Abstract
Study Objectives:
To evaluate non-rapid eye movement (NREM) sleep instability, as measured by the cyclic alternating pattern (CAP), in the first months of life in a group of normal healthy infants, in order to obtain more information on the maturation of arousal mechanisms during NREM sleep and to set normative data of CAP parameters in this age range (from 1 to 4 months of life).
Design:
Retrospective study.
Setting:
Sleep unit of an academic centre.
Participants:
Twenty-three healthy newborns and infants with a mean conceptional age (gestational age plus postnatal age) of 47.6 ± 3.8 weeks, age range 42 to 55 weeks, 10 boys (43.47%), were studied while they slept in the morning between feedings, by means of a 3-hour video-electroencephalographic (EEG)-polygraphic recording. Sleep was visually scored for sleep architecture and CAP in a blinded fashion, using standard criteria.
Measurements and results:
We found 3 different sleep EEG patterns in our infants, according to their age, and we subdivided the entire group into 3 subgroups. Group 1—Tracé alternant mixed with high-voltage slow activity included 9 subjects (3 boys), with a mean conceptional age of 43.9 ± 1.3 weeks; Group 2 (high-voltage slow activity and rudimentary spindles) included 6 subjects (4 boys), with a mean conceptional age of 49.4 ± 3.1 weeks; and Group 3 (slow-wave activity and spindles, scored as NREM sleep) included 8 subjects (3 boys), with a mean conceptional age of 50.4 ± 2.9 weeks. CAP rate was 6.83 ± 3.58 in infants belonging to Group 2 and increased to 12.91 ± 2.21 in Group 3. We found a statistically significant higher A1 index in only Group 3. The relative percentages of the A1, A2, and A3 subtypes showed non significant changes with age. The duration of CAP events and the cortical and subcortical arousal indexes were not statistically different between Groups 2 and 3.
Conclusions:
With this study, we provide the first data on CAP analysis in infants from 1 to 4 months of life, and we found that there is a transitory period when tracè alternant disappears and CAP events begin to occur. Furthermore, we suggest that the more appropriate time of life when CAP analysis can be first performed is related to the appearance of mature stage 2 NREM with spindles and slow delta waves mixed with theta waves, at approximately 3 months of life.
Citation:
Miano S; PiaVilla M; Blanco D; Zamora E; Rodriguez R; Ferri R; Bruni O; Peraita-Adrados R. Development of NREM sleep instability-continuity (cyclic alternating pattern) in healthy term infants aged 1 to 4 months. SLEEP 2009;32(1):83-90.
Keywords: Infant, cyclic alternating pattern, arousal, tracè alternant
AGE IS THE MOST IMPORTANT PHYSIOLOGIC FACTOR MODULATING HUMAN SLEEP AND ALSO INFLUENCES THE ELECTROENCEPHALOGRAPHIC (EEG) PATTERNS and contributes to the occurrence and features of arousals. Arousal can be considered as a short transient intrusion of wakefulness EEG rhythms into sleep and probably has a protective physiologic role in counteracting various endogenous or exogenous stimuli, such as loud noises, tactile stimuli, and temperature changes.1 The analysis of arousals has many clinical implications: an increased level of arousability might be related to sleep fragmentation, excessive arousals from sleep in infants may impair development, and a low level of arousability might play a role in the pathogenesis of the sudden infant death syndrome.1–4
Different criteria for scoring arousals in infants have been reported in the literature.1–4 The International Pediatric Work Group on Arousals, comprising a group of sleep experts, has recently defined a method for the scoring of arousals in infants.3 Scoring includes the differentiation between subcortical activation, with (apparently) no visible changes in the EEG, and cortical arousals associated with EEG changes, during quiet sleep (QS) and active sleep (AS). On the contrary, an American Pediatric Task Force has recently provided an evidence-based review of the age-related development of the polysomnographic features of sleep in neonates, infants, and children and opted not to support scoring of subcortical activations until more evidence is available regarding their clinical significance.1
In the last years, many studies have focused their attention on other methods of scoring arousal not limited to the American Sleep Disorder Association criteria,5 using pulse transit time6 or cyclic alternating pattern (CAP).7–9
It was initially hypothesized that the tracé alternant (TA) could represent a precursor of CAP in neonates, but this has never been demonstrated, and a paper has reported significant EEG structure differences.10 The term TA was used to describe the periodic discontinuity of QS recorded in premature and term babies after 37 weeks conceptional age.11,12 TA usually disappears between 3 and 4 weeks after birth; the disappearance of TA and the appearance of sleep spindles lead to the constitution of the NREM sleep stage 2, reflecting the maturation of the thalamocortical pathways and rostro-caudal pons-thalamus connections.13
Sleep is characterized by an oscillating pattern that exhibits different levels of arousal, mostly during NREM periods, resembling some features of TA. This physiologic oscillating pattern has been coded as CAP and is considered important for sleep building and maintenance.9 Terzano et al14 have published an atlas of CAP with recording techniques and rules for its scoring in human sleep, mostly related to adulthood. CAP is a spontaneous rhythm detectable during NREM sleep in form of EEG amplitude oscillations composed of an EEG transient pattern (phase A of the cycle) separated by intervals of background activity (phase B of the cycle). Three main EEG patterns have been described according to the prevalence of EEG synchrony (subtype A1), prevalence of EEG desynchrony (subtype A3), or a combination of both (subtype A2).14
It has been highlighted that CAP is one of the most systematic and expressive constructs devised to describe arousals, their composition, and their timing during sleep.15 Normative data about CAP parameters during NREM sleep have been provided for preschool-aged children,16 school-aged children,17 and adolescents.18–19
The analysis of CAP in school-aged children is characterized by a high CAP rate during slow wave sleep (SWS) and a high percentage of A1 phases. The distribution of intervals between consecutive A1 phases shows a peak around 25 seconds.17 The analysis of CAP in preschool-aged children shows a lower percentage of A1 with an increase of A2, compared with prepubertal children, reflecting a higher sleep instability in this age group.16 The analysis of the A1-interval distribution shows the same periodicity as that of school-aged children.
