Abstract
Study Objectives:
To develop models based on craniofacial photographic analysis for the prediction of obstructive sleep apnea (OSA).
Design:
Prospective cohort study.
Setting:
Sleep investigation unit in a university teaching hospital.
Patients:
One hundred eighty subjects (95.6% Caucasian) referred for the initial investigation of OSA were recruited consecutively.
Interventions:
Clinical assessment and frontal-profile craniofacial photographic analyses were performed prior to polysomnography. Prediction models for determining the presence of OSA (apnea-hypopnea index [AHI] ≥ 10) were developed using logistic regression analysis and classification and regression trees (CART).
Measurements and Results:
Obstructive sleep apnea was present in 63.3% of subjects. Using logistic regression, a model with 4 photographic measurements (face width, eye width, cervicomental angle, and mandibular length 1) correctly classified 76.1% of subjects with and without OSA (sensitivity 86.0%, specificity 59.1%, area under the receiver operating characteristics curve [AUC] 0.82). Combination of photographic and other clinical data improved the prediction (AUC 0.87), whereas prediction based on clinical assessment alone was lower (AUC 0.78). The optimal CART model provided a similar overall classification accuracy of 76.7%. Based on this model, 59.4% of the subjects were classified as either high or low risk with positive predictive value of 90.9% and negative predictive value of 94.7%, respectively. The remaining 40.6% of subjects have intermediate risk of OSA.
Conclusions:
Craniofacial photographic analysis provides detailed anatomical data useful in the prediction of OSA. This method allows OSA risk stratification by craniofacial morphological phenotypes.
Citation:
Lee RWW; Petocz P; Prvan T; Chan ASL; Grunstein RR; Cistulli PA. Prediction of obstructive sleep apnea with craniofacial photographic analysis. SLEEP 2009;32(1):46-52.
Keywords: Obstructive sleep apnea, craniofacial abnormalities, photogrammetry, prediction
OBSTRUCTIVE SLEEP APNEA (OSA) IS A VERY COMMON DISORDER ASSOCIATED WITH SNORING, REPETITIVE UPPER AIRWAY COLLAPSE DURING SLEEP, OXYGEN desaturation and sleep fragmentation.1,2 It is associated with increased cardiovascular morbidity, motor vehicle accident risk, and overall mortality.3 The diagnosis of OSA is cumbersome because of the need for specialist assessment and overnight monitoring in a sleep laboratory. The latter is expensive, labor intensive, and resource limited.4 As a result, the recognition of OSA in the community is low, and the majority of sufferers of OSA are as yet undiagnosed.5 Hence, there is a critical clinical need to develop methods to improve recognition and diagnosis of OSA in the community.
Prediction algorithms have been developed for risk stratification and screening of subjects for OSA. These algorithms are based mainly on data such as patient demographics, symptoms, and measures of obesity.6,7 While obesity is generally considered the major risk factor for OSA,8 craniofacial morphology is increasingly recognized as an important interacting factor in OSA pathogenesis.9–11 However, craniofacial or intraoral risk factors are included in only a minority of OSA prediction algorithms.12–14 This relates to the impractical nature of the currently available craniofacial assessment techniques. Furthermore, the suboptimal accuracy of these clinical algorithms and complexity of some measurement techniques limit their routine use in the clinical diagnosis of OSA.
We have developed a photographic analysis technique which allows detailed quantitative assessment of craniofacial morphology. These craniofacial photographic measurements appear to capture a number of risk factors relevant to OSA, including skeletal restriction, regional adiposity and obesity (see companion article “Craniofacial Phenotyping in Obstructive Sleep Apnea - A Novel Quantitative Photographic Approach”15). This technique could be useful in a number of clinical and research applications where high throughput is a requirement, such as in epidemiological research. We hypothesized that craniofacial photographic analysis would also be a useful technique in the prediction of OSA. The primary aim of this study was to develop prediction models based on craniofacial photographic measurements for the prediction of OSA, and to compare these to models based on other clinical data.
METHODS
Subjects
Subjects referred for polysomnography to a university teaching hospital for the initial investigation of OSA were recruited consecutively. Exclusion criteria included those with a known history of syndromal craniofacial abnormalities (e.g., Down syndrome), previous craniofacial surgery, and excessive facial hair that significantly obscured facial landmarks. Subjects of all ethnicity (self-reported) were included. Clinical assessment and the standardized photographic procedure were performed on all subjects on the same day as the polysomnography. All data collection and photographic analyses were carried out by a single investigator (RL) who was blinded to the result of polysomnography. Ethics approval was obtained from the institutional ethics committee, and written informed consent was obtained from all subjects.
