Abstract
Study Objectives:
At termination of obstructive apneas, arousal is a protective mechanism that facilitates restoration of upper airway patency and airflow. Treating obstructive sleep apnea (OSA) by continuous positive airway pressure (CPAP) reduces arousal frequency indicating that such arousals are caused by OSA. In heart failure (HF) patients with central sleep apnea (CSA), however, arousals frequently occur several breaths after apnea termination, and there is uncertainty as to whether arousals from sleep are a consequence of CSA. If so, they should diminish in frequency when CSA is attenuated. We therefore sought to determine whether attenuation of CSA by CPAP reduces arousal frequency.
Design:
Randomized controlled clinical trial.
Patients and Setting:
We examined data from 205 HF patients with CSA (apnea-hypopnea index [AHI] ≥ 15, > 50% were central) randomized to CPAP or control who had polysomnograms performed at baseline and 3 months later.
Measurements and Results:
In the control group, there was no change in AHI or frequency of arousals. In the CPAP-treated group, the AHI decreased significantly (from [mean ± SD] 38.9 ± 15.0 to 17.6 ± 16.3, P < 0.001) but neither the frequency of arousals nor sleep structure changed significantly.
Conclusion:
These data suggest that attenuation of CSA by CPAP does not reduce arousal frequency in HF patients. We conclude that arousals were not mainly a consequence of CSA, and may not have been acting as a defense mechanism to terminate apneas in the same way they do in OSA.
Citation:
Ruttanaumpawan P; Logan AG; Floras JS; Bradley TD. Effect of Continuous Positive Airway Pressure on Sleep Structure in Heart Failure Patients with Central Sleep Apnea. SLEEP 2009;32(1):91-98.
Keywords: Arousal, sleep structure, central sleep apnea, sleep physiology
SLEEP STRUCTURE IS INVARIABLY DISRUPTED BY AROUSALS FROM SLEEP IN PATIENTS WITH SLEEP APNEA.1,2 IN OBSTRUCTIVE SLEEP APNEA (OSA), arousals from sleep that typically terminate apneas, trigger activation of the pharyngeal dilator muscles and facilitate resumption of airflow. Indeed, several studies report that 75% to 80% of obstructive events are terminated by arousals.3,4 Accordingly, in OSA, arousals are considered to be an important defense mechanism for reestablishing airway patency, thus preventing asphyxia. On the other hand, this protective mechanism inevitably disrupts sleep. When OSA is alleviated by continuous positive airway pressure (CPAP), the frequency of arousals is immediately reduced in association with consolidation of sleep and an increase in the proportion of both slow wave and REM sleep.5–8 One can therefore conclude that OSA causes arousal from sleep, and that this, in turn, reduces the amounts of slow wave and REM sleep.
In patients with heart failure (HF) and coexisting Cheyne-Stokes respiration with central sleep apnea (CSA), arousals often follow central apneas and hypopneas.2,9,10 However, in contrast to OSA, these arousals often occur several breaths after apnea termination, suggesting that in such cases, they are not an important defense mechanism that contribute to resumption of airflow. Arousals, whether occurring during the apnea-hyperpnea cycle or not, provoke abrupt increases in ventilation and decreases in PCO2. If PCO2 falls below the threshold for apnea, central apnea ensues.11,12 Among patients with CSA, either with or without HF, abrupt increases in ventilation, and falls in PCO2, mainly provoked by arousals, precede more than 90% of episodes of Cheyne-Stokes respiration and repetitive central apneas.11,12 Moreover, in previous studies, it has been shown that CPAP only partially suppresses CSA such that the frequency of apneas and hypopneas/h of sleep (apnea-hypopnea index or AHI) only falls by approximately 30% to 70% in patients with HF.13–21 Perhaps this was because it did not eliminate one stimulus to the Cheyne-Stokes respiratory cycle: arousal-mediated hyperpneas. Therefore, arousals may contribute to causation of CSA.
If arousals are mainly a consequence of CSA in patients with HF, they should diminish if CSA is attenuated by treatment. If, on the other hand, arousals are a cause of, or are incidental to CSA, then suppression of CSA in HF patients may have little or no effect on arousal frequency or sleep structure. There is controversy on this point: some articles reported that CPAP and other interventions that suppress CSA either reduced arousal frequency or increased the amounts of slow wave and REM sleep,13–15 whereas others did not.16–21 However, all of these studies were small (n ≤ 24), or of short duration (1 day to 1 month), and simply reported arousal frequency and sleep structure, but without discussing the significance of whether or not they improved. Our objective, therefore, was to determine in a much larger, longer-term randomized trial, whether attenuation of CSA by CPAP in patients with HF reduces the frequency of arousals from sleep or improves sleep structure. To this end, we analyzed the arousal frequency and sleep structure at baseline and follow-up of patients enrolled in a randomized clinical trial of CPAP to treat CSA in patients with HF (Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure trial [CANPAP]).
METHODS
Study Design
Details of the study design have been published previously.22 Briefly, CANPAP was a prospective randomized, open-label trial with blinded evaluation of outcomes, involving HF patients with CSA in 11 centers (see Appendix). It tested the effects of CPAP on CSA and cardiovascular outcomes. The Research Ethics Board of each institution approved the protocol. Enrollment followed written informed consent.
Subjects
Candidates for participation in CANPAP were men and women aged 18 to 79 years with New York Heart Association (NYHA) Class II to IV HF due to ischemic, hypertensive, or idiopathic dilated cardiomyopathy, stabilized on optimal medical therapy ≥ 1 month; left ventricular ejection fraction (LVEF) < 40% by radionuclide angiography; and CSA, defined as an AHI ≥ 15, with > 50% of apneas and hypopneas central in nature (see below). Exclusion criteria were: pregnancy; myocardial infarction, unstable angina or cardiac surgery within 3 months of enrollment, and obstructive sleep apnea.