The CAP rate tends to increase with age from preschool- to school-aged children, and these data suggest that CAP rate might be very low during the first months of life, considering the maturations of thalamocortical pathways, in contrast with the high frequency of oscillations that characterize TA of newborns. Normative data on CAP parameters in healthy newborns and infants are not available, and the occurrence of CAP patterns during the first months of life has never been evaluated.
The aim of our study was to perform CAP analysis in the first months of life in a group of healthy infants to derive more information on the maturation of arousal mechanisms during NREM sleep and to set normative data of CAP parameters in this age range (from 1 to 4 months of life).
MATERIALS AND METHODS
Subjects
Twenty-three healthy newborns and infants with a mean conceptional age (gestational age plus postnatal age) of 47.6 ± 3.8 weeks, age range 42 to 55 weeks, 10 males (43.47%), were studied while the infants slept in the morning between feedings. They underwent a 3-hour video-EEG-polygraphic recording at the Sleep Unit of the Clinical Neurophysiology Department of the University Hospital Gregorio Marañnéon in Madrid, Spain. At the time of the study, all infants were healthy, not sleep deprived, and receiving no medication; they were born at term and with a normal prenatal and perinatal history (Apgar score ranged from 7–8 at 1 minute to 9–10 and 5 minutes after birth; mean birth weight was 3345.5 ± 438.7 grams, range 2700-4080 grams). They had no family history of sudden infant death syndrome nor of having had apparent life-threatening events; they did not sleep prone at home, as reported by parents.
Video-Polygraphic Recordings
Newborns and infants were admitted to the sleep laboratory for a 3-hour daytime-nap videopolysomnogram. The recordings were performed in a quiet and darkened room, with temperature ranging between 20°C and 23°C. All infants slept supine, without restraints. Recording started around 11:00, after breast or bottle feeding. No infant had a pacifier during recording. The following variables were recorded simultaneously: EEG using the international 10/20 system to place electrodes in standardized scalp locations (F4-C4, C4-T4, T4-O2, F3-C3, C3-T3, T3-O1), electrooculogram (1 channel), submental electromyogram (EMG), and electrocardiogram. Thoracic and abdominal movements were recorded by inductance plethysmography, and air flow pressure by nasal cannula. Oxygen saturation was recorded continuously from a transcutaneous sensor (pulse oximetry). Data were collected on a computerized sleep system (Deltamed SA, Paris, French), with a sampling frequency of 256 Hz. The recordings were subdivided into 30-second epochs.
Data Analysis
Based on the polygraphic recordings, sleep stages and sleep apneas were scored according to standard definitions.20–24 One of the authors (RPA) scored all of the recordings (sleep staging and sleep respiratory analysis).
According to the recommendations of the Pediatric Task Force,1 sleep was scored as QS, AS, and indeterminate sleep (IS), in infants younger than 2 months after term. QS comprises 2 EEG patterns: TA and high-voltage slow activity (HVS). TA is an EEG pattern in which 3- to 8-second bursts of moderate- to high-voltage 0.5- to 3.0-Hz slow waves, intermixed with 2- to 4-Hz sharply contoured waveforms, alternate with 4- to 8-second intervals of attenuated mixed-frequency EEG activity. In contrast, HVS consists of continuous moderately rhythmic 50- to 150-μV, 0.5- to 4-Hz slow activity.
The Pediatric Task Force1 further recommended that stages 1, 2, and SWS should be scored by 4 to 4.5 months post-term, usually by 5 to 6 months. SWS in an infant or child can be called stage NREM 3 and is scored when 20% or more of an epoch consists of waves of 0.5- to 2-Hz frequencies and peak-to-peak amplitude of greater than 75 μV in the frontal derivations. However, since it is difficult to recognize a clear SWS in our age group of children, we decided to score all NREM sleep epochs as stage NREM, avoiding a differentiation between stage 2 and SWS that could be difficult and not appropriate in our sample (Figure 1).1
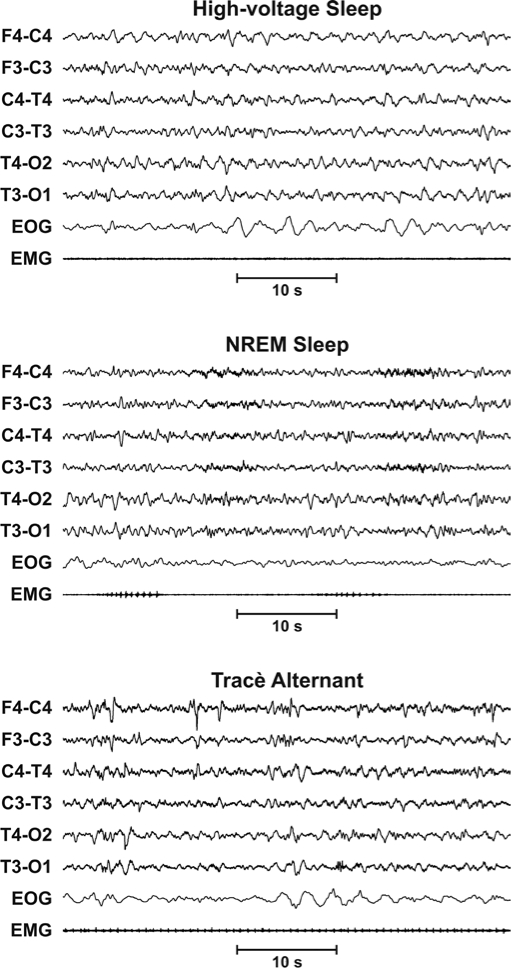
Figure 1.