Standardized Photographic Technique
Frontal and profile photographs of the head and neck were obtained with a standardized setup using a single-lens reflex digital camera (D70 with 18–70mm lens and external flash unit SB-29s; Nikon Corp., Japan). Prior to the photographs, certain bony and cartilaginous landmarks were pre-identified on the subjects by palpation and marked with a white tape. The standardized technique used for subject alignment and its test-retest reliability are described in the companion article “Craniofacial Phenotyping in Obstructive Sleep Apnea - A Novel Quantitative Photographic Approach.”15
Craniofacial Photogrammetry
Using image analysis software (Image J v1.36, NIH, Bethesda, MD), the photographs were examined for landmark digitization. Craniofacial landmarks of interest were captured as pixel coordinates (x, y) of the image which were then transferred to a custom-programmed spreadsheet for the computation of linear, angular, area, and polyhedral volume measurements. Pixel measurements were converted to metric dimensions based on a conversion scale of 52 pixels/cm. In addition to the 71 measurements obtained in the previous study, another 62 related craniofacial measurements were included (133 measurements in total). These measurements represented the dimensions and relationships of the various craniofacial regions including the face, mandible, maxilla, eyes, nose, head, and neck (see supplementary data: Appendix 1).
Clinical Assessment
Subject data on demographics, symptoms of OSA, comorbidities, and Epworth Sleepiness Scale (ESS) were obtained by questionnaire. Anthropometric assessment included neck circumference, waist circumference, and body mass index (BMI). Oropharyngeal assessment was performed with standardized techniques as described in previous studies.12,14,16 These included the assessment of the modified Mallampati class (MMC) (assessed with mouth wide open without protrusion of the tongue: [I] tonsils, pillars and soft palate were clearly visible; [II] uvula, pillars and upper pole were visible; [III] only part of the soft palate was visible; [IV] only the hard palate was visible), pharyngeal grade (assessed with mouth wide open and maximal protrusion of the tongue: [I] palatopharyngeal arch [ppa] intersects at the edge of the tongue; [II] ppa intersects at 25% or more of the tongue diameter; [III] intersects at 50% or more; [IV] intersects at 75% or more), tonsillar grade ([I] previous tonsillectomy or tonsils not seen; [II] tonsils visible behind the anterior pillars; [III] tonsils extended 75% of the way to the midline; [IV] tonsils completely obstructing airway), uvula size (considered enlarged if its approximate length is > 1.5 cm and width > 1 cm), tongue size (considered enlarged if its superior border was above the level of the mandibular occlusal plane, in association with tongue ridging) and the presence of overjet (present if there was a greater than 3 mm anterior-posterior distance between the upper and lower incisors during occlusion).
Polysomnography
Diagnostic polysomnography (PSG) was performed in accordance with previous studies and recommendations.17,18 Sleep staging was determined using standardized definitions.19 Apnea was defined as complete airflow cessation for ≥ 10 seconds with oxygen desaturation of at least 3% and/or associated with arousal. Hypopnea was defined as a reduction in amplitude of airflow or thoracoabdominal wall movement > 50% of the baseline measurement for > 10 seconds with an accompanying oxygen desaturation of at least 3%, and/or associated with arousals. Apnea-hypopnea index (AHI) was calculated as the total number of apneas and hypopneas per hour of sleep. Polysomnography scoring was performed by experienced accredited sleep technologists.
Data and Statistical Analysis
Predictive models for OSA were developed using 2 different statistical approaches, namely logistic regression (SPSS v13.0 for Windows, SPSS Inc., Chicago, IL, USA) and classification and regression tree (CART) analyses (Salford Systems [2006], CART Extended Edition Version 6.0, San Diego, California, USA).20 In both analyses, the presence of OSA was defined by an AHI ≥ 10 events per hour and those without OSA were defined by an AHI of < 10 events per hour.
Logistic Regression
All 133 photographic measurements were initially considered and a multistep process was employed to reduce the number of variables for further analysis. Multi-colinearity reduced the total number of measurements to 105. These were further reduced using forward stepwise regression of the log-transformed AHI (with the addition of 1) for each group of measurements (linear, angles, areas, and volumes). This approach led to the reduced set of 13 variables (see Appendix 1: L27, L61, L62, L65, AN18, AN19, AR3, AR9, AR14, AR20, V2, V13, and V19) for further analysis to derive the OSA prediction models. Forward likelihood ratio logistic regression of the remaining set of variables was employed to generate the photographic prediction model for OSA (Logistic Regression Model 1). Logistic Regression Model 2 was developed by replacing selected variables from Logistic Regression Model 1. Backward likelihood ratio logistic regression was used to develop the clinical and combined clinical/photographic prediction models (Logistic Regression Models 3 and 4). A total of 16 clinical variables were considered (age, sex, BMI, neck circumference, waist circumference, hypertension, diabetes mellitus, alcohol use ≥ 20g/day, witnessed apneas, ESS, MMC, pharyngeal grade, tonsillar grade, enlarged uvula, enlarged tongue, and overjet) for the clinical models. Classification accuracy, model characteristics, predictive values, and receiver operating characteristic (ROC) curves were calculated for each model. The probability threshold used for classification of OSA was 0.50.
