Abstract
Study Objectives:
To examine racial differences in sleep in a large cohort of midlife women and to evaluate whether indices of socioeconomic status (SES) are associated with racial differences in sleep.
Design:
Cross-sectional study.
Setting:
Participants' homes.
Participants:
Caucasian (n = 171), African American (n = 138) and Chinese women (n = 59).
Interventions:
None.
Measurements:
Sleep quality was assessed with the Pittsburgh Sleep Quality Index. Polysomnographically assessed sleep duration, continuity, architecture, and NREM electroencephalograhic (EEG) power were calculated over multiple nights. Sleep disordered breathing and periodic leg movements were measured on a separate night. Linear regression analysis was used to model the independent and synergistic effects of race and SES on sleep after adjusting for other factors that impact sleep in midlife women. Indices of SES were self-reported educational attainment and financial strain.
Results:
Sleep was worse in African American women than Caucasian participants as measured by self-report, visual sleep stage scoring, and NREM EEG power. Slow wave sleep differences were also observed between Chinese and Caucasian participants. Racial differences persisted after adjustment for indices of SES. Although educational attainment was unrelated to sleep, financial strain was associated with decreased sleep quality and lower sleep efficiency. Financial strain-by-race interactions were not statistically significant, suggesting that financial strain has additive effects on sleep, independent of race.
Conclusions:
Independent relationships between race and financial strain with sleep were observed despite statistical adjustment for other factors that might account for these relationships. Results do not suggest that assessed indices of SES moderate the race-sleep relationship, perhaps due to too few women of low SES in the study.
Citation:
Hall MH; Matthews KA; Kravitz HM; Gold EB; Buysse DJ; Bromberger JT; Owens JF; Sowers M. Race and financial strain are independent correlates of sleep in midlife women: the SWAN sleep study. SLEEP 2009;32(1):73-82.
Keywords: sleep, power spectral analysis, PSQI, midlife women, menopause, race, SES
MOUNTING EVIDENCE SUGGESTS THAT SLEEP DIFFERS SIGNIFICANTLY ACROSS RACIAL AND ETHNIC GROUPS IN WAYS THAT MAY BE IMPORTANT TO health and functioning.1–5 Most consistent among these effects is a marked decrease in laboratory-assessed slow wave sleep and a concomitant increase in stages 1 and 2 of NREM sleep in African Americans compared to Caucasians.3–7 Other dimensions of sleep shown to differ between African Americans and Caucasians include sleep duration, continuity and subjective sleep quality, although these relationships are not as strong and consistent as those observed for sleep architecture.8 Far fewer studies have compared sleep across other racial and ethnic groups. Hale and Do9 evaluated data from 32,749 respondents to the 1990 health promotion supplement of the National Health Interview Survey (NHIS) and found that, compared to Caucasians, the prevalence of short sleepers (< 6 hours/night) was higher among all racial and ethnic minorities surveyed including African Americans, Hispanics and non-Hispanic “others.” As measured by one night of in-home polysomnography (PSG) collected in the population-based Sleep Heart Health Study (SHHS), Redline and colleagues reported that American Indians and African Americans had lighter sleep than Caucasians, Hispanics, or Asian Americans.10 Despite growing evidence that sleep differs by race and/or ethnic minority status, few studies have evaluated possible causes or correlates of these differences.
It has been suggested that socioeconomic status (SES), which is closely tied to race and ethnic minority status in many countries, including the United States, may play an important role in the relationship between minority racial/ethnic status and disturbed sleep.5,6,11 Indeed, a number of studies have reported significant associations among subjective sleep complaints and various indices of SES including lower education, occupational status and income, although these studies did not evaluate the influence of race on the SES-sleep relationship.12–17 Three recent studies reported that both race/ethnicity and traditional measures of SES, income, and education, were significant correlates of behavioral or PSG-assessed indices of sleep.2,6,18 For instance, Mezick and colleagues evaluated the independent effects of race and SES on sleep in a cohort of midlife men and women who were self-identified as either non-Hispanic Caucasian or African American. Lower SES, as measured by a composite score of income and education, was associated with greater PSG-assessed wakefulness after sleep onset, after adjusting for other confounding variables, including race. Sleep quality, duration, and architecture were unrelated to SES in the Mezick et al., study. These studies provide some support for the hypothesis that certain dimensions of sleep may be related to traditional markers of SES, independent of race. The extent to which other dimensions of SES affect, or are affected by, sleep have received less empirical attention.
We have hypothesized that financial strain, which is a key chronic stressor associated with lower SES, may be a sensitive marker of the SES-sleep relationship.11 We reported that financial strain, operationalized as difficulties with paying for basics like food and housing, was a significant correlate of increased subjective sleep quality complaints in a sample of 462 midlife women, one-third of whom were African American.11 In multivariate models, financial strain attenuated the relationship between income and sleep quality, which is consistent with the hypothesis that stress pathways are important to the SES-sleep relationship. Stress pathways by which financial strain might interfere with sleep include increased worries and negative affect, as well as endocrine and autonomic dysregulation.19–24 More recently, we demonstrated that chronic and ongoing financial strain was associated with significant decreases in PSG-assessed sleep efficiency in a large sample of community-dwelling elders, after adjusting for a host of variables known to impact sleep in late life.25 Although these studies suggest that financial strain may be an important correlate of sleep, the extent to which financial strain plays a role in the SES-sleep or race-sleep relationship has not been evaluated.
The present study evaluated relationships among race and markers of SES in relation to sleep in a multiracial sample of midlife women enrolled in the SWAN Sleep Study, which was designed to characterize sleep during the menopausal transition. Sleep during the menopausal transition provides an opportune model for evaluating the influence of race and SES on sleep because subjective sleep complaints and some sleep disorders are much more frequent in perimenopausal and menopausal women.26–30 Moreover, sleep disturbances that arise during the menopausal transition may be a marker for the development of later chronic health conditions and declines in general health and functioning occurring in the postmenopausal years. SWAN Sleep Study participants included African American, Caucasian, and Chinese women. Measures of sleep were subjective sleep quality, as measured by the validated Pittsburgh Sleep Quality Index (PSQI),31 and indices of sleep duration, continuity, and architecture including NREM electroencephalographic (EEG) power, as measured by multinight in-home PSG. We hypothesized that African American race would be associated with worse sleep, compared to Caucasian and Chinese participants. We further hypothesized that markers of SES, as measured by educational attainment and financial strain, would affect the race-sleep relationship. Specifically, we hypothesized that lower educational attainment and financial strain would attenuate observed relationships among race and sleep after adjusting for other factors that might confound relationships among race, SES, and sleep in midlife women.