Randomization
Eligible patients were randomized to either a control group continuing optimal medical HF therapy, or a treatment group who, in addition, received CPAP. Randomization was performed by computerized schedule in random blocks of 4 and 6 and was stratified by study center.
Baseline Assessment
Patients underwent clinical assessment followed by overnight polysomnography. All polysomnographic studies were performed and scored according to uniform techniques and standards at all centers. Sleep stages were scored in 30-sec epochs according to standard criteria,23 while arousals were scored according to the American Sleep Disorders Association guidelines, and the frequency of arousals/h of sleep was expressed as the arousal index (ArI).24 Leg movements lasting 0.5–5 sec separated by intervals of 4–90 sec and occurring in a series of ≥ 4 consecutive movements were scored as periodic leg movements (PLM) according to the standard criteria.25 Periodic leg movements were not scored if their onset occurred after the onset of arousals or occured at the resolution of apnea or hypopnea. The periodic leg movement index (PLMI) was defined as the number of PLMs per hour of sleep. Respiratory movements were measured by respiratory inductance plethysmography, and airflow by nasal pressure.22,26 Central apneas were defined as absent tidal volume for ≥ 10 sec without thoracoabdominal motion and central hypopneas as a ≥ 50% reduction in tidal volume from baseline for ≥ 10 sec with in-phase thoracoabdominal motion and without airflow limitation on nasal pressure.21 Apneas and hypopneas were classified as obstructive if out-of-phase thoracoabdominal motion or airflow limitation was present. The diagnosis of CSA required an AHI ≥ 15 with > 50% of the events central in nature.
Initiation of CPAP
CPAP was initiated over 2 to 3 nights in an unmonitored sleep laboratory or hospital bed, starting at 5 cm H2O the first night, then increasing by 2 to 3 cm H2O over the subsequent 1 or 2 nights until 10 cm H2O (a level shown to attenuate CSA and improve LVEF)21,22 or until the highest pressure tolerated was reached. Patients were instructed to use CPAP ≥ 6 h nightly at home during the trial. If necessary, pressure was raised to 10 cm H2O or to the highest level tolerated at the 1 or 3 month follow-up visit. At each clinic visit, hours of CPAP use were downloaded from a mask-on time meter.
Assessments of Outcomes
Clinical assessments were performed 1, 3, and 6 months following randomization, and every 6 months thereafter. Polysomnography was performed at 3 and 24 months, and LVEF at 3, 6, and 24 months post-randomization. Subjects were followed from randomization until death or heart transplant or the end of the study. The primary outcome, the combined rate of all-cause mortality or heart transplantation, has been reported.22,27
To assess the effect of attenuation of CSA by CPAP on arousal frequency and sleep structure, we evaluated those subjects who underwent sleep studies 3 months after enrollment. Since we aimed to assess the effect of CPAP on CSA and sleep structure, we analyzed polysomnographic data from all subjects in the control group, but only subjects using CPAP on the night of the 3-month follow-up sleep study in the CPAP group. The primary analysis focused on sleep structure at the 3-month follow-up assessment because far more data were available at this time than at 2 years (205 subjects at 3 months versus 71 subjects at 2 years). However, we also examined sleep structure at the 2-year follow-up in those patients who completed this assessment.
Statistical Analysis
Although polysomnographic technicians were aware of whether subjects were on CPAP or not at the time of the follow-up sleep studies, these studies were scored by personnel unaware of the treatment allocation. All other tests were both acquired and analyzed without knowledge of treatment allocation. Data are expressed as mean ± SD unless stated otherwise. Analyses were performed using SPSS 13.0 (SPSS Inc., Chicago, Illinois). With respect to baseline data, for continuous variables, unpaired t-tests were used to evaluate between-group differences if the data were normally distributed. Non-normally distributed data were compared by Mann-Whitney test. For nominal and ordinal variables, chi-square or Fisher exact test were used as appropriate. With respect to follow-up data, analysis of covariance was used to compare the changes of continuous outcomes from baseline between the 2 groups. P-values of < 0.05 were considered statistically significant.
RESULTS
Subjects
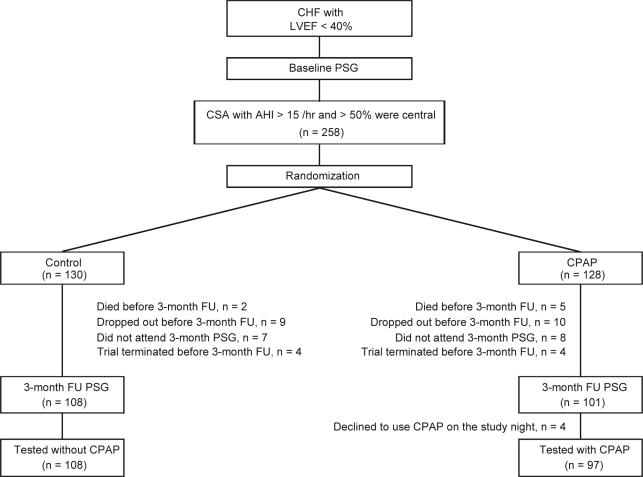
Figure 1 illustrates the flow of subjects through the study. Of 258 patients who were enrolled, 130 were randomized to the control group and 128 to the CPAP group. Three months after randomization, 108 patients in the control group, and 101 in the CPAP group underwent follow-up sleep studies. However, in the latter group, 4 patients randomized to CPAP were no longer using it at this time, and therefore were studied without CPAP. Therefore, 108 and 97 patients in the control and CPAP groups, respectively, underwent sleep studies according to protocol at the 3-month follow-up. The reasons for not undergoing a sleep study at the 3-month follow-up are shown in Figure 1.