Classification and Regression Tree (CART)
Classification and regression tree analysis is a predictive method that uses nonparametric techniques to evaluate data and account for complex relationships.21 In this type of analysis, there is progressive splitting of the population into subgroups that are based on the predictive independent variables. The variables chosen, discriminatory values of the variables, and the order in which the splitting occurs are all produced by the underlying mathematical algorithm to maximize predictive accuracy. A 10-fold cross-validation process was applied during the development of the CART models in order to minimize over-fitting of the data. This cross-validation procedure involved modelling using a proportion (90%) of the data and validation with the remaining (10%), and then repeating with a different one-tenth of the data until all data have been covered. All 133 photographic measurements and every value of splits of these measurements were analyzed with CART in order to construct models that can optimally separate subjects with and without OSA. The classification trees were built by continuing splitting of cases to achieve “terminal nodes” which are clusters of cases with or without OSA. Models using a single photographic measurement, multiple photographic measurements, and combinations of photographic and clinical measurements were constructed.
RESULTS
Subject Characteristics
A total of 180 subjects were included in the analysis; obstructive sleep apnea (AHI ≥ 10) was present in 114 subjects (63.3%). Three subjects were excluded from analysis (2 subjects did not complete the PSG; 1 subject was found to have central sleep apnea). Twelve subjects (5 females, 7 males) declined study participation. Characteristics of the subjects, clinical data, and polysomnographic indices are summarized in Table 1 and Appendix 2 (supplementary data). Details of the logistic regression models are contained in Appendix 3 (Supplementary data available at www.journalsleep.org).
Table 1.
Subject Characteristics
| N (%) | Mean ± SD | Range | |
|---|---|---|---|
| Number of subjects | 180 | - | - |
| Males (%) | 137 (76.1%) | - | - |
| Age (years) | 53.4 ± 14.3 | 20–86 | |
| Ethnicity – Caucasians | 172 (95.6%) | - | - |
| Anthropometry | |||
| BMI (kg/m2) | - | 29.3 ± 5.13 | 19.5–50.9 |
| Neck circumference (cm) | - | 41.3 ± 4.48 | 30.5–55.0 |
| Waist circumference (cm) | - | 105 ± 14.4 | 70.0–148 |
| Symptoms | |||
| Epworth Sleepiness Scale (ESS) | - | 8.82 ± 4.99 | 0–23 |
| Witnessed apneas | 86 (47.8%) | - | - |
| Polysomnography | |||
| Total AHI | - | 22.7 ± 21.7 | 0–110 |
| Minimum SaO2 (%) | - | 83.5 ± 10.1 | 36–99 |
Logistic Regression Analysis
Logistic Regression Model 1 – Calibrated Photographic Measurements
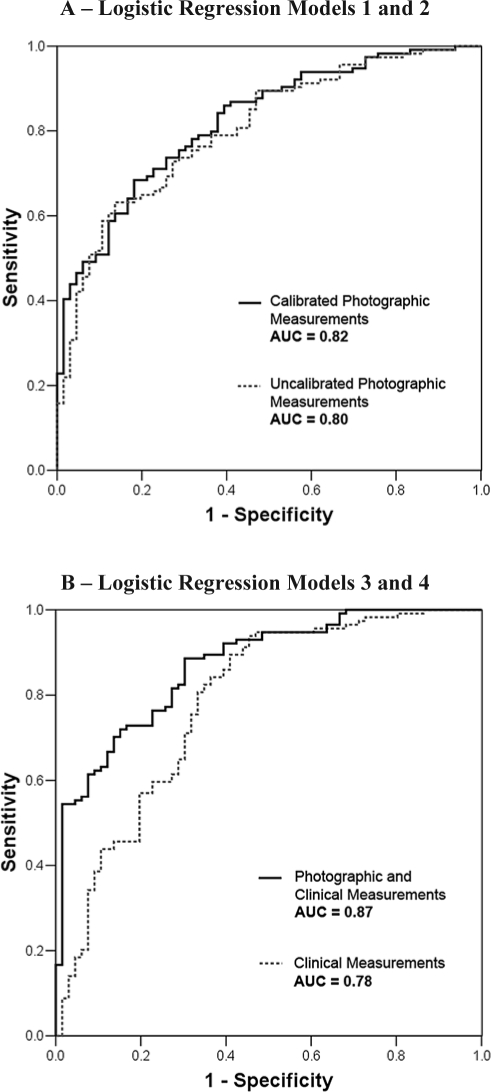
This model had the highest overall correct classification with the least number of variables. Probability of OSA (AHI ≥ 10) can be calculated by the formula: 1 / (1+e−z), where z = −9.235 + 1.442 (face width [cm]) − 2.872 (eye width [cm]) + 0.02 (cervicomental angle [degree]) − 1.224 (mandibular length 1 [cm]). This model classified 76.1% of the subjects correctly. It had a sensitivity of 86.0%, specificity of 59.1%, positive predictive value (PPV) of 78.4% and negative predictive value (NPV) of 70.9%. The area under the ROC curve was 0.82 (Figure 1A). In those who were incorrectly classified, the mean AHI was significantly lower than those who were correctly classified (12.9 versus 25.9 events/hr, P < 0.001); but there was no difference in age, BMI or neck circumference between these groups.