METHODS
Study Participants
The SWAN Sleep Study is a cross-sectional study of sleep in a multiracial sample of midlife women. It is an ancillary study of the Study of Women's Health across the Nation (SWAN), which is a community-based, longitudinal study of the menopausal transition and its consequences to health and functioning. The core SWAN Study was conducted at 7 clinical sites in the United States as previously described.32 The SWAN Sleep Study enrolled a cohort of 370 Caucasian, African American and Chinese participants from 4 study sites: Chicago, IL; Detroit area, MI; Oakland, CA; and Pittsburgh, PA. Exclusions for the Sleep Study were: post-menopausal status; current menopausal hormone replacement therapy (MHT) use; current chemotherapy or radiation; current oral corticosteroid use; regular nocturnal shiftwork; regular consumption of > 4 alcoholic drinks/day; and noncompliance with Core SWAN procedures (missed > 50% of annual visits, refused annual visit blood draw). During the final year of SWAN Sleep Study recruitment, eligibility criteria were revised to allow inclusion of postmenopausal women not currently using MHT. Sleep Study participants did not differ markedly from Core SWAN participants with regard to age, self-assessed sleep quality, self-reported health status, symptoms of depression, hypertension, or diabetes. Sleep Study participants tended to have slightly higher body mass index (BMI) than those who did not participate. Informed consent was obtained in accordance with approved protocols and guidelines of the Institutional Review Board at each participating institution. Participants were paid for their participation.
Study Protocol
The SWAN Sleep Study protocol was conducted across an entire menstrual cycle or 35 days, whichever was shorter. The protocol was initiated within 7 days of the start of menstrual bleeding in participants who were still cycling regularly. Irregularly- and non-cycling women were scheduled at their convenience. Unattended PSG sleep studies were conducted in participants' homes on the first 3 nights of the protocol. In the event of study failure, repeat PSG studies were conducted, when possible. Symptoms of depression, anxiety and stress were measured by self-report.
Sociodemographic Characteristics
Race (Caucasian, African American, Chinese) was defined by self-identification. Each site recruited Caucasian participants while minority population varied by site: African American participants were recruited from the Chicago, Detroit and Pittsburgh sites; all Chinese participants were recruited from the Oakland site. The majority of the Chinese participants were born in the United States (30% emigrated from China). Household income and education were assessed by self-report during the Core SWAN baseline interview. Educational attainment, rather than income, was used as an indicator of SES due to geographic disparities in earnings and cost of living across study sites. Educational attainment was scored categorically as (a) women with less than or possessing a high school dipoloma or its equivalent; (b) women with some college education or possessing an associate's degree; (c) women with a bachelor's degree; and (d) women with an advanced degree (i.e., Master's, PhD, MD). Given its distribution across racial groups, educational attainment was dichotomized as a comparison of those participants with a college or advanced degree (n = 174) versus women without a college degree (n = 171). This latter group consisted of women with a high school degree or equivalent (n = 60) and women with some college or an associate's degree (n = 111). Financial strain11,25 was derived from the Core SWAN interview question “How hard is it for you to pay for the very basics like food, housing, medical care and heating?” For analyses, the 3-level response was dichotomized as “somewhat hard” to “very hard” versus “not hard at all.”
Sleep Characteristics
Sleep data relevant to the current report include subjective sleep quality and PSG-assessed indices of sleep duration, continuity, architecture, power spectral analysis of NREM EEG, sleep disordered breathing, and periodic limb movements. The 19-item Pittsburgh Sleep Quality Index (PSQI)31 was administered twice during the SWAN Sleep Study, once at the beginning of the study and once on the last day of the study. Average global sleep quality ratings (with a possible range of 0–21; higher scores represent more severe sleep complaints) were computed for each participant. Global scores for both PSQI assessments were highly correlated (r = 0.72, P < 0.001). Polysomnographic sleep data were collected with Vitaport-3 (TEMEC VP3) ambulatory monitors. SWAN Sleep Study staff visited participants in their homes on each night of sleep studies to apply electrodes and calibrate monitors. Participants slept in their own beds, under their usual circumstances, at their habitual sleep and wake times, as determined by self-report. Study restrictions precluded participants sleeping in water beds, under electric blankets, or with pets, due to the possible influence of these factors on monitoring equipment. Upon rising in the morning, participants removed study equipment and turned off the recorder. Quality assurance assessments, scoring, and processing of all sleep study records was performed at the University of Pittsburgh Neuroscience - Clinical and Translational Research Center (N-CTRC).
Polysomnographic signals collected on each study night included bilateral central referential EEG channels (C3 and C4, referenced to A1 tied to A2), electro-oculogram (EOG), submentalis electromyogram (EMG), and electrocardiogram (EKG). Additional signals were collected on the first night of sleep studies for the assessment of sleep disordered breathing (SDB; nasal pressure cannula, oral-nasal thermistors, fingertip oximeter, and abdominal and thoracic respiratory effort, as measured by inductance plethysmography) and periodic leg movements (PLM; bilateral EMG of anterior tibialis). Bilateral EEG derivations and data collection on multiple study nights were used to minimize the loss of data due to dislodging of electrodes during the night and technical failures, which can occur during the course of unattended in-home sleep studies. Overall, PSG data loss was less than 5%.
Visual sleep stage scoring was conducted by trained PSG technologists with established reliability (intraclass correlation coefficients for wake, NREM, and REM were each above 0.90), who were blind to participant characteristics (e.g., menopausal status, racial group). Sleep was scored in 20-sec epochs using standard scoring criteria,33 supplemented by apnea-hypopnea criteria derived from American Academy of Sleep Medicine recommendations34 and standard rules for scoring periodic limb movements associated with arousals from sleep.35 Measures related to SDB (apnea-hypopnea index; AHI) and PLMAI (periodic leg movement arousal index) were derived from one night, whereas all other summary sleep variables were the average of non-SDB/PLMAI nights. Other than measures of SDB and PLMAI, data collected on Night 1 was not used in the present analyses due to the disruptive effects of breathing and limb movement sensors on sleep, as well as the first night effect, which has been observed during in-home and laboratory sleep studies.36–38
Summary measures of visually scored sleep included standard indices of sleep duration, continuity and architecture. Time in bed was calculated as time from reported lights out (“good night time;” and confirmation of PSG signals consistent with reduced activity) to time to reported awakening from sleep (“good morning time;” and confirmation of PSG signals consistent with increased activity). Time spent asleep was calculated as total minutes of any sleep stage after sleep onset. Sleep continuity measures included sleep latency (time from beginning of the recording period to the first of 10 consecutive minutes of stage 2 or stage 3−4 sleep interrupted by no more than 2 minutes of stage 1 or wakefulness), wakefulness after sleep onset (WASO; total minutes of wakefulness between sleep onset and good morning time), and sleep efficiency (time spent asleep/time in bed × 100). Number of arousals from sleep was calculated from sleep onset to the final morning awakening. Measures of sleep architecture included percent of time spent asleep in NREM stages 1, 2, and 3+4, as well as REM sleep.