Figure 1.
Flow Diagram Indicating Progress of Eligible Subjects Through the Study
Patient baseline characteristics are summarized in Table 1. There were no significant differences in age, sex distribution, body mass index, cause of HF, NYHA class, LVEF, prevalence of atrial fibrillation/flutter, blood pressure, severity of CSA, or medication use between the 2 groups. The baseline characteristics of the 53 patients who did not have a follow-up sleep study at 3 months did not differ from those who did, except for a higher prevalence of NYHA class III–IV in the former group (46% vs. 30%, P = 0.03).
Table 1.
Characteristics of the Subjects at Baseline
| Control group (n = 108) | CPAP treated group (n = 97) | P-value | |
|---|---|---|---|
| Age, y | 63.5 ± 10.0 | 62.2 ± 9.3 | 0.25 |
| Sex (M : F) | 102 : 6 | 94 : 3 | 0.39 |
| BMI, kg/m2 | 29.2 ± 5.6 | 29.4 ± 5.2 | 0.73 |
| Cause of dilated cardiomyopathy, no (%) | |||
| Ischemic | 73 (68) | 62 (64) | 0.79 |
| Idiopathic | 31 (29) | 32 (33) | 0.79 |
| Hypertensive | 4 (4) | 3 (3) | 0.79 |
| NYHA class, no (%) | |||
| II | 78 (72) | 66 (68) | 0.51 |
| III-IV | 30 (28) | 31 (32) | 0.51 |
| LVEF, % | 24.1 ± 7.6 | 24.7 ± 7.7 | 0.53 |
| Atrial fibrillation/flutter, no (%) | 20 (19) | 24 (25) | 0.28 |
| Blood pressure, mm Hg | |||
| Systolic | 115 ± 18 | 115 ± 19 | 0.77 |
| Diastolic | 71 ± 11 | 71 ± 11 | 0.72 |
| Severity of CSA | |||
| Total AHI, no/h | 37.8 ± 15.1 | 38.9 ± 15.0 | 0.59 |
| Central AHI, no/h | 33.0 ± 15.0 | 35.4 ± 15.1 | 0.26 |
| Mean SaO2, % | 93.1 ± 3.3 | 93.2 ± 3.6 | 0.70 |
| Lowest SaO2, % | 81.9 ± 6.3 | 81.4 ± 8.1 | 0.82 |
| Medication use, no (%) | |||
| ACEIs and/or ARBs | 105 (97) | 92 (95) | 0.38 |
| Loop diuretics | 98 (91) | 83 (86) | 0.25 |
| Beta blockers | 85 (79) | 75 (77) | 0.81 |
| Digoxin | 61 (56) | 50 (52) | 0.48 |
| Aspirin | 57 (53) | 49 (51) | 0.75 |
| Statins | 43 (40) | 41 (42) | 0.72 |
| Spironolactone | 41 (38) | 33 (34) | 0.56 |
| Amiodarone | 19 (18) | 22 (23) | 0.36 |
| Calcium channel blockers | 16 (15) | 13 (14) | 0.77 |
Values are means ± SD or number (%).
Totals in table may not equal 100% due to rounding.
Abbreviations: ACEIs = angiotensin converting enzyme inhibitors, AHI = apnea-hypopnea index, ARBs = angiotensin II receptor blockers, BMI = body mass index, CSA = central sleep apnea, LVEF = left ventricular ejection fraction, NYHA = New York Heart Association, SaO2 = oxygen saturation.
Effects of CPAP on CSA and Sleep Structure
As displayed in Table 2, none of the baseline polysomnographic variables differed significantly between the 2 groups (P ≥ 0.08 for all variables), and there was no correlation between the ArI and PLMI (R = −0.04, P = 0.61). Patients randomized to CPAP used it at an average pressure of 8.8 ± 1.8 cm H2O for 4.6 ± 2.1 h/day. After 3 months, the CPAP group experienced a significant reduction in total AHI (by 55%), and in central and obstructive AHIs that was greater than in the control group (P < 0.001 for all variables), who had no significant reduction in the AHI. Since we did not use a desaturation criterion for hypopnea, the fall in the AHI on CPAP also indicates attenuation of periodic breathing without desaturation. The mean and lowest SaO2 also increased more in the CPAP group than in the control group (P < 0.001 for both). The increases in mean and lowest SaO2 were significant within the CPAP group (P < 0.001 for both). Within the control group, there were also slight but significant increases in mean and lowest SaO2 (P = 0.04 for both).
Table 2.