Top panel: Example of the electroencephalographic (EEG) pattern exhibited by Group 1: Trace alternant consisting of 3- to 8-second bursts of moderate- to high-voltage, 0.5- to 3.0-Hz slow waves, intermixed with 2- to 4-Hz sharply contoured waveforms, alternate with 4- to 8-second intervals of attenuated mixed-frequency EEG activity. Middle panel: Example of the EEG pattern exhibited by Group 2: High-voltage slow activity consisting of continuous moderately rhythmic 50- to 150-μV, 0.5- to 4-Hz slow activity. Bottom panel: Example of the EEG pattern exhibited by Group 3: NREM sleep EEG consisting of slow EEG activity and sleep spindles.
Apneas were scored when they lasted 3 seconds or longer. They were classified as central apnea when flat tracings were obtained simultaneously from the strain gauges and the nasal cannula. Periodic breathing was defined as the succession of more than 2 central apneas separated from each other by less than 20 seconds. Obstructive apneas were defined as continuous deflections from the strain gauges, with a flat tracing recorded from the nasal cannula. Mixed apnea was defined as a central apnea directly followed by an obstructive episode, and mixed apneas were scored together with the obstructive episodes. The recordings were analyzed visually by 1 of the investigators (RPA).
Cortical Arousal and Subcortical Activation
Arousals were subdivided into subcortical activations or cortical arousals, according to the consensus on arousal scoring in healthy infants younger than 6 months of age.3 A subcortical activation was scored if no apparent change in the EEG was seen, while at least 2 of the following changes occurred: a gross body movement detected by movement sensors or seen as an artefact movement in the somatic channels, changes in heart rate (at least 10% of baseline values), or changes in breathing pattern (frequency and/or amplitude). A cortical arousal was scored using the above criteria, with the addition of the occurrence of an abrupt change in EEG background frequency of at least 1 Hz, for a minimum of 3 seconds. EEG arousals in young infants may provoke an abrupt diffuse decrease in EEG amplitude called a “decremental response,” and this was scored as an arousal.
CAP Scoring
CAP was scored following the criteria published by Terzano et al. 14 The following CAP parameters were measured: CAP time (temporal sum of all CAP sequences) in QS; CAP rate (percentage of total QS time occupied by CAP sequences); number and duration of CAP cycles; number and duration of CAP sequences; number, duration, and percentage of A phases (including the phase A subtypes); A1 index (number of A1 phases per hour of QS or NREM sleep); A2 index (number of A2 phases per hour of QS or NREM sleep); A3 index (number of A3 phases per hour of QS or NREM sleep); and number and duration of B phases.
All of these variables were visually detected, and their parameters measured by means of the Hypnolab 1.0 sleep software analysis (SWS Soft, Italy). CAP was visually scored by 1 of the investigators (SM), without knowledge of the subject's age or sex, and the sleep parameters derived were tabulated for subsequent statistical analysis.
Two-dimensional plotting of the interval (in seconds) distribution of the A1 and A2+A3 phase was also obtained to establish the mean interval values and to evaluate if a preferential distribution could be observed, different from random distributions for Group 2 and Group 3 (see below).
Rules for Scoring CAP in babies aged 1 to 4 months
To adapt CAP scoring for the age of the subjects included in this study, we made some modifications to the rules for identifying the different CAP A phase subtypes in children and adults. EEG arousals in young children often include rhythms slower than those seen in adults, usually represented by frequencies in the range of theta and other faster rhythms. In infants, an arousal can also include frequencies ranging in the delta band, according to the definition of an arousal in this age range: an arousal is defined as an abrupt change in EEG background frequency of at least 1 Hz, even into the delta band.3 Therefore, we also modified the CAP scoring criteria and considered these EEG frequencies as components potentially characterizing the A3 and, partially, the A2 subtypes of CAP. During the scoring of sleep stages, we had already noticed that most EEG arousals associated with an increase in EMG presented with a theta or delta switch of EEG frequencies, as shown in Figure 2.
Figure 2.
Top panel: Cyclic alternating pattern (CAP) Subtype A1—Slow electroencephalographic (EEG) synchrony is the predominant activity, mostly composed by high-voltage delta bursts. Middle panel: CAP Subtype A2—Mixture of slow and fast EEG activities, including bursts of theta rhythms, and other faster rhythms superimposed, associated or not with EMG activation. Bottom panel: CAP Subtype A3—EEG activity with predominant fast low-voltage rhythms; more than 50% of phase A is occupied by fast EEG activities, including EEG arousals, polyphasic bursts, and high-voltage delta waves with an amplitude one-third higher, or more, than the background activity, followed by theta and other faster rhythms.
CAP cannot be scored in a recording if it is not possible to recognize K complexes, delta bursts, and/or spindles. Spindles are usually first present by 46 to 48 weeks conceptional age, whereas K complexes first appear 5 months postterm and slow wave activity of SWS is first seen as early as 2 to 3 months postterm and is usually present 4 to 4.5 months postterm.3 Because these EEG patterns are not well developed at the time of TA, we could exclude that TA is the precursor of CAP, since the “bricks” that constitute the basic structure of CAP are not present. Therefore, we analyzed tracings and scored CAP only when at least rudimentary spindles appeared and slow wave activity emerged from HVS.