Additionally, spectral analysis of the EEG was performed to quantify power in the δ (0.05–4.0 Hz) and β (16–32 Hz) bands during NREM sleep. Briefly, modified periodograms were computed using the Fast Fourier transform (FFT) of non-overlapping 4-sec epochs of the sleep EEG. This software includes a validated automated artifact rejection routine.39 EEG spectra were obtained for each artifact-free 4-sec epoch and were then aligned with 20-sec visually scored sleep stage data. The δ band was selected for analysis due to previous reports of decreased visually scored slow wave sleep in African Americans compared to Caucasians.8 Beta power was selected as a measure of hyperarousal during sleep and has been associated with psychological stress and insomnia.40–45 Relative power (each band was divided by total power) was used in the present analyses in order to account for individual differences in overall EEG power (QEEG).45
Covariates
Age at the time of the sleep study was used to adjust for age-related changes in subjective and PSG-assessed sleep.46 Menopausal status, which has been associated with sleep complaints,27,47,48 was determined by bleeding patterns reported during Core SWAN assessments. Participants were categorized as premenopausal, early perimenopausal, late perimenopausal, and postmenopausal.49 Due to the limited number of premenopausal participants (n = 21), pre- and early perimenopausal participants were combined into one group, which was used as the referent category for the late perimenopausal and postmenopausal groups in data analysis. Participants whose bleeding patterns were altered by prior menopausal hormone (MHT) use or hysterectomy (n = 17) were categorized as postmenopausal. Vasomotor symptoms were measured by sleep diary entries on sleep study nights. Participants were asked to report the number of hot flashes and the number of night sweats they experienced on the previous night of sleep. Vasomotor symptoms were averaged for PSG nights and dichotomized as none reported versus at least one reported, due to the distributional properties of vasomotor symptoms in this sample. Body mass index (BMI), which is a significant correlate of sleep,50 was calculated as weight in kilograms/height in meters-squared during the Core SWAN assessment and treated as a continuous variable in analyses. Self-reported symptoms of depression were measured concurrently with in-home sleep studies using the 16-item Inventory of Depressive Symptomatology (IDS).51 The IDS, minus sleep items, was calculated as a continuous variable. Perceived health was dichotomized as “fair” to “poor” versus “good” to “excellent” based on the distribution of responses to the single-item general health rating of the SF-36.52 Daily medication use (prescription and over-the-counter), recorded at Sleep Study protocol inception, was coded according to the World Health Organization ATC classification (http://www.whocc.no/atcddd). For this study “sleep medications” were considered to be those products associated with the following ATC classification codes: N02A (opioids), N03A (antiepileptics), N05B (anxiolytics), N05C (hypnotics and sedatives), N06A (antidepressants) and R06A (antihistamines). Medication use was dichotomized as “present” or “absent.”
Statistical Analysis
Descriptive statistics were used to characterize the study sample and evaluate distributions of the data. Skewed variables were transformed prior to analyses. Pearson Chi-Squares and analysis of variance (ANOVA) were used to examine race differences in key sample covariates. Analysis of covariance, adjusting for participant age, menopausal status, vasomotor symptoms, BMI, symptoms of depression, perceived health and medication use was used to evaluate the hypothesis that subjective and PSG-assessed measures of sleep differed by race. Tukey's post hoc tests were used to identify statistically significant racial differences in sleep outcomes. Linear regression analyses were used to test the hypothesis that educational attainment and financial strain would attenuate the race-sleep relationship in midlife women. Independent variables were race, educational attainment, financial strain and covariates including age, menopausal status, vasomotor symptoms, BMI, symptoms of depression, perceived health and use of medications that affect sleep. In separate analyses, interaction terms were used to evaluate whether educational attainment or financial strain moderated relationships among race and sleep. Sensitivity analyses were conducted in participants with apnea-hypopnea values < 15 (264 participants had AHI values < 15) to confirm that sleep disordered breathing did not confound study results. A conservative α level was set at P < 0.01 for all analyses due to the number of statistical tests computed. Traditional α level (P < 0.05) is denoted in tables and figures for descriptive purposes only. The present report includes 368 participants as PSG-assessed sleep was not available for 2 SWAN Sleep Study participants.
RESULTS
Characteristics of the sample are shown in Table 1. On average, SWAN Sleep Study participants perceived their overall physical health as “good” to “excellent” and reported few symptoms of depression. Most were early perimenopausal, as determined by bleeding patterns. The sample as a whole was fairly well educated: only 17% of participants did not report education beyond high school. By study design, 38.5% of participants were African American and 15.4% Chinese. Racial differences were observed for vasomotor symptoms, BMI, educational attainment and financial strain. More than 50% of African American participants reported vasomotor symptoms on sleep study nights compared to 33% of Caucasian and 25% of Chinese women. African American women had a significantly greater mean BMI than Caucasian women, who, in turn, had a higher mean BMI than Chinese women. Fewer African American participants reported obtaining college or advanced degrees, and more African Americans reported financial strain as defined by difficulty paying for the basics including food, housing, and medical care. Few Chinese participants reported financial strain.
Table 1.
Background Characteristics for Full Sample and by Race
| All n = 368 | Caucasian n = 171 | African American n = 138 | Chinese n = 59 | Test Statistic1 | |
|---|---|---|---|---|---|
| Mean (SD) age, years | 50.72 (2.02) | 50.70 (2.04) | 50.64 (2.00) | 50.95 (2.03) | 0.49 |
| Menopausal status | 9.42 | ||||
| No. (%) pre or early perimenopausal | 227 (61.7%) | 110 (64.3%) | 80 (58.0%) | 37 (62.7%) | |
| No. (%) late perimenopausal | 76 (20.7%) | 30 (17.5%) | 33 (23.9%) | 13 (22.0%) | |
| No. (%) postmenopausal | 65 (17.6%) | 31 (18.2%) | 25 (18.1%) | 9 (15.3%) | |
| Vasomotor symptoms | 17.48*** | ||||
| No. (%) reporting none | 221 (60.9%) | 113 (66.9%) | 64 (47.4%) | 44 (74.6%) | |
| No. (%) reporting ≥1 | 142 (39.1%) | 56 (33.1%) | 71 (52.6%) | 15 (25.4%) | |
| Mean (SD) body mass index (BMI) | 29.96 (7.68) | 29.56 (7.22) | 33.31 (7.69) | 23.27 (7.79) | 44.13*** |
| Mean (SD) Inventory of | |||||
| Depressive Symptomatology (IDS)2 | 7.94 (3.11) | 7.80 (2.64) | 7.99 (3.65) | 8.23 (2.43) | 0.34 |
| Perceived health | 7.75* | ||||
| No. (%) fair to poor | 47 (13.0%) | 13 (7.7%) | 24 (17.8%) | 10 (17.2%) | |
| No. (%) very good to excellent | 314 (87.0%) | 155 (92.3%) | 111 (82.2%) | 48 (82.8%) | |
| No. (%) taking medications that affect sleep | 99 (27.3%) | 50 (29.4%) | 36 (26.9%) | 13 (22.4%) | 1.09 |
| Educational attainment | 31.27*** | ||||
| No. (%) less than or earned a high school degree or equivalent | 62 (17.1%) | 21 (12.4%) | 29 (21.6%) | 12 (20.3%) | |
| No. (%) some college or assoc. degree | 115 (31.8%) | 46 (27.2%) | 58 (43.3%) | 11 (18.6%) | |
| No. (%) college degree or advanced degree | 185 (51.1%) | 102 (60.4%) | 47 (35.1%) | 36 (61.0%) | |
| Difficulty paying for basics | 24.74*** | ||||
| No. (%) not difficult at all | 252 (72.6%) | 131 (79.4%) | 70 (56.9%) | 51 (72.6%) | |
| No. (%) somewhat to very difficult | 95 (27.4%) | 34 (20.6%) | 53 (43.1%) | 8 (13.6%) |
Test statistic = Chi square for all but age, BMI, and symptoms of depression (ANOVA F test);
Square root transformed prior to analyses; for tests of statistical significance, * P < 0.05, **P < 0.01, ***P < 0.001.
