Abstract
In our study, the prevalence of nasopharyngeal Streptococcus pyogenes was 130 (14.3%) of 909 healthy children. Isolates were found to be susceptible to all antibiotics tested. Pulsed-field gel electrophoresis and arbitrarily primed PCR revealed that 34 (32.4%) of the 105 isolates and 41 (40.6%) of the 101 isolates typed, respectively, were clonally indistinguishable.
Streptococcus pyogenes group A streptococcus (GAS) strains colonized in the upper respiratory tracts of children play an important role in the spread of this bacterial infection, especially among children at school, day-care centers, orphanages, and home. Study of the prevalence of healthy S. pyogenes carriers and the molecular epidemiology of the isolates may provide useful information about the origin and spread of this infectious agent, allowing for more effective control measures. Pulsed-field gel electrophoresis (PFGE) (3, 4, 14) has been used as a standard technique for surveying epidemiology of S. pyogenes infections. Although arbitrarily primed PCR (AP-PCR)-based fingerprinting performed with the M13 primer has been widely used for molecular epidemiology of gram-negative (1, 2, 10) and gram-positive bacteria (11), there had been no study about its efficiency in typing S. pyogenes strains.
The aims of the present study were to investigate the rate of pharyngeal colonization, drug susceptibility, and the molecular epidemiology of GAS isolated from healthy children and to compare PCR-based fingerprinting with PFGE in the investigation of clonal relatedness among the GAS isolates.
Study groups.
The study groups included 800 primary schoolchildren and 109 children living in an orphanage in Malatya, Turkey. An otorhinology specialist rubbed sterile swabs over the posterior nasopharyngeal walls of the 909 children, who had no symptoms or signs of pharyngitis. The samples were inoculated on sheep's blood agar plates. After incubation overnight at 37°C, beta-hemolytic streptococci were identified with a bacitracin disk (0.04 U) and a latex test for the identification of streptococcal groups A, B, C, D, F, and G (streptococcal grouping kit and diagnostic reagent; Oxoid Limited, Basingstoke, England).
Susceptibility testing.
The antimicrobial susceptibilities of the GAS isolates were investigated by the disk diffusion method according to the criteria of the National Committee for Clinical Laboratory Standards (13). The antibiotic disks (Oxoid) used were penicillin (10 U), erythromycin (15 μg), vancomycin (30 μg), chloramphenicol (30 μg), clindamycin (2 μg), cefepime (30 μg), ceftriaxone (30 μg), ofloxacin (5 μg), and levofloxacin (5 μg).
Molecular typing of the strains.
Both AP-PCR and PFGE typing was performed on 101 strains which had available stocks. PFGE was also carried out on five additional strains. For AP-PCR typing, isolation of DNA by using lysozyme and proteinase K and extraction were performed by following the protocol of Welsh and McClelland (19). Then AP-PCR, which had previously been optimized (1, 2), was performed with the M13 primer (10). For PFGE, isolation and deproteinization of the genomic DNA were done by following the protocol of Elliott et al. (8) but with lysostaphin (5 U/ml) used instead of mutanolysin. The genomic DNA in the plugs was incubated with 24 U of SmaI (Promega Corporation, Madison, Wis.) for 24 h at 25°C in a water bath. DNA fragments were separated on 1% agarose gels run in 0.5× Tris-borate-EDTA buffer by using a CHEF-DR II system (Bio-Rad Laboratories, Nazareth, Belgium). The conditions for electrophoresis were 14°C at 6 V/cm for 24 h. The initial and final switch times were 5 and 40 s, respectively. The band patterns of the strains obtained with both typing procedures were analyzed with GelCompar software (version 3.0; Applied Maths, Sint-Martens-Latem, Belgium). According to the interpretative criteria of Tenover et al. (17), the isolates were classified as indistinguishable (cluster), closely related, possibly related, or different.