Taking this into account, we modified the CAP-subtype scoring criteria for infants as follows (Figure 2):
Subtype A1—A phases in which slow EEG synchrony is the predominant activity, mostly comprising high-voltage delta bursts. Phasic activities initiating a phase A must be one-third higher than the background voltage (calculated during the 2 seconds before the onset and 2 seconds after the offset of a phase A). Compared with the background EEG delta rhythms of QS, delta bursts tend to be lower in frequency.
Subtype A2— A phases that contain a mixture of slow and fast EEG activities, including bursts of theta rhythms, associated or not with EMG activation; delta wave bursts followed by theta; and other faster rhythms. Fast EEG activities generally represent 20% to 50% of the entire phase A2. Subtype A2 can be linked with a moderate increase of muscle tone, cardiorespiratory rate, or both. As stated before, in our recordings, we found several phasic events represented mainly by bursts of high-voltage delta activity (Figure 2). They were often associated with a mild EMG activation.
Subtype A3—A phases in which the EEG activity is predominantly fast low-voltage rhythms with more than 50% of phase A occupied by fast EEG activities, including EEG arousals, polyphasic bursts, and high-voltage delta waves with an amplitude one-third higher, or more, than the background activity, followed by theta and other faster rhythms. All A3 phases are very often associated with remarkable enhancement of muscle tone, cardiorespiratory rate, or both. It is not common to find A3 CAP subtypes without EMG activation infants. As stated by the International Pediatric Work Group on Arousals, EEG arousals in young infants may provoke an abrupt diffuse decrease in EEG amplitude called a “decremental response,” and we also scored this transient event as A3.
We detected A phases if they appeared at least in the anterior channels (F3-C3, F4-C4); meanwhile, the events scored as A phases rarely appeared simultaneously in the anterior and posterior regions, especially for the A1 phases.
Statistical Analysis
For the statistical analysis of the differences in sleep architecture and CAP parameters found in the groups of infants included in this study, the nonparametric Mann-Whitney test for independent datasets, followed by the Bonferroni correction for multiple comparisons when necessary, was used.
Moreover, all intervals between subsequent CAP A phases were subdivided into 25 duration classes (< 5 s, ≥ 5 < 10 s, ≥ 10 < 15 s …. ≥ 115 < 120 s, ≥ 120 < 125 s) and counted in each subject; this count was used to draw individual normalized interval distribution graphs. The normalization was obtained by calculating the percentage of each class with respect to the total individual count. In this way, data from different individuals could be pooled together. This analysis was carried out only in Group 2 and Group 3, and the differences between the 2 groups were evaluated by means of the nonparametric Mann-Whitney test for independent datasets, followed by the Bonferroni correction for multiple comparisons.
Differences were considered statistically significant at P < 0.05. The commercially available software STATISTICA (data analysis software system), version 6, StatSoft, Inc. (2001) was used for all statistical tests.
RESULTS
Analysis of sleep EEG patterns
During the scoring session, it emerged that the sleep patterns were different and fell into 3 distinct groups. Those infants who had a specific pattern were then allocated to each group,and the statistics were performed according to this specific pattern, described below in detail.
Group 1
Group 1 (TA mixed with HVS), the first EEG pattern of QS, was characterized by TA, which was mixed with HVS and mixed frequency activity. Nine subjects (3 boys) exhibited this EEG pattern, and they had a mean conceptional age of 43.9 ± 1.3 weeks, (range: 42-46 weeks). In this group, CAP scoring was not performed.
Group 2
Group 2 (HVS and rudimentary spindles), the second EEG pattern, was characterized by HVS and rarely sleep spindles. Six subjects (4 boys) exhibited this EEG pattern, and they had a mean conceptional age of 49.4 ± 3.1 weeks (range: 46–54 weeks).
Group 3
Group 3 (SWS and spindles, scored as NREM sleep), the third EEG pattern, was characterized by the appearance of sleep spindles mixed with delta and theta waves, with more oscillations than continuous sleep (HVS), and it has been scored as NREM sleep. Eight subjects (3 boys) exhibited this EEG pattern, and they had a mean conceptional age of 50.4 ± 2.9 weeks (range: 46–55 weeks).
Examples of the 3 patterns are presented in Figure 1. CAP scoring was performed in groups 2 and 3. Table 1 reports the sleep architecture characteristics of all groups, and Table 2 shows the CAP parameters and arousal indexes.
Table 1.