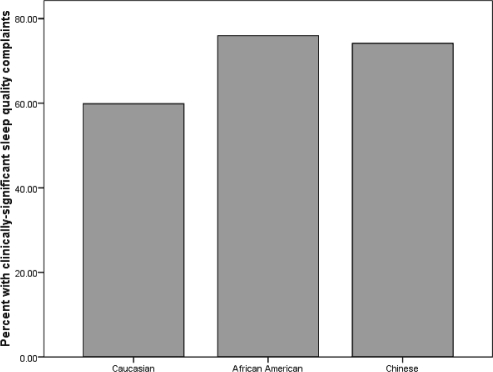
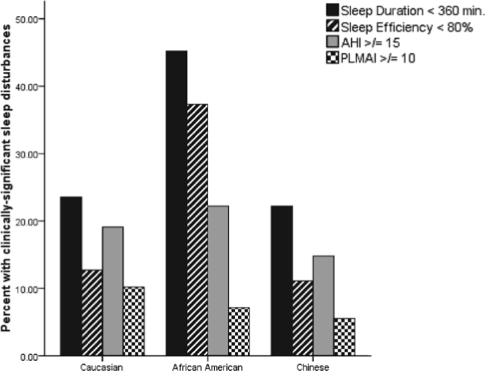
The majority of SWAN Sleep Study participants (66%) reported clinically significant subjective sleep quality complaints as defined by PSQI scores > 5.31 Although participants spent an average of 7½ hours in bed, 31.3% of the sample had < 6 hours of total sleep as measured by PSG. Sleep latencies > 30 min were observed in 17% of the sample, while 21% of the sample had sleep efficiency values < 80%. Clinically significant levels of sleep apnea and periodic leg movements were also observed; 20% of the sample had AHI values > 15, and nearly 8% had PLMAI values > 10. The prevalence of clinically significant subjective sleep quality complaints and of sleep disturbances by race are shown in Figures 1a and 1b, respectively.
Figure 1a.
Prevalence of clinically significant subjective sleep quality complaints (PSQI > 5) by race.
Figure 1b.
Prevalence of PSG-assessed clinically significant sleep disturbances by race.
African American participants reported greater subjective sleep quality complaints and spent less time asleep than Caucasian participants (see Table 2). With respect to PSG-assessed indices of sleep continuity, African American women took longer to fall asleep and spent more time awake after sleep onset, which translated into poorer overall sleep efficiency values, compared to both Caucasian and Chinese participants. Racial differences were similarly observed for measures of sleep architecture. After adjusting for covariates, slow wave sleep was decreased in both African American and Chinese participants, compared to Caucasians. These differences were observed in visually scored slow wave sleep (percent stage 3+4) as well as relative EEG power in the delta band during NREM sleep. Cortical hyperarousal, as measured by relative EEG power in the β band during NREM sleep, was higher in African American compared to Caucasian participants. Sensitivity analyses confirmed that results were the same for the subsample of participants with an AHI < 15. Stages 1 and 2 of NREM sleep, REM sleep, number of arousals from sleep, AHI, and PLMAI did not differ by race.
Table 2.
Unadjusted Values for Sleep Measures for Full Sample and by Race
| All Mean (SD) | Caucasian1 Mean (SD) | African American2 Mean (SD) | Chinese3 Mean (SD) | ANOVA7 F test | Tukey's HSD | |
|---|---|---|---|---|---|---|
| SELF-REPORT | ||||||
| Sleep Quality Complaints (PSQI) | 6.56 (2.41) | 5.96 (2.06) | 7.42 (2.63) | 6.27 (2.25) | 10.96*** | 2 >1*** |
| POLYSOMNOGRAPHY | ||||||
| Sleep Duration | ||||||
| Time in bed (minutes) | 453.63 (64.37) | 458.18 (55.37) | 450.34 (76.75) | 448.12 (56.95) | 1.03 | — |
| Time spent asleep (minutes) | 381.93 (59.12) | 393.92 (50.50) | 363.29 (67.41) | 390.37 (51.19) | 10.36*** | 2 < 1,3** |
| Sleep Continuity | ||||||
| Sleep latency (minutes)4 | 19.61 (19.02) | 17.52 (15.46) | 24.51 (24.12) | 14.36 (11.11) | 5.51** | 2 < 1,3** |
| Wakefulness after sleep onset (minutes)4 | 52.05 (34.50) | 47.66 (25.28) | 62.54 (43.70) | 43.39 (27.30) | 4.85** | 2 < 1,3** |
| Sleep efficiency (%)5 | 84.34 (8.19) | 86.05 (6.18) | 80.95 (9.85) | 80.95 (6.31) | 14.88*** | 2 < 1,3*** |
| Number of arousals | 20.66 (7.29) | 20.15 (7.58) | 20.17 (6.85) | 22.02 (7.39) | 1.50 | — |
| Sleep Architecture | ||||||
| Percent stage 16 | 7.23 (5.87) | 6.51 (5.38) | 8.14 ( 6.68) | 7.18 (5.10) | 1.39 | — |
| Percent stage 26 | 64.57 (8.02) | 64.13 (8.07) | 66.00 (8.52) | 63.71 (6.47) | 0.52 | — |
| Percent stage 3+46 | 3.53 (4.47) | 4.44 (4.73) | 2.53 (3.81) | 3.24 (4.66) | 11.26*** | 2,3 < 1*** |
| Percent REM | 24.67 (5.22) | 24.92 (5.02) | 23.85 (5.74) | 25.87 (4.23) | 2.47 | — |
| Relative δ Power (Hz)4 | 27.32 (15.53) | 29.24 (11.45) | 26.28 (20.57) | 24.18 (11.55) | 9.81*** | 2,3 < 1*** |
| Relative β Power (Hz)4 | 1.65 (0.30) | 1.58 (0.14) | 1.75 (0.43) | 1.63 (0.24) | 8.22*** | 2 > 1*** |
| Apnea-Hypopnea Index4 | 10.43 (15.08) | 11.17 (17.00) | 10.56 (14.55) | 8.03 (9.46) | 2.87 | — |
| Periodic Leg Movement Arousal Index | 3.93 (5.37) | 4.47 (6.41) | 3.83 (4.59) | 2.56 (2.98) | 1.71 | — |
Caucasian;
African American;
Chinese;
Log transformed (Ln) prior to analyses;
Reverse scored and log transformed (Ln) prior to analyses such that lower values reflect higher sleep efficiency;
Square root transformed prior to analyses;
Analyses are adjusted for age, menopausal status, vasomotor symptoms, BMI, symptoms of depression, perceived health and use of medications that affect sleep; for tests of statistical significance, *P < 0.05, **P < 0.01, ***P < 0.001.