The means and standard deviations (SD) were calculated and data were analyzed using the statistical suite SPSS (version 10). The chi-square test was used to determine the significance of difference of the parameters tested.
Analysis of the results.
The mean age of the 909 children was 8.17 years (SD, 3.89; range, 4 to 13 years). Of the study group, 491 (54%) were boys and 418 (46%) were girls. The global prevalence of healthy S. pyogenes carriers was 14.3% (130 carriers), which was considerably higher than for previous findings (2.5 to 6.8%) (9, 12, 15). Although age, gender, hospitalization during the last 3 months, antibiotic use, sanitary conditions, and being with family members and/or school workers are risk factors for S. pyogenes colonization (16), we found that the rates of carriers for boys and girls were similar (13.6 and 15.1%, respectively; P > 0.05) and that there was no significant difference between the mean age of the carriers (7.74 years; SD, 1.91) and that of the noncarriers (8.23 years; SD, 4.13). The frequency was similar in all age groups of schoolchildren (P > 0.05), but it was significantly higher in children aged 4 to 6 years living in the orphanage (P < 0.05) (Table 1). The prevalence of carriers was significantly higher in the orphanage (P < 0.001), suggesting that group living for a long period has an important effect on the spread of GAS.
TABLE 1.
Frequency of S. pyogenes carriers based on study groups
| Group | Total no. of children | No. of children who were:
|
Frequency (%) of carriers in indicated age group
|
||||
|---|---|---|---|---|---|---|---|
| Tested (sampling rate %) | Carriers (%) | 4-6 | 7-8 | 9-10 | >10 years | ||
| Schoolchildren | 1284 | 800 (62.3) | 101 (12.6) | 12.1 | 11.7 | 13.0 | |
| Orphanage children | 135 | 109 (80.7) | 29 (26.6) | 50.0 | 24.0 | 17.1 | 15.4 |
| Total | 1419 | 909 (64.1) | 130 (14.3) | 21.0 | 13.5 | 13.3 | 15.4 |
Similar to the results of previous studies (5, 7, 12), all GAS tested in the present study were susceptible to first-line drugs such as penicillin and erythromycin; moreover, we did not find any strains resistant to other antibiotics tested. These results show that even upper respiratory tract cultures yield S. pyogenes. Susceptibility testing is not necessary.
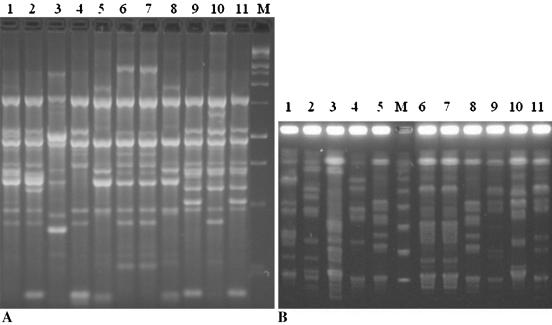
Although many investigators use the AP-PCR method in combination with PFGE for investigation of molecular epidemiology of GAS in a community (4, 5, 6, 18), to date there have been no published data on the clonal diversity of S. pyogenes isolates obtained from asymptomatic carriers or symptomatic patients in Turkey. In our study, PFGE yielded 85 different patterns among 105 S. pyogenes isolates. Of the isolates, 34 (32.4%) were clustered in 14 groups, 4 (3.8%) were possibly related, and 67 (63.8%) were epidemiologically unrelated. Seventy-seven AP-PCR banding patterns were obtained among 101 isolates. The PCR-based typing procedure revealed that 41 (40.6%) of the isolates were indistinguishable (clustered in 17 groups), 13 (12.9%) were closely related, 12 (11.9%) were possibly related, and 35 (34.6%) were different. The mean numbers of detectable bands obtained by PFGE and AP-PCR were 11.6 (range, 5 to 22) and 10.6 (range, 5 to 21), respectively. Figure 1 provides examples of the band profiles obtained by AP-PCR and PFGE.