Sleep Architecture Parameters
| Group |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 (n = 9) (TA and HVS) |
2 (n = 6) (HVS and rudimentary spindles) |
3 (n = 8) (SW and spindles) |
Bonferroni-corrected Mann-Whitney test (P <) |
||||||
| Mean | SD | Mean | SD | Mean | SD | 1 vs 2 | 1 vs 3 | 2 vs 3 | |
| Conceptional age, wk | 43.9 | 1.29 | 49.4 | 3.10 | 50.4 | 2.86 | 0.0045 | 0.0015 | NS |
| Sao2 | 96.3 | 1.65 | 96.7 | 0.74 | 96.4 | 0.35 | NS | NS | NS |
| Sleep architecture parameters | |||||||||
| TIB, min | 120.7 | 13.53 | 135.3 | 7.74 | 121.1 | 17.9 | NS | NS | NS |
| SPT, min | 99.3 | 19.60 | 98.9 | 0.55 | 100.2 | 24.8 | NS | NS | NS |
| TST, min | 74.6 | 19.26 | 66.2 | 5.48 | 85.6 | 19.4 | NS | NS | NS |
| SOL, min | 18.8 | 15.00 | 29.4 | 0.89 | 15.6 | 9.26 | NS | NS | NS |
| FASL, min | 1.1 | 3.17 | 5.5 | 0.89 | 6.1 | 13.7 | NS | NS | NS |
| SS, no./h | 19.9 | 5.76 | 14.8 | 5.60 | 11.7 | 3.64 | NS | 0.03 | NS |
| AWN, no./h | 7.8 | 3.88 | 7.9 | 15.71 | 5.0 | 3.39 | NS | NS | NS |
| MT, no./h | 1.8 | 1.23 | 1.0 | 0.55 | 1.2 | 1.24 | NS | NS | NS |
| SE, % | 61.5 | 12.95 | 48.7 | 0.67 | 71.2 | 14.9 | NS | NS | 0.045 |
| AS periods, no. | 10.3 | 4.18 | 8.3 | 7.15 | 7.7 | 2.55 | NS | NS | NS |
| WASO, % | 24.8 | 11.33 | 31.7 | 14.09 | 13.8 | 7.68 | NS | NS | NS |
| TA, % | 24.6 | 9.73 | - | - | - | - | - | - | - |
| HVS, % | 7.8 | 6.45 | 31.4 | 6.38 | - | - | 0.005 | - | - |
| NREM sleep, % | - | - | - | - | 44.7 | 5.7 | - | - | - |
| IS,% | 5.1 | 4.18 | 4.1 | 3.19 | 4.6 | 3.57 | NS | NS | NS |
| AS,% | 37.7 | 14.11 | 32.8 | 8.99 | 36.9 | 9.69 | NS | NS | NS |
Abbreviations: FASL refers to latency to first active sleep; TIB, time in bed; SPT, sleep period time; TST, total sleep time; SOL, sleep onset latency; SS, stage shifts; AWN, awakenings; SE, sleep efficiency; WASO, wake time after sleep onset; HVS, high-voltage slow activity; AS, active sleep; TA, tracè alternant, IS, indeterminate sleep; AS, active sleep; NS, not significant.
Table 2.
Cyclic Alternating Pattern and Arousal Parameters
| Group |
|||||||
|---|---|---|---|---|---|---|---|
| 1 (n = 9) (TA and HVS) |
2 (n = 6) (HVS and rudimental spindles) |
3 (n = 8) (SW and spindles) |
Mann-Whitney test (P<) |
||||
| Mean | SD | Mean | SD | Mean | SD | 2 vs 3 | |
| CAP rate, % | NS | ||||||
| in HVS | 7.4 | 3.57 | |||||
| in NREM sleep | 12.9 | 2.21 | |||||
| A1,% | 69.7 | 31.34 | 85.2 | 11.5 | NS | ||
| A2,% | 17.4 | 18.97 | 10.3 | 7.1 | NS | ||
| A3,% | 4.3 | 3.76 | 4.4 | 5.34 | NS | ||
| A1 index, no./h | 6.7 | 3.81 | 19.8 | 4.55 | 0.005 | ||
| A2 index, no./h | 5.1 | 6.59 | 2.8 | 1.62 | NS | ||
| A3 index, no./h | 0.7 | 0.84 | 0.5 | 0.75 | NS | ||
| A mean duration, s | 6.4 | 0.94 | 6.8 | 2.92 | 7.7 | 1.54 | NS |
| B mean duration, s | 10.01 | 2.31 | 23.0 | 5.05 | 19.8 | 3.67 | NS |
| A1 mean duration, s | 6.0 | 2.63 | 6.5 | 1.51 | NS | ||
| A2 mean duration, s | 5.9 | 4.9 | 11.6 | 6.27 | NS | ||
| A3 mean duration, s | 10.6 | 8.84 | 13.4 | 16.7 | NS | ||
| Cortical arousal index | 16.0 | 3.28 | 11.9 | 10.65 | 10.5 | 3.49 | NS |
| Subcortical activation index | 2.6 | 2.09 | 3.1 | 0.84 | 4.2 | 2.75 | NS |
Abbreviations: HVS refers to high-voltage slow activity; SW, = slow waves; NS, not significant.
Sleep architecture
Considering the 3 groups, we found a trend toward an increase of latency to first AS, but it was not significantly different between groups (P < 0.05). The percentage of AS and of IS remained almost similar in the 3 groups, whereas HVS increased in Group 2 after the disappearance of TA. Sleep efficiency was higher in Group 3 associated with the lowest wake time after sleep onset (13.83 ± 7.68), as compared with the other 2 groups.
The sleep period time was approximately 1.5 hours, and the IS was approximately 4% to 5% in all groups. The cortical arousal index tended to decrease with the maturation of EEG patterns: 16.04 ± 3.28 in Group 1, 11.87 ± 10.65 in the Group 2, and 10.48 ± 3.49 in Group 3.
Sleep Respiratory Analysis
No episodes of periodic breathing or obstructive apneas or hypopneas were found in any of the subjects, except for some isolated central apnea lasting less than 5 seconds during AS and not associated with oxygen desaturation in 4 subjects.
CAP Analysis
Although it was not possible to score CAP in Group 1, we evaluated the A phases of TA that appeared simultaneously in the anterior and posterior region, calculating the mean duration of the A phases of TA (6.38 seconds) and of B phases (10.01 seconds). In Group 1, after the disappearance of TA, the monomorphic delta rhythms during QS emerged in the posterior regions; this slow wave activity was different from CAP oscillations that usually involve the central and frontal regions.