Regression coefficients for race and selected sleep outcomes, adjusting for age, menopausal status, vasomotor symptoms, BMI, symptoms of depression, perceived health, and use of medications that affect sleep, are displayed in Table 3 (see Model 1 results). With the exception of sleep latency, race-sleep relationships remained statistically significant when educational attainment and financial strain were added to the model (see Model 2 results). Compared to Caucasian participants, African American women reported more subjective sleep complaints and had shorter, more disrupted, and lighter sleep after adjusting for educational attainment, financial strain, and model covariates. In both models, Chinese women tended to have less visually scored slow wave sleep and lower NREM EEG δ power than Caucasian participants.
Table 3.
Multivariate Linear Regression Models of Relationships Among Race, Educational Attainment, Financial Strain, and Selected Sleep Outcomes
| Race |
Educational Attainment |
Financial Strain |
||
|---|---|---|---|---|
| African American1 | Chinese1 | Some college or high school education2 | Difficulty paying for the very basics3 | |
| Beta | Beta | Beta | Beta | |
| Sleep Quality Complaints (PSQI) | ||||
| Model 14 | 0.24*** | 0.08 | ||
| Model 25 | 0.21*** | 0.09 | 0.03 | 0.15** |
| Time Spent Asleep (minutes) | ||||
| Model 14 | −0.26*** | −0.03 | ||
| Model 25 | −0.27*** | −0.04 | −0.05 | 0.02 |
| Sleep Latency (minutes)6 | ||||
| Model 14 | 0.16** | −0.08 | ||
| Model 25 | 0.11 | −0.08 | 0.06 | 0.13* |
| Wakefulness After Sleep Onset (minutes)6 | ||||
| Model 14 | 0.16** | −0.05 | ||
| Model 25 | 0.18** | −0.05 | −0.08 | 0.14* |
| Sleep Efficiency (percent)7 | ||||
| Model 14 | 0.28*** | −0.08 | ||
| Model 25 | 0.28*** | −0.07 | −0.03 | 0.16** |
| Percent Stage 3+48 | ||||
| Model 14 | −0.26*** | −0.15* | ||
| Model 25 | −0.28*** | −0.14* | 0.01 | 0.05 |
| Relative δ Power (Hz)6 | ||||
| Model 14 | −0.25*** | −0.14* | ||
| Model 25 | −0.28*** | −0.13* | 0.02 | 0.10 |
| Relative β Power (Hz)6 | ||||
| Model 14 | 0.24*** | 0.07 | ||
| Model 25 | 0.22*** | 0.08 | −0.01 | 0.05 |
Referent was Caucasian race;
Referent was “college or advanced degree;”
Referent was “not difficult at all to pay for the very basics;”
Model 1 predictor variables were race and covariates (age, menopausal status, vasomotor symptoms, BMI, symptoms of depression, perceived health and use of medications that affect sleep);
Model 2 predictor variables were race, markers of SES (educational attainment, financial strain) and covariates;
Log transformed (Ln) prior to analyses;
Reverse scored and log transformed (Ln) prior to analyses such that lower scores reflect higher sleep efficiency;
Square root transformed prior to analyses; for tests of statistical significance, *P < 0.05, **P < 0.01, ***P < 0.001.
Educational attainment, defined by the dichotomous variable of reporting a high school education or some college compared to reporting having earned a college or advanced degree, was unrelated to sleep (Model 2 results, Table 3) and the race-by-educational attainment interaction was also nonsignificant (P values > 0.05, interaction data not shown). In contrast to educational attainment, financial strain was a significant correlate of subjective sleep quality and PSG-assessed sleep continuity, after adjusting for covariates, race and educational attainment. Participants who reported that it was “somewhat” to “very hard” to pay for basics like food, housing, medical care and heating had greater subjective sleep quality complaints and poorer PSG-assessed sleep efficiency, compared to participants for whom paying for basics was “not difficult at all.” Race-by-financial strain interactions were tested in Caucasian and African American women only, due to the low prevalence of financial strain in Chinese participants. None of the race-by-financial strain interactions were significant (P values > 0.05, data not shown). Thus, financial strain had a similar impact on sleep in African American and Caucasian participants. Sensitivity analyses confirmed that results of regression analyses were unchanged for the subsample of participants with an AHI < 15.
DISCUSSION
We evaluated relationships among race, markers of SES and multiple dimensions of sleep including subjective sleep quality and in-home PSG in a community sample of midlife women. As hypothesized, sleep was worse in African American women compared with Caucasian and Chinese participants. Differences in slow wave sleep were also noted between Chinese and Caucasian participants. Contrary to our hypothesis, markers of SES did not markedly attenuate the race-sleep relationship, nor were race-SES interactions statistically significant. Although educational attainment was unrelated to sleep, financial strain was a significant correlate of sleep quality and continuity. African American and Caucasian participants who endorsed financial strain had increased subjective sleep complaints and lower sleep efficiency compared to their counterparts who reported no financial strain. Independent relationships among race, financial strain, and sleep were observed despite statistical adjustment for other factors that might account for these relationships including age, menopausal status, vasomotor symptoms, BMI, symptoms of depression, perceived health, and use of medications that affect sleep.