Sleep Data at Baseline and 3 Months after Randomization
| Control group (n = 108) |
CPAP treated group (n = 97) |
Between group P-value | |||
|---|---|---|---|---|---|
| Baseline | 3 months | Baseline | 3 months | ||
| Effect on CSA | |||||
| Total AHI, no/h | 37.8 ± 15.1 | 37.6 ± 25.7 | 38.9 ± 15.0 | 17.6 ± 16.3* | < 0.001 |
| Central AHI, no/h | 33.0 ± 15.0 | 30.7 ± 23.6 | 35.4 ± 15.1 | 16.1 ± 16.3* | < 0.001 |
| Obstructive AHI, no/h | 4.8 ± 4.9 | 6.9 ± 10.7 | 3.5 ± 4.1 | 1.6 ± 3.9* | < 0.001 |
| Mean SaO2, % | 93.1 ± 3.3 | 93.5 ± 2.8† | 93.2 ± 3.6 | 94.9 ± 2.7* | < 0.001 |
| Lowest SaO2, % | 81.9 ± 6.3 | 83.0 ± 6.0† | 81.4 ± 8.1 | 86.3 ± 6.4* | < 0.001 |
| Effect on Arousals | |||||
| Arousal index, no/h | 24.8 ± 19.3 | 26.3 ± 18.6 | 28.8 ± 23.7 | 24.3 ± 19.5 | 0.15 |
| Effect on Sleep Structure | |||||
| Time in bed, min | 452.4 ± 51.4 | 457.5 ± 48.9 | 454.5 ± 51.7 | 454.5 ± 44.9 | 0.49 |
| Sleep period time, min | 421.4 ± 52.7 | 423.7 ± 55.9 | 428.8 ± 54.4 | 423.7 ± 48.7 | 0.57 |
| Total sleep time, min | 310.5 ± 84.3 | 308.9 ± 77.6 | 318.2 ± 75.6 | 318.0 ± 73.9 | 0.55 |
| Sleep efficiency, % | 68.4 ± 16.5 | 67.8 ± 16.6 | 70.3 ± 16.4 | 70.2 ± 15.7 | 0.45 |
| Sleep onset latency, min | 30.8 ± 30.5 | 33.7 ± 36. | 25.3 ± 27.7 | 30.6 ± 29.3 | 0.99 |
| Wake after sleep onset, min | 111.0 ± 65.4 | 114.8 ± 70.0 | 110.6 ± 70 | 105.7 ± 65.5 | 0.29 |
| Stage 1 percentage, % | 18.2 ± 12.8 | 18.9 ± 12.9 | 19.1 ± 13.5 | 16.8 ± 15.3 | 0.14 |
| Stage 2 percentage, % | 58.8 ± 13.5 | 58.0 ± 14.4 | 56.6 ± 15.5 | 59.0 ± 15.3 | 0.27 |
| SWS percentage, % | 9.6 ± 11.0 | 10.3 ± 10.8 | 10.8 ± 10.1 | 10.8 ± 11.7 | 0.77 |
| REM sleep percentage, % | 13.1 ± 6.8 | 12.7 ± 7.9 | 13.6 ± 7.0 | 13.3 ± 7.0 | 0.69 |
| PLMI, no/h | 25.8 ± 38.9 | 24.1 ± 33.2 | 24.9 ± 40.4 | 26.4 ± 38.7 | 0.48 |
Values are expressed as means ± SD.
Abbreviations: AHI = apnea-hypopnea index, CSA = central sleep apnea, PLMI = periodic leg movement index, SaO2 = oxygen saturation, SWS = slow wave sleep;
P < 0.001 compared to their baseline;
P = 0.04 compared to their baseline
With respect to sleep structure, there were no significant changes in the ArI, total sleep time, sleep efficiency, sleep onset latency, PLMI, or the percentage of any of the sleep stages in the CPAP group compared to the control group (P ≥ 0.14 for all variables). To determine if suppression of CSA affected arousal frequency and sleep structure, we analyzed data in a subgroup of 58 subjects in whom CPAP reduced the AHI to < 15 after 3 months (CPAP-CSA-suppressed group, Table 3). Although the AHI fell by 82% in this subgroup, ArI did not change significantly compared to the control or the CPAP-CSA-unsuppressed groups (P = 0.15). Similarly, none of the sleep structure variables improved in CPAP-CSA-suppressed group compared to the other two groups (P ≥ 0.08 for all variables). Even among the subset of 22 patients in whom CPAP reduced the AHI to < 5 (from 30.1 ± 12.4 to 2.5 ± 1.5, P < 0.001), there was no significant reduction in the ArI (from 24.9 ± 16.3 to 19.3 ± 15.9, P = 0.17).
Table 3.
Sleep Data at Baseline and 3 Months after Randomization According to CPAP-Response
| Control group (n = 108) |
CPAP-CSA-suppressed (n = 58) |
CPAP-CSA-unsuppressed (n = 39) |
Between group P-value | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 3 months | Baseline | 3 months | Baseline | 3 months | ||
| Effect on CSA | |||||||
| Total AHI, no/h | 37.8 ± 15.1 | 37.6 ± 25.7 | 33.8 ± 12.7 | 6.2 ± 3.9 | 46.9 ± 14.9 | 34.6 ± 12.4 | < 0.001* |
| Effect on Arousals | |||||||
| Arousal index, no/h | 24.8 ± 19.3 | 26.3 ± 18.6 | 24.0 ± 16.6 | 18.4 ± 15.5 | 36.4 ± 30.3 | 33.1 ± 21.5 | 0.15 |
| Effect on Sleep Structure | |||||||
| Time in bed, min | 452.4 ± 51.4 | 457.5 ± 48.9 | 456.1 ± 52.2 | 450.8 ± 44.6 | 452.8 ± 51.8 | 464.3 ± 44.9 | 0.14 |
| Sleep period time, min | 421.4 ± 52.7 | 423.7 ± 55.9 | 426.6 ± 57.3 | 420.9 ± 47.1 | 432.4 ± 50.3 | 432.0 ± 47.4 | 0.47 |
| Total sleep time, min | 310.5 ± 84.3 | 308.9 ± 77.6 | 321.4 ± 77.0 | 326.1 ± 65.3 | 314.9 ± 74.7 | 309.9 ± 83.0 | 0.88 |
| Sleep efficiency, % | 68.4 ± 16.5 | 67.8 ± 16.6 | 70.6 ± 16.2 | 72.4 ± 13.3 | 70.1 ± 16.9 | 67.3 ± 18.3 | 0.40 |
| Sleep onset latency, min | 30.8 ± 30.8 | 33.7 ± 36.5 | 28.9 ± 31.0 | 29.7 ± 29.6 | 19.9 ± 21.1 | 32.0 ± 29.4 | 0.08 |
| Wake after sleep onset, min | 111.0 ± 65.4 | 114.8 ± 70.0 | 105.3 ± 62.8 | 94.8 ± 51.5 | 117.4 ± 79.8 | 122.1 ± 80.1 | 0.36 |