FIG. 1.
Representative band patterns of 11 isolates obtained by both typing methods. (A) AP-PCR profiles. Lane M is a molecular weight marker (QX174 DNA/HaeIII); lanes 6 and 7 are isolates with identical DNA profiles. (B) PFGE profiles. Lane M contains a concatemer of lambda DNA (catalog no. D2416; Sigma); lanes 6 and 7 are isolates with identical profiles.
A total of 38 (36.1%) and 66 (65.3%) of the isolates typed by PFGE and AP-PCR, respectively, were clonally related. The clonal relationships determined by PFGE (81.6%) and AP-PCR (75.8%) typing confirm conventional epidemiological data that indicate being in the same school and classroom or living in an orphanage increases the prevalence of carriers. When we considered only clustering isolates, concordance between conventional and molecular epidemiology increased to 85.3% for both typing procedures.
Comparison of the typing procedures showed that 76.5% of the strains defined as indistinguishable by PFGE had identical AP-PCR profiles. Thirty-five (92.1%) of the 38 strains defined as clonally related by PFGE also showed clonal relationships by AP-PCR typing. On the other hand, 91.4% of the strains which yielded distinct profiles by AP-PCR were also different by PFGE. Our results revealed that the clustering results of AP-PCR typing need confirmation; however, strains found distinct by this method most probably do not need retesting.
Molecular typing results showed that there was no association between clustering rate and age and gender of the study populations; however, similar to results for colonization, the isolates of the children in the orphanage had significantly higher clustering rates. As was expected, these data reveal that an increase in time spent with other children leads to an increase in the risk of transmission.
In conclusion, high rates of colonization and clustering obtained by both typing procedures show that the streptococcal populations circulating in children of school age tend to be highly related. The AP-PCR method used in this study is useful in eliminating epidemiologically unrelated isolates.
REFERENCES
- 1.Ayan, M., C. Kuzucu, R. Durmaz, E. Aktas, and Z. Cizmeci. 2003. Analysis of three outbreaks due to Klebsiella species in a neonatal intensive care unit. Infect. Control Hosp. Epidemiol. 24:495-500. [DOI] [PubMed] [Google Scholar]
- 2.Ayan, M., R. Durmaz, E. Aktas, and B. Durmaz. 2003. Bacteriological, clinical and epidemiological characteristics of nosocomial Acinetobacter baumannii infections in a teaching hospital. J. Hosp. Infect. 54:39-45. [DOI] [PubMed] [Google Scholar]
- 3.Cockerill, F. R., III, K. L. MacDonald, R. L. Thompson, F. Roberson, P. C. Kohner, J. Besser-Wiek, J. M. Manahan, J. M. Musser, P. M. Schlievert, J. Talbot, B. Frankfort, J. M. Steckelberg, W. R. Wilson, and M. T. Osterholm. 1997. An outbreak of invasive group A streptococcal disease associated with high carriage rates of the invasive clone among school-aged children. JAMA 277:38-43. [PubMed] [Google Scholar]
- 4.Cresti, S., M. Lattanzi, A. Zanchi, F. Montagnani, S. Pollini, C. Cellesi, and G. M. Rossolini. 2002. Resistance determinants and clonal diversity in group A streptococci collected during a period of increasing macrolid resistance. Antimicrob. Agents Chemother. 46:1816-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Melo, M. C., A. M. Sa Figueiredo, and B. T. Ferreira-Carvalho. 2003. Antimicrobial susceptibility patterns and genomic diversity in strains of Streptococcus pyogenes isolated in 1978-1997 in different Brazilian cities. J. Med. Microbiol. 52:251-258. [DOI] [PubMed] [Google Scholar]