CAP oscillations, in the other 2 groups, appeared mostly simultaneously in anterior and posterior regions or with a very brief asynchrony between the right and left hemispheres. Based on the age of our groups, the age range when it was possible to see the first appearance of CAP events seemed to be around 46 to 55 weeks (Group 2).
CAP analysis in Groups 2 and 3 showed that CAP rate was 6.83 ± 3.58 in infants belonging to Group 2 and increased to 12.9 ± 2.21 in Group 3. We found a statistically significant increase of the A1 index only in Group 3. The percentage of A1, A2, and A3 showed non significant variation with age. The duration of CAP events was similar in all age groups considered. Similarly, the cortical and subcortical arousal indexes were not statistically different.
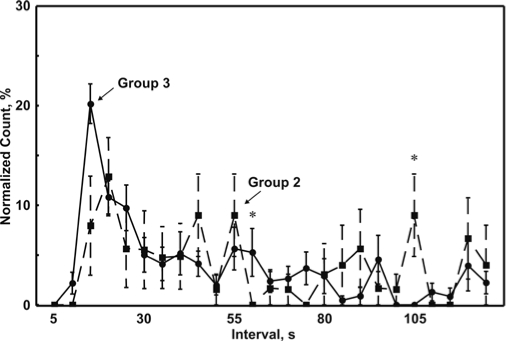
Figure 3 shows the comparison between the normalized CAP interval distribution graphs obtained from Group 2 and Group 3. In this comparison, a statistically significant difference can be seen only for the normalized number of intervals ≥ 55 < 60 seconds and ≥ 105 < 110 seconds. However, this analysis seems to indicate the presence of a temporal distribution with a peak around 15 to 20 only in Group 3.
Figure 3.
Comparison between the normalized cyclic alternating pattern (CAP) interval distribution graphs obtained from Group 2 (dashed line) and Group 3 (continuous line). Statistically significant differences (P < 0.05) are indicated by asterisks.
DISCUSSION
Our study is the first attempt to provide normative CAP parameters for infants during the first months of life. The age range of the infants when it was possible to see the first appearance of CAP events seems to be around 46 to 55 weeks conceptional age. In this age range, we found 2 different EEG patterns, one immature constituted by HVS with or without spindles and another more mature pattern constituted by a clear-cut spindle activity and slow waves mixed with theta activity that can be considered as NREM sleep, as has been suggested by Guilleminault et al21 and Coons and Guilleminault.25 These authors reported that, in normal infants younger than 6 months of age, sleep spindles first appear at approximately 8 to 12 weeks postterm and “true slow waves with delta waves” between 3 and 6 months postterm.25 The variability of the appearance of EEG features belonging to NREM sleep explains why we found an overlap of age in infants with HVS and infants with NREM sleep and spindles. Also, the interindividual variability in the maturation of sleep patterns is likely to play a role in this overlapping.
QS in infants is characterized by 1 of 2 EEG patterns: TA or HVS activity. HVS consists of continuous moderately rhythmic 50- to 150-μV, 0.5- to 4-Hz slow activity, without the bursting activity of TA. HVS represents the more mature pattern of QS in infants.26,27
We suggest that EEG maturation is crucial for the appearance of CAP events: after the disappearance of TA, infants with HVS (Group 2) begin to show some events that might be coded as phase A, but, with the appearance of slow waves, delta bursts, and immature K complexes (Group 3), CAP events could be clearly recognized as a distinct pattern from the background NREM/QS EEG activity. Therefore, we might state that the appearance of slow waves and spindles is indicative of a more mature pattern and allowed us to score CAP events.
It has been suggested that apnea, periodic leg movements, and other sleep pathology may be gated by a brain-generated CAP, which is a natural underlying tendency to arouse from sleep at regular intervals.15 The analysis of CAP events in the first months of life might be useful to study and eventually confirm the low level of arousability that has been implicated in the pathogenesis of the sudden infant death syndrome and in apparent life-threatening events. Moreover, CAP analysis might provide further insights on the mechanism of arousability in sleep-breathing disorders during the early period of life.
Parrino et al28 initially hypothesized that the CAP rate shows a U-shaped curve across the life span, supposing that, at birth, the CAP rate should be close to 100 %, in the view that CAP is similar to TA. However the first studies on children by Bruni et al,16,17 Lopes et al, and Parrino et al18,19 did not confirm this hypothesis, showing instead that the high values of CAP rate in adolescents was followed by a steep decrease in school- and preschool-aged children. Therefore, the low CAP rate in school- and preschool-aged children does not fit the original hypothesis of the age-related U-shaped curve but, instead, seems to suggest that the CAP rate decreases in younger children and infants, with the lowest level in neonates.15 However, since there are no studies available in the age group between 6 months and 2 years of age, we cannot definitely confirm this trend and need more information on this age range from future studies.
It has been demonstrated that the EEG shows dramatic changes across early development: a salient feature of the QS/NREMS spectrum is the emergence of a peak in the sigma band (12–14 Hz) at 2 months that corresponds with the appearance of sleep spindles. In infants between 2 and 9 months of age, low-frequency delta activity (0.75–1.75 Hz) shows an alternating pattern with a high level occurring in every other QS/NREMS episode. The appearance of slow waves and sleep spindles in infants promote the formation of thalamocortical networks by providing endogenous neural signals with repetitive and synchronized activity.29 This pattern of maturation probably would lead to the appearance of the phasic events during sleep that we were able to recognize as phase A1 of CAP.