| Stage 1 percentage, % | 18.2 ± 12.8 | 18.9 ± 12.9 | 18.4 ± 12.0 | 15.2 ± 14.2 | 20.3 ± 15.5 | 18.8 ± 16.9 | 0.27 |
| Stage 2 percentage, % | 58.8 ± 13.5 | 58.0 ± 14.4 | 55.8 ± 14.6 | 57.3 ± 14.8 | 57.8 ± 16.7 | 61.9 ± 16.1 | 0.20 |
| SWS percentage, % | 9.6 ± 11.0 | 10.3 ± 10.8 | 11.4 ± 10.7 | 12.6 ± 12.2 | 9.8 ± 9.3 | 8.4 ± 10.4 | 0.37 |
| REM sleep percentage, % | 13.1 ± 6.8 | 12.7 ± 7.9 | 14.4 ± 6.6 | 14.8 ± 6.3 | 12.3 ± 7.4 | 10.9 ± 7.3 | 0.45 |
| PLMI, no/h | 25.8 ± 38.9 | 24.1 ± 33.2 | 22.2 ± 32.8 | 23.3 ± 28.5 | 29.8 ± 49.8 | 32.3 ± 50.4 | 0.37 |
Values are expressed as means ± SD.
Abbreviations: AHI = apnea-hypopnea index, CSA = central sleep apnea, PLMI = periodic leg movement index, SWS = slow wave sleep.
Compared the changes over time between; Control vs. CPAP responder P < 0.001, Control vs. CPAP non responder P = 0.01, CPAP responder vs. CPAP non responder P = 0.002.
In the patients who underwent the 2 year assessment, the ArI in those randomized to CPAP (n = 33) did not change significantly from baseline compared to the control group (n = 38) (from 26.3 ± 18.3 to 24.6 ± 18.4 vs. from 27.1 ± 16.0 to 32.0 ± 16.5, respectively, P = 0.11), despite a reduction in AHI (from 38.7 ± 16.1 to 15.5 ± 16.2 vs. from 36.1 ± 15.8 to 34.8 ± 16.6, respectively, P = 0.001). There was also no significant change in total, slow wave, or REM sleep time, or sleep efficiency compared to the control group (all P ≥ 0.18).
DISCUSSION
In this multicenter randomized controlled trial, we found that treating CSA in HF patients with CPAP for 3 months reduced the AHI by 55%, but that this was not accompanied by any significant reduction in the frequency of arousals from sleep or any increases in the amounts of total, slow wave, or REM sleep, or improvement in sleep efficiency. Even among the subgroup in whom CPAP suppressed the AHI by 82% to below 15 after 3 months, the ArI did not decrease significantly. These data suggest that in HF patients with CSA, arousal from sleep is not mainly a consequence of central apneas and hypopneas, but may be incidental to, or play a causative role in the development of CSA by rendering the respiratory control system unstable.11,12,28
Previous studies have reported inconsistent effects of CPAP and other interventions on arousal frequency and sleep structure in HF patients with CSA. In some studies, these interventions lowered the AHI by 40% to 87% in association with 47% to 75% decrease in ArI and an increase in the proportion of both slow wave and REM sleep.13–15 In contrast, other studies reported that although CPAP and other treatments reduced the AHI by 62% to 89%, they had no effect on ArI or the amounts of slow wave or REM sleep.16–21 In this regard, the most interesting study was that of Mansfield and colleagues.29 They studied 13 HF patients with CSA in an uncontrolled study before and 6 months after cardiac transplantation. Their LVEF increased from 19% to 54% and their AHI decreased significantly from 28 to 7. However, neither arousal frequency nor sleep structure changed. These findings suggest the possibility that in some HF patients with CSA, there is an underlying arousal disorder, accompanied by sleep disruption that is neither mainly a consequence of CSA, nor of impaired cardiac function.
The reasons for discrepancies on the effects of treating CSA on arousal frequency and sleep structure between the above studies are not clear. One possibility is that some studies reported only “respiratory-related” arousals. It follows that if the AHI is reduced by an intervention, then respiratory-related arousals must also fall, even if the overall frequency of arousals is unchanged. This can be misleading since, in that case, arousals deemed respiratory-related at baseline would be considered spontaneous when a treatment lowered the AHI. This suggests that some arousals deemed respiratory-related at baseline were not caused by apneas and hypopneas, but that their spontaneous nature was unmasked by treatment of CSA.17 Because all the above studies were small, single-centered, and of short duration, and in none was the significance of arousal frequency and sleep structure in response to interventions discussed as an outcome, the discrepancy in their findings remains a source of controversy, which we have addressed.