Our results replicate and extend previous reports of increased sleep disturbances in African Americans compared to Caucasians (for a review, see Durrence8) in 4 important ways. First, differences between African American and Caucasian participants were observed for each domain of sleep evaluated, whether assessed by self-report or multinight in-home PSG. The consistency of this effect across sleep domains may be related to the significant degree of sleep disturbance evident in all three groups of women studied. Although several risk factors for disturbed sleep including vasomotor symptoms, increased BMI, and poorer perceived health were elevated in African American compared to Caucasian and Chinese women, they did not account for the increased sleep disturbances observed in African American participants.53 Second, to our knowledge, this is the first study to document race differences in sleep using power spectral analysis of the EEG. The observation that EEG power was decreased in the δ band and increased in the β band during NREM sleep provides quantitative support for the observation that sleep is lighter in African Americans than Caucasians and that race/ethnicity is a robust correlate of slow wave sleep.10 The β power finding is particularly intriguing, given that elevated NREM EEG β power is seen in patients with primary insomnia and has also been associated with chronic psychological stress.23,41,45,54 Also notable is that observed differences among African Americans and Caucasians in sleep quality, duration, continuity, and architecture were not secondary to indices of sleep pathologies, including sleep disordered breathing or periodic limb movements. It was somewhat surprising that sleep disordered breathing was not elevated in African American women, given previous reports of race/ethnic differences in sleep disorderd breathing as well as the prevalence of obesity (BMI ≥ 30) in the African American participants (65%) compared to Caucasian (39.8%) and Chinese participants (1.7%).55 Finally, contrary to hypotheses, educational attainment and financial strain did not attenuate the effects of African American race on sleep. Other potential mechanisms that might account for poorer sleep in African Americans compared to Caucasians include genetic factors that might predispose towards lighter sleep or other environmental, cultural, social, or behavioral factors that might interfere with sleep and were not evaluated in the present study. Systematic evalaution of these factors is needed in future studies to identify the mechanisms that contribute to marked differences in sleep in African Americans compared to Caucasians and the extent to which these differences may contribute to disparities in health and functioning.
Chinese participants were thinner, and a smaller proportion of them reported financial strain than African American and Caucasian participants. Moreover, previous reports in the full SWAN Study Study have generally reported lower levels of depressive symptoms and lower proportions of women reporting vasomotor symptoms among Chinese participants.53,56 It might, thus, be expected that Chinese women would have better sleep profiles than their Caucasian and African American counterparts, yet this was not the case. Overall, mean sleep values for Chinese participants fell between and did not differ significantly from those observed for African Americans and Caucasians, with two exceptions. Chinese participants had better sleep continuity on average than did African Americans and less slow wave sleep than did Caucasians. Similar to our findings, the Sleep Heart Health Study (SHHS) reported higher sleep efficiency values in “Asian-American” compared to African American participants.10 The magnitude of the difference in sleep efficiency between Chinese and African American participants was nearly identical in both studies (5.9% in the SHHS cohort and 6.3% in the SWAN Sleep Study). These differences are not surprising in light of the increased number of risk factors for poorer sleep continuity observed among African American compared to Chinese SWAN Sleep Study participants (e.g., increased BMI and greater financial strain among African American compared to Chinese participants), although these factors did not significantly attenuate the effect of race on sleep efficiency. Vasomotor symptoms including night sweats and hot flashes, which were reported by a greater proportion of African American compared to Chinese participants, did not account for the increased sleep continuity disturbances in African American compared to Chinese participants.53 The difference in slow wave sleep between Chinese and Caucasian participants was observed for both visually scored sleep and quantitative EEG. Although the magnitude of the difference in slow wave sleep between Chinese and Caucasian participants was not clinically significant (1.2%), it has been reported before.4 The mechanisms that contribute to lower slow wave sleep in Chinese compared to Caucasian women in these samples is unclear and should be considered a preliminary finding given that sleep architecture differences were not observed among Asian-Americans and Causcasians in the multisite SHHS cohort.10
Although educational attainment and financial strain differed by race, race-sleep relationships remained unchanged whether or not one earned a college or advanced degree and whether or not one reported financial strain. It must be noted that the SWAN Sleep Study cohort was relatively well educated and financially stable; 80% of the sample graduated from high school and obtained at least some college or advanced technical education, and roughly 30% reported financial strain. The effects of SES on sleep may be more evident at the lower end of the socioeconomic spectrum where the assurance of a safe, comfortable place to sleep can be more elusive. While these sample characteristics limit the generalizability of findings related to educational attainment, financial strain and sleep, the data indicate that SES, as measured by educational attainment and financial strain, did not “explain” the race-sleep relationship observed in the present study. That financial strain is a more sensitive marker of the SES-sleep relationship compared to educational attainment is not surprising given previous reports from our laboratory as well as other studies that have linked work-related and financial worries to disturbed sleep.11,19,25,57,58 Moreover, our results are consistent with emerging evidence that indices of health and functioning, including sleep, may be more strongly linked to subjective and sociocultural aspects of SES than to traditional measures of SES such as income and education.16,21,59,60 Future studies would thus be advised to consider alternative pathways and indicators by which indices of SES might affect or be affected by sleep.
Several limitations of the SWAN Sleep Study should be noted. Hispanics who, together with African Americans, comprise the majority of racial and ethnic minority groups in the United States were not represented in the SWAN Sleep Study sample, nor were other racial and ethnic minority groups such as American Indians, Alaska natives, or Japanese women. Without knowing the mechanisms underlying known racial/ethnic differences in sleep, generalization to other racial/ethnic groups is not justified. Nor can these results be generalized to men, whose sleep differs significantly from women across the lifespan.46 The issue of generalizability is also important in light of SWAN Sleep Study eligibility criteria that might reasonably be expected to bear on questions of race, SES, and sleep. For instance, women who were regularly employed on the night shift were excluded from participation because of the influence of shiftwork on sleep and circadian rhythms. Given that US labor statistics indicate that the majority of shiftworkers are members of a minority racial or ethnic group and are at the lower end of the social gradient, the SWAN Sleep Study may have excluded a significant portion of African American and Chinese women who may have been of lower SES by virtue of their work schedules. Nor can results be generalized to younger or older women whose sleep differs markedly from that of midlife women.46 As a final comment on generalizability, all participants had a demonstrated history of participation in research. At the time of the sleep study, SWAN participants had completed between five and eight annual visits. The sample was, thus, composed of highly motivated research participants who might not be representative of the full spectrum of racial and SES categories of midlife women.
In conclusion, the present study revealed prevalent subjective sleep complaints and clinically significant sleep continuity disturbances in a multiracial community sample of midlife women. Race and financial strain were independent correlates of these sleep characteristics. The extent to which race and financial strain contribute to the potential adverse health effects associated with disturbed sleep in midlife women merits investigation.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Buysse has consulted for Actelion, Arena, Cephalon, Eli Lilly, GlaxoSmithKline, Merck, Neurocrine, Pfizer, Respironics, Sanofi-Aventis, Sepracor, Somnus Therapuetics, Stress Eraser, Takeda, and Transcept Pharmaceuticals. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Funding for the SWAN Sleep Study is from the National Institute on Aging (Grants AG019360, AG019361, AG019362, AG019363). The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health, DHHS, through the National Institute on Aging, the National Institute of Nursing Research and the NIH Office of Research on Women's Health (Grants NR004061, AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). Sleep data were processed with the support of RR024153.