In this respect, Ferri et al30 reported that the CAP slow components (A1) might be considered as the cortical expression of synchronizing mechanisms during NREM sleep subserved by thalamocortical pathways. The progressive maturation of thalamocortical networks could lead to the gradual appearance of an oscillating pattern of slow EEG activities (different from that of TA) that represents the first prototype of CAP appearing at 46 to 55 weeks conceptional age. The progressive emergence of these slow EEG patterns/bursts parallels, at older ages, the reported increase of A1 percentage from preschool-aged to school-aged children.16,17 This is indirectly in agreement with the idea that CAP represents a marker of the thalamocortical interactions and of the maturation of these cerebral activities.
Another indirect sign of maturation of thalamocortical interactions might be considered to be the peak of A1 interval around 15 to 20 seconds that we found in only our Group 3. The cortical arousal and subcortical activation indexes found in our subjects seem to be lower than the indexes provided by Kato et al4; a possible explanation might be that these authors used a different method of analysis (overnight polysomnography) and analyzed a larger sample of subjects in a different age range, similar to our Group 1.
Finally, this study confirms that age-related maturation of CAP patterns seems to involve not only its quantitative aspects, expressed by CAP rate and the other CAP parameters, but also the time structure of CAP sequences, as shown by our analysis of CAP A phase interval distribution. We know that in children and adults this distribution is characterized by a log-normal–like aspect, with a major peak toward the left side of the graph (shorter intervals between 20 and 30 seconds), declining gradually with increasing interval length.31 The time distribution of CAP indicates that its components are the expression of a timely ordered process that exhibits specific sleep stage-related features and undergoes age-related modifications. The new findings of the present study indicate that this time structure is barely detectable in subjects in an early developmental stage, such as those included in our Group 2, and starts to be somewhat evident in those in our Group 3; however, the time structure of CAP of Group 3 is not yet as clear-cut as in older children aged 3 years or more.31
Our preliminary study in a relatively small group of normal healthy infants needs to be replicated in a larger number of infants, and normative data on infants between 6 and 12 months of life need to be added. In our opinion, normative data on arousal and CAP parameters in the first year if life are needed and might be useful to understand and to prevent several pathologies related to arousal mechanisms, such as apparent life threatening events, sudden infants death syndrome, and sleep-disordered breathing.
The limits of our study were the relatively small number of polysomnographic recordings and the choice of 2 complete wakefulness-sleep cycles (120 minutes) in the morning between feedings, rather than an all-night polysomnography. The reason was that we wanted to improve the compliance of families of healthy babies with undergoing a relatively intrusive analysis.
With this study, we provide the first data on CAP analysis in infants from 1 to 4 months of life, and we found that there is a transitory period when TA disappears and CAP events begin to occur. Furthermore, we suggest that the more appropriate time of life when CAP analysis can be first performed is related to the appearance of mature stage 2 NREM sleep with spindles, slow delta waves mixed with theta waves, at approximately 3 months of life.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Grigg-Damberger M, Gozal D, Marcus CL, et al. The visual scoring of sleep and arousal in infants and children. J Clin Sleep Med. 2007;3:201–40. [PubMed] [Google Scholar]
- 2.McNamara F, Wulbrand H, Thach BT. Habituation of the infant arousal response. Sleep. 1999;22:320–6. [PubMed] [Google Scholar]
- 3.The International Paediatric Work Group on Arousals. The scoring of arousals in healthy term infants (between the ages of 1 and 6 months) J Sleep Res. 2005;14:37–41. doi: 10.1111/j.1365-2869.2004.00426.x. [DOI] [PubMed] [Google Scholar]
- 4.Kato I, Scaillet S, Groswasser J, et al. Spontaneous arousability in prone and supine position in healthy infants. Sleep. 2006;29:785–90. doi: 10.1093/sleep/29.6.785. [DOI] [PubMed] [Google Scholar]
- 5.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 6.Katz ES, Lutz J, Black C, Marcus CL. Pulse transit time as a measure of arousal and respiratory effort in children with sleep-disordered breathing. Pediatr Res. 2003;53:580–88. doi: 10.1203/01.PDR.0000057206.14698.47. [DOI] [PubMed] [Google Scholar]
- 7.Terzano MG, Parrino L. Clinical applications of cyclic alternating pattern. Physiol Behav. 1993;54:807–13. doi: 10.1016/0031-9384(93)90096-x. [DOI] [PubMed] [Google Scholar]
- 8.Terzano MG, Parrino L. Origin and significance of the cyclic alternating pattern (CAP) Sleep Med Rev. 2000;4:101–23. doi: 10.1053/smrv.1999.0083. [DOI] [PubMed] [Google Scholar]