Our study has several strengths. First, it specifically addressed, in HF patients with CSA, the long-term effects of an intervention that reduces the AHI (i.e., CPAP) on arousal frequency and sleep structure as a trial outcome, and considered the physiological and clinical ramifications of these findings. Our data are consistent with those previous studies, discussed above, in which interventions that suppressed CSA had no effect on ArI, or the amounts of slow wave and REM sleep. Second, because ours was a prospective, randomized, multicenter (11 sites) trial with the largest number of subjects studied to date (n = 205), our results are liable to be generalizable to the broad population of HF patients with CSA. Because there was a slight tendency for ArI to decrease from baseline to 3 months in the CPAP group, it is possible that if we had studied more patients a significant difference might emerge. However, even at 2 years in a smaller sample, there was no reduction in the ArI in CPAP treated patients, and given the high variance of the measurement, the sample size required to detect such a small difference would have to be several-fold greater than in our study. In addition, if the magnitude of the difference in such a scenario was similar to that in the present study, it would be of little or no physiological or clinical significance. Third, we used uniform, state-of-the-art noninvasive techniques for classifying apneas and hypopneas at all centers with use of respiratory inductive plethysmography and nasal pressure cannulae. A limitation of our study was that we did not classify arousals as being respiratory or non-respiratory related, and did not examine their timing. Nevertheless, since the arousal index did not decrease in the CPAP-treated group despite a 55% reduction in the AHI, our findings imply that HF patients with CSA might have a predisposition to hyperarousability.
In contrast to CSA, in randomized trials involving CPAP-treated patients with OSA, but without HF, reductions in the AHI of 88% to 94% were consistently accompanied by reductions in ArI of 25% to 74%.6–8 CPAP also reduced the amount of stage 1 sleep and increased the amounts of slow wave and REM sleep.8,30 Similarly, in HF patients with OSA, CPAP reduced the AHI significantly by 74% to 79% in association with a significant 53% to 59% reduction in ArI after one month.31–33 Interestingly, however, unlike patients without HF, the CPAP-induced reduction in ArIs were not accompanied by any increases in the amounts of slow wave or REM sleep. These observations support the findings of Artz et al34 that even in the absence of sleep apnea, HF patients have poor sleep structure with reduced sleep efficiency, and amounts of total, slow wave and REM sleep compared to the general population. Thus factors other than sleep apnea, such as pulmonary congestion during the night, other comorbidities or medications, may contribute to poor sleep quality in patients with HF.
Although not all obstructive apneas and hypopneas are terminated by arousals, and upper airway opening can occur in their absence in a small minority of cases,35 arousal is an important defense mechanism for terminating obstructive apneas. The combination of inspiratory efforts against the occluded airway,36 hypoxemia,37 and hypercapnia38 provoke arousal both in spontaneous and experimentally induced obstructive apneas.36,39,40 In contrast to obstructive apneas, central apneas are not accompanied by negative intrathoracic pressure swings, cause less severe oxygen desaturation,41 and are typically accompanied by hypocapnia41 rather than hypercapnia. Therefore, stimuli for arousals from central apneas are generally less potent than those arising from obstructive apneas. In keeping with this view, the ratio of the ArI to the AHI was only 68% in our patients, which is lower than the ratio of 85% to 90% reported in HF patients with OSA in our previous studies.31–33 Furthermore, in HF patients with CSA, arousal is often not necessary for resumption of airflow following central apneas.9,42 In the present study, however, we did not analyze the timing of arousals.
Arousals from sleep provoke respiratory control system instability. They augment ventilation abruptly by increasing the ventilatory response to chemical respiratory stimuli and by causing sudden reinstitution of the non-chemical waking neural drive to breathe.28 This sudden increase in ventilation can cause hypopcapnic apnea.11,12 Indeed, in HF patients with CSA, more than 90% of episodes of Cheyne-Stokes respiration with cyclic hyperpneas and central apneas are precipitated by arousals from sleep and hyperventilation.11 Similarly, it has been postulated that arousals following obstructive apneas might also destabilize the respiratory control system and predispose to upper airway collapse by causing withdrawal of upper airway dilator muscle activity in patients with OSA.35 Thus arousals may act both as a defense mechanism to terminate obstructive events, and perhaps in some instances, as an agent that provokes respiratory instability and facilitates upper airway collapse. However, there is little experimental evidence to support this latter possibility.
Previous small studies, in which attempts were made to suppress arousals, and thus to dampen hyperventilation, yielded inconsistent results. Bonnet et al43 studied the effects of triazolam on arousals and AHI in patients with CSA, but without HF. They observed that a triazolam-induced reduction in the frequency of arousals was accompanied by a decrease in the frequency of central respiratory events. In contrast, in a study involving HF patients with CSA, administration of temazepam decreased the arousal index but did not suppress CSA.44 Taken together, these findings suggest that the presence of arousals in HF patients with CSA is not entirely a consequence of central apneas and hypopneas, but that such arousals may be incidental to, or play a causative or aggravating role in the pathogenesis of CSA in HF patients. Another possibility is that even though CPAP reduced the AHI, and might have reduced respiratory-related arousals, this was offset by arousals caused by discomfort from the CPAP mask. However, this seems unlikely for 3 reasons. First, subjects had 3 months to 2 years during which to habituate to CPAP and used it, on average, for 4.6 h per night during this time, indicating that it was well tolerated. Second, preserved amounts of total, slow wave, and REM sleep times, and sleep efficiency while on CPAP compared to baseline, suggest that CPAP was not disrupting sleep. Third, other interventions such as inhaled CO2 and O2 that reduced the AHI in patients with CSA, but which did not require a tight-fitting face mask, were also reported not to reduce the frequency of arousals or improve sleep structure.45,46
In conclusion, our data demonstrate that despite lowering of the AHI, CPAP had no significant effect on the frequency of arousals, sleep efficiency, or on the amounts of total, slow wave, or REM sleep in HF patients with CSA. Thus, in such patients, arousals from sleep do not appear to be primarily a consequence of central apneas and hypopneas, and thus may not be acting as a defense mechanism to terminate these events in the same way they do obstructive apneas in OSA. It therefore seems possible that HF is a condition that predisposes to pathological hyperarousability or sleep disruption, even in the absence of sleep related breathing disorders.34 In addition, the lack of improvement in sleep structure we observed may be one factor that contributed to the lack of effect that CPAP had on quality of life that we previously reported, despite lowering of the AHI, and improvements in left ventricular systolic function and exercise capacity.22 It would therefore be interesting to further examine interventions that alleviate arousals to see whether this would attenuate CSA, and improve sleep structure.