Figure 1.
Receiver Operating Characteristic (ROC) Curves for Logistic Regression Models. A – Logistic Regression Models 1 (Calibrated Photographic Measurements) and 2 (Uncalibrated Photographic Measurements); B – Logistic Regression Models 3 (Clinical Measurements) and 4 (Photographic and Clinical Measurements). AUC = area under the curve.
Logistic Regression Model 2 – Uncalibrated Photographic Measurements
In order to allow uncalibrated photographs to be used for OSA prediction, Logistic Regression Model 1 was simplified by using ratio and angular measurements instead of calibrated metric measurements. This model used the face width-eye width ratio to replace the individual measurements and mandibular-nasion angle 2 instead of mandibular length 1. Probability of OSA can be calculated using z = −4.516 + 1.528 (FER [face width-eye width ratio]) + 0.025 (cervicomental angle [degree]) − 0.262 (mandibular-nasion angle 2 [degree]). This model classified 71.1% of the subjects correctly. It had a sensitivity of 80.7%, specificity of 54.5%, PPV of 75.4%, and NPV of 62.1%. The area under the ROC curve was 0.80 (Figure 1A).
Logistic Regression Model 3 – Clinical Measurements
This prediction model for OSA was built using all the clinical variables. Age, BMI, and witnessed apneas were identified as independent predictors for OSA. This model classified 76.1% of the subjects correctly. It had a sensitivity of 86.0%, specificity of 59.1%, PPV of 78.4%, and NPV of 70.9%. The area under the ROC curve was 0.78 (Figure 1B), which was smaller than either of the photographic models (Logistic Regression Models 1 and 2).
Logistic Regression Model 4 – Photographic and Clinical Measurements
This model was developed with the reduced set of 13 photographic measurements and all the clinical variables. Similar to Logistic Regression Model 1, face width, eye width, and mandibular length 1 remained independent photographic predictors for OSA. Witnessed apnea and MMC were the only clinical variables further contributing to the model, although the contribution of MMC was small. This combined photographic and clinical model classified 79.4% of the subjects correctly. It had a sensitivity of 85.1%, specificity of 69.7%, PPV of 82.9%, and NPV of 73.0%. The area under the ROC curve was highest at 0.87 (Figure 1B).
Classification and Regression Tree (CART) Analysis
CART Model 1 – Single Photographic Measurement
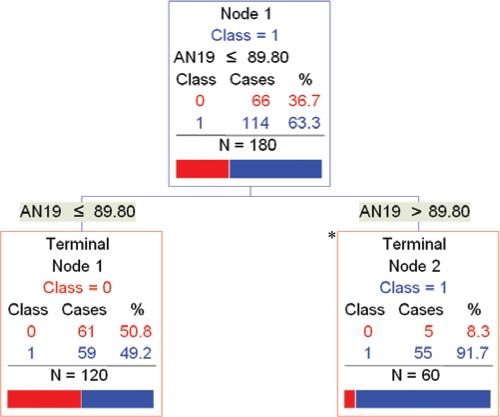
The simplest CART model used a single photographic measurement (mandibular width-length angle) to classify 64.4% of the subjects correctly with 2 terminal nodes (Figure 2). In this model, if the mandibular width-length angle was > 89.8 degrees, 55 out of 60 subjects (91.7%) had OSA. If the angle was ≤ 89.8 degrees, 61 of 120 subjects (50.8%) did not have OSA. This model had a sensitivity of 48.2%, specificity of 92.4%, PPV of 91.7%, and NPV of 50.8%. In other words, one-third of the cases (60 of 180 subjects) were classified as having a high risk of OSA of 91.7%.
Figure 2.
CART Model 1: Single Photographic Measurement. Fifty-five out of 60 (91.7%) subjects in terminal node 2 (*) had OSA. (AN19 = mandibular width-length angle [degrees]. Class 0 = No OSA; Class 1 = OSA.)