Institutions where work was performed:
Rush University Medical Center, Chicago, IL
University of California-Davis, Davis, CA
University of Michigan, Ann Arbor, MI
University of Pittsburgh, Pittsburgh, PA
REFERENCES
- 1.Jean-Louis G, Magai C, Casimir GJ, et al. Insomnia symptoms in a multiethnic sample of american women. J Womens Health (Larchmt ) 2008;17:15–25. doi: 10.1089/jwh.2006.0310. [DOI] [PubMed] [Google Scholar]
- 2.Lauderdale DS, Knutson KL, Yan LL, et al. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 3.Profant J, Ancoli-Israel S, Dimsdale JE. Are there ethnic differences in sleep architecture? Am J Hum Biol. 2002;14:21–326. doi: 10.1002/ajhb.10032. [DOI] [PubMed] [Google Scholar]
- 4.Rao U, Poland RE, Lutchmansingh P, Ott GE, McCracken JT, Lin KM. Relationship between ethnicity and sleep patterns in normal controls: implications for psychopathology and treatment. J Psychiatr Res. 1999;33:419–26. doi: 10.1016/s0022-3956(99)00019-9. [DOI] [PubMed] [Google Scholar]
- 5.Stepnowsky CJ, Moore PJ, Dimsdale JE. Effect of ethnicity on sleep: complexities for epidemiologic research. Sleep. 2003;26:329–32. doi: 10.1093/sleep/26.3.329. [DOI] [PubMed] [Google Scholar]
- 6.Mezick EJ, Matthews KA, Hall M, et al. Influence of race and socioeconomic status on sleep: Pittsburgh SleepSCORE project. Psychosom Med. 2008;70:410–16. doi: 10.1097/PSY.0b013e31816fdf21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas KS, Bardwell WA, Ancoli-Israel S, Dimsdale JE. The toll of ethnic discrimination on sleep architecture and fatigue. Health Psychol. 2006;25:635–42. doi: 10.1037/0278-6133.25.5.635. [DOI] [PubMed] [Google Scholar]
- 8.Durrence HH, Lichstein KL. The sleep of African Americans: a comparative review. Behav Sleep Med. 2006;4:29–44. doi: 10.1207/s15402010bsm0401_3. [DOI] [PubMed] [Google Scholar]
- 9.Hale L, Do P. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30:1096–103. doi: 10.1093/sleep/30.9.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164:406–418. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- 11.Hall M, Bromberger JT, Matthews KA. Socioeconomic status as a correlate of sleep in African-American and Caucasian women. In: Adler NE, Marmot M, McEwen BS, Stewart J, editors. Socioeconomic status and health in industrial nations: social, psychological, and biological pathways. New York: Annals of the New York Academy of Sciences; 1999. pp. 427–30. [DOI] [PubMed] [Google Scholar]
- 12.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262:1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 13.Gellis LA, Lichstein KL, Scarinci IC, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Socioeconomic status and insomnia. J Abnorm Psychol. 2005;114:111–18. doi: 10.1037/0021-843X.114.1.111. [DOI] [PubMed] [Google Scholar]
- 14.Geroldi C, Frisoni GB, Rozzini R, De Leo D, Trabucchi M. Principal lifetime occupation and sleep quality in the elderly. Gerontology. 1996;42:163–69. doi: 10.1159/000213788. [DOI] [PubMed] [Google Scholar]
- 15.Moore PJ, Adler NE, Williams DR, Jackson JS. Socioeconomic status and health: the role of sleep. Psychosom Med. 2002;64:337–44. doi: 10.1097/00006842-200203000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Paine SJ, Gander PH, Harris R, Reid P. Who reports insomnia? Relationships with age, sex, ethnicity, and socioeconomic deprivation. Sleep. 2004;27:1163–69. doi: 10.1093/sleep/27.6.1163. [DOI] [PubMed] [Google Scholar]
- 17.Paine SJ, Gander PH, Travier N. The epidemiology of morningness/eveningness: influence of age, gender, ethnicity, and socioeconomic factors in adults (30–49 years) J Biol Rhythms. 2006;21:68–76. doi: 10.1177/0748730405283154. [DOI] [PubMed] [Google Scholar]
- 18.Fiorentino L, Marler M, Stepnowsky C, Johnson S, Ancoli-Israel S. Sleep in older African Americans and Caucasians at risk for sleep-disordered breathing. Behav Sleep Med. 2006;4:164–78. doi: 10.1207/s15402010bsm0403_3. [DOI] [PubMed] [Google Scholar]
- 19.Akerstedt T, Fredlund P, Gillberg M, Jansson B. Work load and work hours in relation to disturbed sleep and fatigue in a large representative sample. J Psychosom Res. 2002;53:585–88. doi: 10.1016/s0022-3999(02)00447-6. [DOI] [PubMed] [Google Scholar]
- 20.Akerstedt T, Kecklund G, Axelsson J. Impaired sleep after bedtime stress and worries. Biol Psychol. 2007;76:170–73. doi: 10.1016/j.biopsycho.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Gallo LC, Matthews KA. Understanding the association between socioeconomic status and physical health: do negative emotions play a role? Psychol Bull. 2003;129:10–51. doi: 10.1037/0033-2909.129.1.10. [DOI] [PubMed] [Google Scholar]
- 22.Hall M, Buysse DJ, Dew MA, Prigerson HG, Kupfer DJ, Reynolds CF. Intrusive thoughts and avoidance behaviors are associated with sleep disturbances in bereavement-related depression. Depress Anxiety. 1997;6:106–12. [PubMed] [Google Scholar]
- 23.Hall M, Buysse DJ, Nowell PD, et al. Symptoms of stress and depression as correlates of sleep in primary insomnia. Psychosom Med. 2000;62:227–30. doi: 10.1097/00006842-200003000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Hall M, Vasko R, Buysse DJ, et al. Acute stress affects heart rate variability during sleep. Psychosom Med. 2004;66:56–62. doi: 10.1097/01.psy.0000106884.58744.09. [DOI] [PubMed] [Google Scholar]
- 25.Hall M, Buysse DJ, Nofzinger EA, et al. Financial strain is a significant correlate of sleep continuity disturbances in late-life. Biol Psychol. 2008;77:217–22. doi: 10.1016/j.biopsycho.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freedman RR, Roehrs TA. Sleep disturbance in menopause. Menopause. 2007;14:826–29. doi: 10.1097/GME.0b013e3180321a22. [DOI] [PubMed] [Google Scholar]
- 27.Kravitz HM, Ganz PA, Bromberger J, Powell LH, Sutton-Tyrrell K, Meyer PM. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19–28. doi: 10.1097/00042192-200310010-00005. [DOI] [PubMed] [Google Scholar]