DISCLOSURE STATEMENT
This study was supported in part by Respironics, ResMed, and Tyco Healthcare. The authors have indicated no other financial conflicts of interest.
ACKNOWLEDGMENTS
The CANPAP Trial was supported by peer-reviewed grant from the Canadian Institutes of Health Research (CIHR). In accordance with CIHR policy, one-third of the funding was provided by the CIHR and two-thirds by industry partners (Respironics Inc, ResMed Inc and Tyco Healthcare Inc). P Ruttanaumpawan was supported by a research fellowship from Siriraj Hospital, Mahidol University, Bangkok, Thailand, and from Toronto Rehabilitation Institute, T.D. Bradley by a Senior Scientist Award from the CIHR, F. Series by a National Scientist Award from the Fonds de la Recherche en Sante du Quebec, and JS Floras by a Canada Research Chair in Integrative Cardiovascular Biology and a Career Investigator Award from the Heart and Stroke Foundation of Ontario.
Trial Registration # ISRCTN25258560 at www.controlled-trails.com
APPENDIX
CANPAP Administration
Executive Committee:
T.D. Bradley (Chair), A.G. Logan and J.S. Floras.
Advisory Committee:
N. Anthonisen and E.R. Smith.
Data and Safety Monitoring Committee:
M. Bourassa (Chair), D. Stewart, and L. Magee.
CANPAP Sites and Investigators
End Point Adjudication Committee:
J.S Floras (Chair), I. Belenkie and J. Howlett.
In Canada: University of Calgary, Calgary: I. Belenkie and W. Whitelaw, University of Alberta, Edmonton: M. Heule, Dalhousie University, Halifax: D. Morrison and J. Howlett, University of Western Ontario, London: K.A. Ferguson, McGill University, Montreal: R.J. Kimoff and M. Smilovitch, Laval University, Quebec City: F. Series and M.H. Leblanc, University of Toronto, Toronto: Site A - Toronto General Hospital/University Health Network: T.D. Bradley, A.G. Logan, J.S. Floras, H. Ross, D. Delgado and R.S.T. Leung, Site B – St. Michael's Hospital: P.J. Hanly, University of British Columbia, Vancouver: J. Fleetham, K. Gin and J. Wilson, University of Manitoba, Winnipeg: S. Corne and E. Azevedo.
In Germany: University of Regensburg, Regensburg: M. Pfeifer, S. Montalvan-Dobmayr, T. Schichtl and M. Arzt.
CANPAP Managers: F.S. Fitzgerald, N. Catherine and G. Hopkins.
Data Management Centre: The Prosserman Centre for Health Research, Samuel Lunenfeld Research Institute of the Mount Sinai Hospital, University of Toronto, Toronto, Ontario (D. Ng, Data Manager).
Statistician: G. Tomlinson.
Footnotes
See appendix for a complete list of the investigators and their affiliations
REFERENCES
- 1.Collard P, Dury M, Delguste P, Aubert G, Rodenstein DO. Movement arousals and sleep-related disordered breathing in adults. Am J Respir Crit Care Med. 1996;154:454–9. doi: 10.1164/ajrccm.154.2.8756822. [DOI] [PubMed] [Google Scholar]
- 2.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–6. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 3.Dingli K, Fietze I, Assimakopoulos T, Quispe-Bravo S, Witt C, Douglas NJ. Arousability in sleep apnoea/hypopnoea syndrome patients. Eur Respir J. 2002;20:733–40. doi: 10.1183/09031936.02.00262002. [DOI] [PubMed] [Google Scholar]
- 4.Martin SE, Engleman HM, Kingshott RN, Douglas NJ. Microarousals in patients with sleep apnoea/hypopnoea syndrome. J Sleep Res. 1997;6:276–80. doi: 10.1111/j.1365-2869.1997.00276.x. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–5. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 6.Lam B, Sam K, Mok WY, et al. Randomised study of three non-surgical treatments in mild to moderate obstructive sleep apnoea. Thorax. 2007;62:354–9. doi: 10.1136/thx.2006.063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loredo JS, Ancoli-Israel S, Dimsdale JE. Effect of continuous positive airway pressure vs placebo continuous positive airway pressure on sleep quality in obstructive sleep apnea. Chest. 1999;116:1545–9. doi: 10.1378/chest.116.6.1545. [DOI] [PubMed] [Google Scholar]
- 8.McArdle N, Douglas NJ. Effect of continuous positive airway pressure on sleep architecture in the sleep apnea-hypopnea syndrome: a randomized controlled trial. Am J Respir Crit Care Med. 2001;164:1459–63. doi: 10.1164/ajrccm.164.8.2008146. [DOI] [PubMed] [Google Scholar]
- 9.Hanly PJ, Millar TW, Steljes DG, Baert R, Frais MA, Kryger MH. Respiration and abnormal sleep in patients with congestive heart failure. Chest. 1989;96:480–8. doi: 10.1378/chest.96.3.480. [DOI] [PubMed] [Google Scholar]
- 10.Ancoli-Israel S, Engler RL, Friedman PJ, Klauber MR, Ross PA, Kripke DF. Comparison of patients with central sleep apnea with and without Cheyne-Stokes respiration. Chest. 1994;106:780–6. doi: 10.1378/chest.106.3.780. [DOI] [PubMed] [Google Scholar]