CART Model 2 – Multiple Photographic Measurements
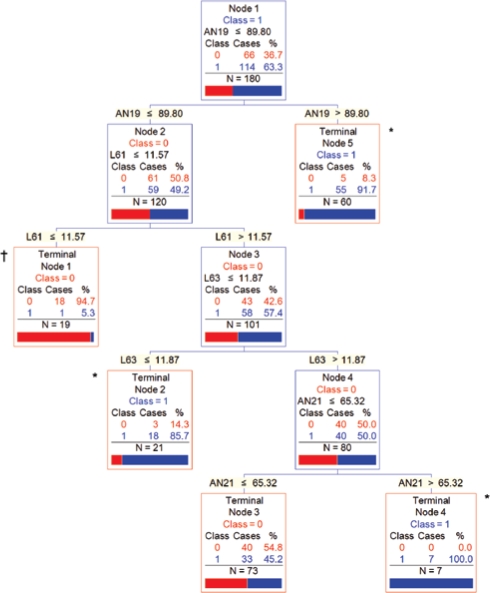
This model used 4 photographic measurements (mandibular width-length angle, neck depth, mandible width, face width-lower face depth angle) to classify 76.7% of the subjects correctly with 5 terminal nodes (Figure 3). This model had a sensitivity of 70.2%, specificity of 87.9%, PPV of 90.9%, and NPV of 63.0%. Based on this model, 80 of 88 subjects (90.9%) in terminal nodes 2, 4, and 5 collectively had OSA and 18 of 19 subjects (94.7%) in terminal node 1 did not have OSA. The remaining 73 subjects at terminal node 3 had intermediate risk of OSA of 45.2%. In other words, 59.4% (107 of 180) of the subjects were classified as either high or low risk with PPV of 90.9% and NPV of 94.7%, respectively.
Figure 3.
CART Model 2: Multiple Photographic Measurements. Collectively, 80 out of 88 (90.9%) subjects in terminal nodes 2, 4, and 5 (*) had OSA and 18 of 19 (94.7%) subjects in terminal node 1 (†) did not have OSA. (AN19 = mandibular width-length angle [degrees]; L61 = neck depth [cm]; L63 = mandible width [cm]; AN21 = face width-lower face depth angle [degrees]. Class 0 = No OSA; Class 1 = OSA.)
Other CART Models
A more complex CART Model (with 8 terminal nodes) based on 2 uncalibrated photographic measurements (FER [face width-eye width ratio] and mandibular-nasion angle 2) classified 76.1% of the subjects correctly. Another CART model combining clinical and multiple photographic measurements (mandibular width-length angle, FER, anterior neck space area, total face height - vertical and witnessed apneas) classified 81.1% of the subjects correctly.
DISCUSSION
Craniofacial photographic analysis provides detailed quantitative information of the craniofacial morphology. This study demonstrates that anatomical data useful for the prediction of OSA can be obtained from photographic analysis. Both methods of modelling provide correct subject classification in approximately 76%, based solely on the photographic measurements. A summary of all the prediction models is presented in Table 2.
Table 2.
Summary of the Logistic Regression and Classification and Regression Tree (CART) Models in the Prediction of OSA
| Number of Photographic Variables | Number of Clinical Variables | Overall Correct Classification | ROC AUC | |
|---|---|---|---|---|
| Logistic Regression Model 1 – Calibrated Photographic Measurements | 4 | - | 76.1% | 0.82 |
| Logistic Regression Model 2 – Uncalibrated Photographic Measurements† | 4 | - | 71.1% | 0.80 |
| Logistic Regression Model 3 – Clinical Measurements | - | 3 (Age, BMI, WA) | 76.1% | 0.78 |
| Logistic Regression Model 4 – Photographic and Clinical Measurements | 3 | 2 (WA, MMC) | 78.3% | 0.87 |
| CART Model 1 – Single Photographic Measurement | 1 | - | 64.4% | 0.68 |
| CART Model 2 – Multiple Photographic Measurements | 4 | - | 76.7% | 0.84 |
model built using measurements that do not require calibration (e.g., ratios and angular measurements); ROC AUC = receiver operating characteristic, area under the curve; BMI = body mass index; WA = witnessed apneas; MMC = modified Mallampati class
Prediction of OSA using only photographic measurements performs better than a clinical model as suggested by the ROC analysis. Combination of the photographic and clinical data may further improve prediction, although in both modelling techniques, witnessed apneas was the only questionnaire item of additional predictive value. Notably, the combined photographic and clinical models did not identify any demographic data, obesity-related (e.g., BMI or neck circumference) or oropharyngeal measurements (except a small contribution of the MMC in logistic regression model 4) as independent predictors. This suggests craniofacial photographic measurements, which capture composite elements of craniofacial structure and regional adiposity, could be more important predictors for OSA.