- 6.Descheemaeker, P., S. Chapelle, C. Lammens, M. Hauchecorne, M. Wijdooghe, P. Vandamme, M. Leven, and H. Goossens. 2000. Macrolide resistance and erythromycin resistance determinants among Belgian Streptococcus pyogenes and Streptococcus pneumoniae isolates. J. Antimicrob. Chemother. 45:167-173. [DOI] [PubMed] [Google Scholar]
- 7.Detcheva, A., R. R. Facklam, and B. Beall. 2002. Erythromycin-resistant group A streptococcal isolates recovered in Sofia, Bulgaria, from 1995 to 2001. J. Clin. Microbiol. 40:3831-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott, J. A., K. D. Farmer, and R. R. Facklam. 1998. Sudden increase in isolation of group B streptococci, serotype V, is not due to emergence of a new pulsed-field gel electrophoresis type. J. Clin. Microbiol. 36:2115-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Lama, Z., J. J. Gonzalez, P. Lupiola, and M. T. Tejedor. 2000. Carriers of beta hemolytic streptococci from groups A, B, and C among schoolchildren in Las Palmas. Enferm. Infect. Microbiol. Clin. 18:271-273. (In Spanish.) [PubMed]
- 10.Grundmann, H. J., K. J. Towner, L. Dijkshoorn, P. Gerner-Smidt, M. Mahmer, H. Seifert, and M. Vaneechoutte. 1997. Multicenter study using standardized protocols and reagents for evaluation of reproducibility of PCR-based fingerprinting of Acinetobacter spp. J. Clin. Microbiol. 35:3071-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guducuoglu, H., M. Ayan, R. Durmaz, M. Berktas, H. Bozkurt, and Y. Bayraktar. 2002. Epidemiological analysis of Staphylococcus aureus strains from nasal carriers in a teaching hospital. New Microbiol. 25:421-426. [PubMed] [Google Scholar]
- 12.Herruzo, R., L. Chamorro, M. E. Garcia, M. C. Gonzalez, A. M. Lopez, N. Mancenido, and L. Yebenes. 2001. Prevalence and antimicrobial-resistance of S. pneumoniae and S. pyogenes in healthy children in the region of Madrid. Int. J. Pediatr. Otorhinolaryngol. 65:117-123. [DOI] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards (NCCLS). 2001. Performance standards for antimicrobial disk susceptibility tests. Approved standard M2-A7. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.Petersen, J. P., M. S. Kaltoft, J. C. Misfeldt, H. Schumacher, and H. C. Schonheyder. 2003. Community outbreak of perianal group A streptococcal infection in Denmark. Pediatr. Infect. Dis. J. 22:105-109. [DOI] [PubMed] [Google Scholar]
- 15.Pichichero, M. E., S. M. Marsocci, A. M. L. Murphy, W. Hoeger, J. L. Green, and A. Sorrento. 1999. Incidence of streptococcal carriers in private pediatric practice. Arch. Pediatr. Adolesc. Med. 153:624-628. [DOI] [PubMed] [Google Scholar]
- 16.Principi, N., P. Marchisio, G. C. Schito, and S. Manelli. 1999. Risk factor for carriage of respiratory pathogens in the nasopharynx of healthy children. Pediatr. Infect. Dis. J. 18:517-523. [DOI] [PubMed] [Google Scholar]
- 17.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Muray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valisena, S., C. Falci, A. Mazzariol, G. Cornaglia, C. E. Cocuzza, P. Nicoletti, R. Rescaldani, and R. Fontana. 1999. Molecular typing of erythromycin-resistant Streptococcus pyogenes strains with the M phenotype isolated in Italy. Eur. J. Clin. Microbiol. Infect. Dis. 18:260-264. [DOI] [PubMed] [Google Scholar]
- 19.Welsh, J., and M. McClelland. 1993. Characterization of pathogenic microorganisms by genomic fingerprinting using arbitrarily primed PCR, p. 595-602. In D. H. Persing, T. F. Smith, F. C. Tenover, and T. J. White (ed.), Diagnostic molecular microbiology: principles and applications. American Society for Microbiology, Washington, D. C.