- 28.Kuh DL, Wadsworth M, Hardy R. Women's health in midlife: the influence of the menopause, social factors and health in earlier life. Br J Obstetr Gynaecol. 1997;104:923–33. doi: 10.1111/j.1471-0528.1997.tb14352.x. [DOI] [PubMed] [Google Scholar]
- 29.Obermeyer CM, Reher D, Saliba M. Symptoms, menopause status, and country differences: a comparative analysis from DAMES. Menopause. 2007;14:788–97. doi: 10.1097/gme.0b013e318046eb4a. [DOI] [PubMed] [Google Scholar]
- 30.Owens JF, Matthews KA. Sleep disturbance in healthy middle-aged women. Maturitas. 1998;30:41–50. doi: 10.1016/s0378-5122(98)00039-5. [DOI] [PubMed] [Google Scholar]
- 31.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 32.Sowers MF, Crawford S, Sternfeld B, et al. SWAN: A multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Lobo R, Marcus R, Kelsey J, editors. Menopause. New York: Academic Press; 2000. [Google Scholar]
- 33.Rechtschaffen A, Kales A. Washington, D.C.: U.S.Government Printing Office, Department of Health Education and Welfare; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects NIH Publication 204. [DOI] [PubMed] [Google Scholar]
- 34.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 35.American Sleep Disorders Association Atlas Task Force. Recording and scoring leg movements. Sleep. 1993;16:749–759. [PubMed] [Google Scholar]
- 36.Drewes AM, Nielsen KD, Taagholt SJ, Svendsen L, Bjerregard K, Nielsson L, Kristensen L. Ambulatory polysomnography using a new programmable amplifier system with on-line digitization of data: technical and clinical findings. Sleep. 1996;19:347–54. [PubMed] [Google Scholar]
- 37.Edinger JD, Marsh GR, McCall WV, Erwin CW, Lininger AW. Sleep variability across consecutive nights of home monitoring in older mixed DIMS patients. Sleep. 1991;14:13–17. [PubMed] [Google Scholar]
- 38.Edinger JD, McCall WV, Marsh GR, Radtke RA, Erwin CW, Lininger A. Periodic limb movement variability in older DIMS patients across consecutive nights of home monitoring. Sleep. 1992;15:156–61. [PubMed] [Google Scholar]
- 39.Brunner DP, Vasko RC, Detka CS, Monahan JP, Reynolds CF, Kupfer DJ. Muscle artifacts in the sleep EEG: Automated detection and effect on all-night EEG power spectra. J Sleep Res. 1996;5:155–64. doi: 10.1046/j.1365-2869.1996.00009.x. [DOI] [PubMed] [Google Scholar]
- 40.Freedman RR. EEG power spectra in sleep-onset insomnia. Electroencephalogr Clin Neurophysiol. 1986;63:408–13. doi: 10.1016/0013-4694(86)90122-7. [DOI] [PubMed] [Google Scholar]
- 41.Hall M, Thayer JF, Germain A, et al. Psychological stress is associated with heightened physiological arousal during NREM sleep in primary insomnia. Behav Sleep Med. 2007;5:178–93. doi: 10.1080/15402000701263221. [DOI] [PubMed] [Google Scholar]
- 42.Krystal AD, Edinger JD, Wohlgemuth WK, Marsh GR. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25:630–40. [PubMed] [Google Scholar]
- 43.Merica H, Blois R, Gaillard JM. Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci. 1998;10:1826–34. doi: 10.1046/j.1460-9568.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- 44.Perlis ML, Smith MT, Andrews PJ, Orff H, Giles DE. Beta/Gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24:110–17. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- 45.Perlis ML, Merica H, Smith MT, Giles DE. Beta EEG activity and insomnia. Sleep Med Rev. 2001;5:363–74. doi: 10.1053/smrv.2001.0151. [DOI] [PubMed] [Google Scholar]
- 46.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 47.Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31:979–90. [PMC free article] [PubMed] [Google Scholar]
- 48.Ohayon MM. Severe hot flashes are associated with chronic insomnia. Arch Intern Med. 2006;166:1262–68. doi: 10.1001/archinte.166.12.1262. [DOI] [PubMed] [Google Scholar]
- 49.World Health Organization Scientific Group. Research on the Menopause in the 1990s. Geneva: World Health Organization; 1996. [PubMed] [Google Scholar]
- 50.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28:1289–96. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 51.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 52.Ware JE, Sherbourne CD. The MOS-36-Item short form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 53.Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women's health across the nation. Am J Public Health. 2006;96:1226–35. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ribary U. Dynamics of thalamo-cortical network oscillations and human perception. Prog Brain Res. 2005;150:127–142. doi: 10.1016/S0079-6123(05)50010-4. [DOI] [PubMed] [Google Scholar]
- 55.O'Connor GT, Lind BK, Lee ET, et al. Variation in symptoms of sleep-disordered breathing with race and ethnicity: the Sleep Heart Health Study. Sleep. 2003;26:74–9. [PubMed] [Google Scholar]
- 56.Bromberger JT, Matthews KA, Schott LL, et al. Depressive symptoms during the menopausal transition: the Study of Women's Health Across the Nation (SWAN) J Affect Disord. 2007;103:267–72. doi: 10.1016/j.jad.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akerstedt T, Knutsson A, Westerholm P, Theorell T, Alfredsson L, Kecklund G. Sleep disturbances, work stress and work hours: a cross-sectional study. J Psychosom Res. 2002;53:741–748. doi: 10.1016/s0022-3999(02)00333-1. [DOI] [PubMed] [Google Scholar]
- 58.Akerstedt T, Fredlund P, Gillberg M, Jansson B. A prospective study of fatal occupational accidents -- relationship to sleeping difficulties and occupational factors. J Sleep Res. 2002;11:69–71. doi: 10.1046/j.1365-2869.2002.00287.x. [DOI] [PubMed] [Google Scholar]
- 59.Ghaed SG, Gallo LC. Subjective social status, objective socioeconomic status, and cardiovascular risk in women. Health Psychol. 2007;26:668–74. doi: 10.1037/0278-6133.26.6.668. [DOI] [PubMed] [Google Scholar]
- 60.Singh-Manoux A, Marmot MG, Adler NE. Does subjective social status predict health and change in health status better than objective status? Psychosom Med. 2005;67:855–61. doi: 10.1097/01.psy.0000188434.52941.a0. [DOI] [PubMed] [Google Scholar]