The photographic measurements identified in the logistic regression models appear to capture a range of anatomical risk factors for OSA.22–25 These include general and regional obesity (face width), fat deposition on the anterior neck (cervicomental angle), mandibular deficiency (mandibular length), and possibly inferior hyoid position (cervicomental angle). Eye width as a predictor for OSA has not been previously reported. This measurement may reflect the dimensions of the bony orbit, which is in turn closely related to the cranial base; the latter is well described to exert considerable influence on craniofacial development.26
Classification and regression tree (CART) analysis identified photographic measurements that similarly capture anatomical risk factors for OSA. For example, mandibular width-length angle and face width-lower face depth angle capture composite anatomy of regional facial obesity and mandibular restriction. These analyses also provide a decision tree approach for OSA risk stratification, such that those at high or low risk (∼60% of subjects in CART Model 2) and intermediate risk (∼40%) can be identified. In addition, these analyses also illustrate that in subjects with OSA, there are distinct morphological phenotypes with different craniofacial characteristics (e.g., subject clusters at terminal nodes 2, 4, and 5 in CART Model 2). Methods of subject stratification by craniofacial morphological phenotypes may potentially have novel research and clinical applications, such as a craniofacial phenotyping tool in epidemiological research or a diagnostic screening tool in clinical practice. However, to realize such applications requires the simplification of the technique we have described.
Prediction models for OSA have been developed largely based on demographic data, symptoms and anthropometric measures of obesity.6,7,27–29 While these parameters are easier to obtain, the computation of these algorithms remains complex for the clinical setting. Predictive ability varies greatly between these models, with sensitivities ranging from 76% to 96% and specificities ranging from 13% to 54%.30 This may be related to differences in the variables used, statistical techniques, and subject population in which the models were developed. Overall, given the limitations and suboptimal predictive abilities of such clinical models, their routine use in practice remains limited.
Despite the importance of craniofacial and intra-oral factors in determining OSA risk, these factors are only included in a few prediction algorithms.12–14,31 Techniques available for obtaining these measurements remain restricted with regard to their qualitative nature,12 radiation risk31 and complexity.13 Furthermore, a simplified bedside method of craniofacial assessment appeared to leave a significant proportion (∼60%) of subjects unclassified.14
The photographic technique used in this study overcomes some of these limitations. In addition to its safety and relative availability, photographic analysis provides detailed quantitative data across various craniofacial regions. Using these photographic data with the CART models could distinctly divide subjects into different OSA risk categories. Those with high or low clinical risk of OSA may undergo alternative diagnostic evaluations, thereby potentially reducing the need for polysomnography. While models presented in this study represented the optimal balance of test characteristics, the ultimate choice of cut-off probability (logistic regression models) or discriminatory values of measurements (CART) to maximize sensitivity or specificity will depend on the clinical context in which the models are to be used. Craniofacial photographic prediction of OSA may also have higher accuracy, compared to the clinical models, in certain ethnic populations where OSA risk is less attributed to obesity.32,33 Uncalibrated measurements such as craniofacial ratios and angles can also provide OSA prediction. This method may potentially allow prediction of OSA with photographs taken using any camera (e.g., photographs taken by patients or by driver license authority), although further validation is required.
Further studies will be needed to address some of the limitations of this study. Firstly, prospective validation of the models will need to be undertaken in both sleep clinic and community populations to assess clinical utility. Secondly, the photographic models will need to be tested at different AHI levels in combination with OSA symptoms with an aim to develop diagnostic pathways involving portable sleep monitoring. Subjects in this study were at higher risk for OSA as they were recruited from a sleep laboratory population, although the prevalence of OSA in this study was comparable to those from other institutions. Overall, our cohort of subjects had lower ESS and BMI and thus might be at the milder end of the spectrum of disease, including a proportion of subjects who were minimally symptomatic. However, as all the subjects recruited had been referred for polysomnography after assessment through usual clinical care involving their primary care physician and sleep physician or pulmonologist, we believe the sample was representative of the local population demographics. While ethnicity is an important consideration, the majority of the subjects in this study were of Caucasian background. Future studies will need to examine the utility of the photographic models in non-Caucasian populations. Limitations in regard to the craniofacial photographic analysis technique may include possible errors from subject alignment, camera lens distortion or projection errors. While these issues may affect the accuracy of the technique, they are generally minor as demonstrated in our previous study (Craniofacial Phenotyping in Obstructive Sleep Apnea - A Novel Quantitative Photographic Approach15). The non-linear nature of craniofacial anatomy cannot be captured by these measurements. In addition, measurements relate mainly to size rather than shapes. The latter may provide additional insights into the OSA craniofacial phenotype.
