M, Serrano-Mollar A, Farre R, Panes J, Segui J, Montserrat JM. Recurrent obstructive apneas trigger early systemic inflammation in a rat model of sleep apnea. Respir Physiol Neurobiol. 2007;155:93–6. doi: 10.1016/j.resp.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Burt RK, Loh Y, Pearce W, et al. Clinical applications of blood-derived and marrow-derived stem cells for nonmalignant diseases. JAMA. 2008;299:925–36. doi: 10.1001/jama.299.8.925. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt A, Bierwirth S, Weber S, Platen P, Schinköthe T, Bloch W. Short intensive exercise increases the migratory activity of mesenchymal stem cells. Br J Sports Med. doi: 10.1136/bjsm.2007.043208. Forthcoming 2008. [DOI] [PubMed] [Google Scholar]
- 9.Crisostomo PR, Wang Y, Markel TA, Wang M, Lahm T, Meldrum DR. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am J Physiol Cell Physiol. 2008;294:C675–82. doi: 10.1152/ajpcell.00437.2007. [DOI] [PubMed] [Google Scholar]
- 10.Farré R, Nácher M, Serrano-Mollar A, et al. Rat model of chronic recurrent airway obstructions to study the sleep apnea syndrome. Sleep. 2007;30:930–3. doi: 10.1093/sleep/30.7.930. [DOI] [PMC free article] [PubMed] [Google Scholar]