- 9.Terzano MG, Parrino L, Boselli M, Spaggiari MC, Di Giovanni G. Polysomnographic analysis of arousal responses in OSAS by means of the cyclic alternating pattern (CAP) J Clin Neurophysiol. 1996;13:145–55. doi: 10.1097/00004691-199603000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Ferri R, Chiaramonti R, Elia M, Musumeci SA, Ragazzoni A, Stam CJ. Nonlinear EEG analysis during sleep in premature and full-term newborns. Clin Neurophysiol. 2003;114:1176–80. doi: 10.1016/s1388-2457(03)00089-0. [DOI] [PubMed] [Google Scholar]
- 11.Dreyfus-Brisac C., Monod N. The EEG of full-term newborns and premature infants. In: Lairy R, editor. Handbook of EEG and Clinical Neurophysiology. Amsterdam, the Netherlands: Elsevier; 1975. pp. 6–23. [Google Scholar]
- 12.Curzi-Dascalova L, Mirmiran M, editors. Manual of Methods for Recording and Analyzing Sleep-Wakefulness States in Preterm And Full-Term Infant. Paris, France: Editions Inserm; 1996. [Google Scholar]
- 13.Louis J. Maturation du sommeil pendant le deux premières années de vie: aspects quantitatif, structurel et circadien. Neurophysiol Clin. 1998;28:477–91. doi: 10.1016/s0987-7053(99)80017-3. [DOI] [PubMed] [Google Scholar]
- 14.Terzano MG, Parrino L, Smerieri A, et al. Consensus Report. Atlas, rules, and recording techniques for the scoring of cyclic alternating pattern (CAP) in human sleep. Sleep Med. 2001;2:537–53. doi: 10.1016/s1389-9457(01)00149-6. [DOI] [PubMed] [Google Scholar]
- 15.Chervin RD. The cyclic alternating pattern in children. Sleep. 2005;28:163–4. [PubMed] [Google Scholar]
- 16.Bruni O, Ferri R, Miano S, et al. Sleep cyclic alternating pattern in normal preschool-aged children. Sleep. 2005;28:220–30. doi: 10.1093/sleep/28.2.220. [DOI] [PubMed] [Google Scholar]
- 17.Bruni O, Ferri R, Miano S, et al. Sleep cyclic alternating pattern in normal school-age children. Clin Neurophysiol. 2002;113:1806–14. doi: 10.1016/s1388-2457(02)00265-1. [DOI] [PubMed] [Google Scholar]
- 18.Parrino L, Boselli M, Spaggiari MC, Smerieri A, Terzano MG. Cyclic alternating pattern (CAP) in normal sleep: polysomnographic parameters in different age groups. Electroencephalogr Clin Neurophysiol. 1998;107:439–50. doi: 10.1016/s0013-4694(98)00108-4. [DOI] [PubMed] [Google Scholar]
- 19.Lopes MC, Rosa A, Roizenblatt S, et al. Cyclic alternating pattern in peripubertal children. Sleep. 2005;28:215–9. doi: 10.1093/sleep/28.2.215. [DOI] [PubMed] [Google Scholar]
- 20.Guilleminault C, Peraita R, Souquet M, Dement WC. Apneas during sleep in infants. Possible relation with the sudden infant death syndrome: facts and hypotheses. Science. 1975;190:677–79. doi: 10.1126/science.1188364. [DOI] [PubMed] [Google Scholar]
- 21.Guilleminault C, Souquet M. Sleep states and related pathology. In: Korobkin R, Guilleminault C, editors. Advances in Perinatal Neurology. New York, NY: Spectrum Publications; 1979. pp. 225–47. [Google Scholar]
- 22.Guilleminault C, Souquet M, Ariagno RL, Korobkin R, Simmons FB. Five cases of near-miss sudden infant death syndrome and development of obstructive apnea syndrome. Pediatrics. 1984;73:71–8. [PubMed] [Google Scholar]
- 23.Kelly DH, Shannon DC. Periodic breathing in infants with near-miss sudden infant death syndrome. Pediatrics. 1979;63:355–60. [PubMed] [Google Scholar]
- 24.Kelly DH, Golub H, Carley D, Shannon DC. Pneumograms in infants who subsequently died of sudden infant death syndrome, J. Pediatr. 1986;109:249–54. doi: 10.1016/s0022-3476(86)80380-8. [DOI] [PubMed] [Google Scholar]
- 25.Coons S, Guilleminault C. Development of sleep-wake patterns and non-rapid eye movement sleep stages during the first six months of life in normal infants. Pediatrics. 1982;69:793–8. [PubMed] [Google Scholar]
- 26.Parmelee AH, Jr., Schulte FJ, Akiyama Y, Wenner WH, Schultz MA, Stern E. Maturation of EEG activity during sleep in premature infants. Electroencephalogr Clin Neurophysiol. 1968;24:319–29. doi: 10.1016/0013-4694(68)90193-4. [DOI] [PubMed] [Google Scholar]
- 27.Anders TF, Sadeh A, Appareddy V. Normal sleep in neonates and children. In: Ferber R, Kryger M, editors. Principles and practice of sleep medicine in the child. Philadelphia, PA: WB Saunders; 1995. pp. 7–18. [Google Scholar]
- 28.Parrino L, Boselli M, Spaggiari MC, et al. Cyclic alternating pattern (CAP) in normal sleep: polysomnographic parameters in different age groups. Electroencephalogr Clin Neurophysiol. 1998;107:439–50. doi: 10.1016/s0013-4694(98)00108-4. [DOI] [PubMed] [Google Scholar]
- 29.Jenni OG, Borbely A, Achermann P. Development of the nocturnal sleep electroencephalogram in human infants. Am J Physiol Regul Integr Comp Physiol. 2004;286:R528–38. doi: 10.1152/ajpregu.00503.2003. [DOI] [PubMed] [Google Scholar]
- 30.Ferri R, Bruni O, Miano S, Terzano MG. Topographic mapping of the spectral components of the cyclic alternating pattern (CAP) Sleep Med. 2005;6:29–36. doi: 10.1016/j.sleep.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Ferri R, Bruni O, Miano S, et al. The time structure of the cyclic alternating pattern during sleep. Sleep. 2006;29:693–9. doi: 10.1093/sleep/29.5.693. [DOI] [PubMed] [Google Scholar]