In relation to the statistical approaches, CART analysis provided an alternate approach to examine the data based on progressive binary splitting of data, therefore avoided the problems of colinearity and parametric assumptions with logistic regression analysis. Furthermore, a distinct advantage of CART is that it is well suited to the generation of decision rules.34,35 Notably, both statistical approaches of modelling resulted in similar predictive accuracy and concordance. While any methods of modelling will produce a better fit of the current data compared to new datasets, the 10-fold cross-validation procedure in the CART modelling would minimize over-fitting of the data. In addition, the cross-validation process provided an assessment of the accuracy of the “within model” prediction, which was in the range of 61% to 76%. A subsequent prospective study will be required to examine the accuracy and clinical utility of all the prediction models. Other methods of data analysis (e.g., principal component analysis) may provide new insights into the influence of craniofacial phenotype on OSA risk.
In summary, using a novel craniofacial photographic analysis technique, we have developed potentially useful clinical prediction models for the identification of OSA based on craniofacial morphological phenotypes. This approach may potentially have other research and clinical applications in OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Lee is listed as an inventor on a patent application covering aspect of the material in this manuscript, the rights of which are owned by Dr. Lee's institution (Northern Sydney Central Coast Area Health Service, NSW, Australia). Dr. Cistulli is listed as co-inventor on a patent application covering aspect of the material in this manuscript, the rights of which are owned by Dr. Cistulli's institution (Northern Sydney Central Coast Area Health Service, NSW, Australia). Dr. Cistulli contributed to the development of an oral appliance for the treatment of OSA which is being commercialized by SomnoMed Ltd. Dr. Cistulli has consulted for and been on the advisory board of SomnoMed and has a financial interest in the company. Dr. Cistulli has received research support from ResMed and the use of equipment from ResMed and SomnoMed. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Staff of the Sleep Investigation Unit, Department of Respiratory Medicine, Royal North Shore Hospital
Scholarship supported by the National Health and Medical Research Council (NHMRC) of Australia
REFERENCES
- 1.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–45. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 4.Flemons WW, Douglas NJ, Kuna ST, Rodenstein DO, Wheatley J. Access to diagnosis and treatment of patients with suspected sleep apnea. Am J Respir Crit Care Med. 2004;169:668–72. doi: 10.1164/rccm.200308-1124PP. [DOI] [PubMed] [Google Scholar]
- 5.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–6. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 6.Maislin G, Pack AI, Kribbs NB, et al. A survey screen for prediction of apnea. Sleep. 1995;18:158–66. doi: 10.1093/sleep/18.3.158. [DOI] [PubMed] [Google Scholar]
- 7.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 8.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–9. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 9.Dempsey JA, Skatrud JB, Jacques AJ, et al. Anatomic determinants of sleep-disordered breathing across the spectrum of clinical and nonclinical male subjects. Chest. 2002;122:840–51. doi: 10.1378/chest.122.3.840. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson KA, Ono T, Lowe AA, Ryan CF, Fleetham JA. The relationship between obesity and craniofacial structure in obstructive sleep apnea. Chest. 1995;108:375–81. doi: 10.1378/chest.108.2.375. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe T, Isono S, Tanaka A, Tanzawa H, Nishino T. Contribution of body habitus and craniofacial characteristics to segmental closing pressures of the passive pharynx in patients with sleep-disordered breathing. Am J Respir Crit Care Med. 2002;165:260–5. doi: 10.1164/ajrccm.165.2.2009032. [DOI] [PubMed] [Google Scholar]
- 12.Friedman M, Tanyeri H, La Rosa M, et al. Clinical predictors of obstructive sleep apnea. Laryngoscope. 1999;109:1901–7. doi: 10.1097/00005537-199912000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Kushida CA, Efron B, Guilleminault C. A predictive morphometric model for the obstructive sleep apnea syndrome. Ann Intern Med. 1997;127:581–7. doi: 10.7326/0003-4819-127-8_part_1-199710150-00001. [DOI] [PubMed] [Google Scholar]
- 14.Tsai WH, Remmers JE, Brant R, Flemons WW, Davies J, Macarthur C. A decision rule for diagnostic testing in obstructive sleep apnea. Am J Respir Crit Care Med. 2003;167:1427–32. doi: 10.1164/rccm.200112-110OC. [DOI] [PubMed] [Google Scholar]
- 15.Lee RWW, Chan ASL, Grunstein RR, Cistulli PA. Craniofacial phenotyping inobstructive sleep apnea—a novel quantitative photographic approach. Sleep. 2009;32:37–45. [PMC free article] [PubMed] [Google Scholar]
- 16.Schellenberg JB, Maislin G, Schwab RJ. Physical findings and the risk for obstructive sleep apnea. The importance of oropharyngeal structures. Am J of Respir Crit Care Med. 2000;162:740–8. doi: 10.1164/ajrccm.162.2.9908123. [DOI] [PubMed] [Google Scholar]
- 17.Mehta A, Qian J, Petocz P, Darendeliler MA, Cistulli PA. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1457–61. doi: 10.1164/ajrccm.163.6.2004213. [DOI] [PubMed] [Google Scholar]
- 18.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 19.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 20.San Diego, California, USA: Salford Systems; CART Extended Edition Version 6.0 (2006) http://www.salford-systems.com. [Google Scholar]
- 21.Lemon SC, Roy J, Clark MA, Friedmann PD, Rakowski W. Classification and regression tree analysis in public health: methodological review and comparison with logistic regression. Ann Behav Med. 2003;26:172–81. doi: 10.1207/S15324796ABM2603_02. [DOI] [PubMed] [Google Scholar]
- 22.Miles PG, Vig PS, Weyant RJ, Forrest TD, Rockette HE., Jr. Craniofacial structure and obstructive sleep apnea syndrome—a qualitative analysis and meta-analysis of the literature. Am J Orthod Dentofacial Orthop. 1996;109:163–72. doi: 10.1016/s0889-5406(96)70177-4. [DOI] [PubMed] [Google Scholar]
- 23.Mortimore IL, Marshall I, Wraith PK, Sellar RJ, Douglas NJ. Neck and total body fat deposition in nonobese and obese patients with sleep apnea compared with that in control subjects. Am J Respir Crit Care Med. 1998;157:280–3. doi: 10.1164/ajrccm.157.1.9703018. [DOI] [PubMed] [Google Scholar]
- 24.Riha RL, Brander P, Vennelle M, Douglas NJ. A cephalometric comparison of patients with the sleep apnea/hypopnea syndrome and their siblings. Sleep. 2005;28:315–20. [PubMed] [Google Scholar]
- 25.Stradling JR, Crosby JH. Predictors and prevalence of obstructive sleep apnoea and snoring in 1001 middle aged men. Thorax. 1991;46:85–90. doi: 10.1136/thx.46.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nie X. Cranial base in craniofacial development: developmental features, influence on facial growth, anomaly, and molecular basis. Acta Odontol Scand. 2005;63:127–35. doi: 10.1080/00016350510019847. [DOI] [PubMed] [Google Scholar]
- 27.Flemons WW, Whitelaw WA, Brant R, Remmers JE. Likelihood ratios for a sleep apnea clinical prediction rule. Am J Respir Crit Care Med. 1994;150:1279–85. doi: 10.1164/ajrccm.150.5.7952553. [DOI] [PubMed] [Google Scholar]
- 28.Hoffstein V, Szalai JP. Predictive value of clinical features in diagnosing obstructive sleep apnea. Sleep. 1993;16:118–22. [PubMed] [Google Scholar]
- 29.Rodsutti J, Hensley M, Thakkinstian A, D'Este C, Attia J. A clinical decision rule to prioritize polysomnography in patients with suspected sleep apnea. Sleep. 2004;27:694–9. doi: 10.1093/sleep/27.4.694. [DOI] [PubMed] [Google Scholar]
- 30.Rowley JA, Aboussouan LS, Badr MS. The use of clinical prediction formulas in the evaluation of obstructive sleep apnea. Sleep. 2000;23:929–38. doi: 10.1093/sleep/23.7.929. [DOI] [PubMed] [Google Scholar]
- 31.Xu Z, Cheuk DKL, Lee SL. Clinical evaluation in predicting childhood obstructive sleep apnea. Chest. 2006;130:1765–71. doi: 10.1378/chest.130.6.1765. [DOI] [PubMed] [Google Scholar]
- 32.Ip MS, Lam B, Lauder IJ, et al. A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong. Chest. 2001;119:62–9. doi: 10.1378/chest.119.1.62. [DOI] [PubMed] [Google Scholar]
- 33.Udwadia ZF, Doshi AV, Lonkar SG, Singh CI. Prevalence of sleep-disordered breathing and sleep apnea in middle-aged urban Indian men. Am J Respir Crit Care Med. 2004;169:168–73. doi: 10.1164/rccm.200302-265OC. [DOI] [PubMed] [Google Scholar]
- 34.Avila PC, Segal MR, Wong HH, Boushey HA, Fahy JV. Predictors of late asthmatic response. Logistic regression and classification tree analyses. Am J Respir Crit Care Med. 2000;161:2092–5. doi: 10.1164/ajrccm.161.6.9909056. [DOI] [PubMed] [Google Scholar]
- 35.Fonarow GC, Adams KF, Jr, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–80. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]