- 11.Naughton M, Benard D, Tam A, Rutherford R, Bradley TD. Role of hyperventilation in the pathogenesis of central sleep apneas in patients with congestive heart failure. Am Rev Respir Dis. 1993;148:330–8. doi: 10.1164/ajrccm/148.2.330. [DOI] [PubMed] [Google Scholar]
- 12.Xie A, Wong B, Phillipson EA, Slutsky AS, Bradley TD. Interaction of hyperventilation and arousal in the pathogenesis of idiopathic central sleep apnea. Am J Respir Crit Care Med. 1994;150:489–95. doi: 10.1164/ajrccm.150.2.8049835. [DOI] [PubMed] [Google Scholar]
- 13.Hu K, Li QQ, Yang J, Chen XQ, Hu SP, Wu XJ. The role of high-frequency jet ventilation in the treatment of Cheyne-Stokes respiration in patients with chronic heart failure. Int J Cardiol. 2006;106:224–31. doi: 10.1016/j.ijcard.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Kohnlein T, Welte T, Tan LB, Elliott MW. Assisted ventilation for heart failure patients with Cheyne-Stokes respiration. Eur Respir J. 2002;20:934–41. doi: 10.1183/09031936.00.02622001. [DOI] [PubMed] [Google Scholar]
- 15.Teschler H, Dohring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164:614–9. doi: 10.1164/ajrccm.164.4.9908114. [DOI] [PubMed] [Google Scholar]
- 16.Krachman SL, D'Alonzo GE, Berger TJ, Eisen HJ. Comparison of oxygen therapy with nasal continuous positive airway pressure on Cheyne-Stokes respiration during sleep in congestive heart failure. Chest. 1999;116:1550–7. doi: 10.1378/chest.116.6.1550. [DOI] [PubMed] [Google Scholar]
- 17.Javaheri S. Effects of continuous positive airway pressure on sleep apnea and ventricular irritability in patients with heart failure. Circulation. 2000;101:392–7. doi: 10.1161/01.cir.101.4.392. [DOI] [PubMed] [Google Scholar]
- 18.Arzt M, Schulz M, Wensel R, et al. Nocturnal continuous positive airway pressure improves ventilatory efficiency during exercise in patients with chronic heart failure. Chest. 2005;127:794–802. doi: 10.1378/chest.127.3.794. [DOI] [PubMed] [Google Scholar]
- 19.Granton JT, Naughton MT, Benard DC, Liu PP, Goldstein RS, Bradley TD. CPAP improves inspiratory muscle strength in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med. 1996;153:277–82. doi: 10.1164/ajrccm.153.1.8542129. [DOI] [PubMed] [Google Scholar]
- 20.Naughton MT, Benard DC, Liu PP, Rutherford R, Rankin F, Bradley TD. Effects of nasal CPAP on sympathetic activity in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med. 1995;152:473–9. doi: 10.1164/ajrccm.152.2.7633695. [DOI] [PubMed] [Google Scholar]
- 21.Naughton MT, Liu PP, Bernard DC, Goldstein RS, Bradley TD. Treatment of congestive heart failure and Cheyne-Stokes respiration during sleep by continuous positive airway pressure. Am J Respir Crit Care Med. 1995;151:92–7. doi: 10.1164/ajrccm.151.1.7812579. [DOI] [PubMed] [Google Scholar]
- 22.Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–33. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 23.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring for sleep stages of human subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 24.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 25.Recording and scoring leg movements.: The ASDA Atlas Task Force. 1993. Dec, [PubMed]
- 26.Staats BA, Bonekat HW, Harris CD, Offord KP. Chest wall motion in sleep apnea. Am Rev Respir Dis. 1984;130:59–63. doi: 10.1164/arrd.1984.130.1.59. [DOI] [PubMed] [Google Scholar]
- 27.Arzt M, Floras JS, Logan AG, et al. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP) Circulation. 2007;115:3173–80. doi: 10.1161/CIRCULATIONAHA.106.683482. [DOI] [PubMed] [Google Scholar]
- 28.Yumino D, Bradley TD. Central sleep apnea and Cheyne-Stokes respiration. Proc Am Thorac Soc. 2008;5:226–36. doi: 10.1513/pats.200708-129MG. [DOI] [PubMed] [Google Scholar]
- 29.Mansfield DR, Solin P, Roebuck T, Bergin P, Kaye DM, Naughton MT. The effect of successful heart transplant treatment of heart failure on central sleep apnea. Chest. 2003;124:1675–81. doi: 10.1378/chest.124.5.1675. [DOI] [PubMed] [Google Scholar]
- 30.Bardwell WA, Ancoli-Israel S, Berry CC, Dimsdale JE. Neuropsychological effects of one-week continuous positive airway pressure treatment in patients with obstructive sleep apnea: a placebo-controlled study. Psychosom Med. 2001;63:579–84. doi: 10.1097/00006842-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233–41. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 32.Ruttanaumpawan P, Gilman MP, Usui K, Floras JS, Bradley TD. Sustained effect of continuous positive airway pressure on baroreflex sensitivity in congestive heart failure patients with obstructive sleep apnea. J Hypertens. 2008;26:1163–8. doi: 10.1097/HJH.0b013e3282fb81ed. [DOI] [PubMed] [Google Scholar]
- 33.Ryan CM, Usui K, Floras JS, Bradley TD. Effect of continuous positive airway pressure on ventricular ectopy in heart failure patients with obstructive sleep apnoea. Thorax. 2005;60:781–5. doi: 10.1136/thx.2005.040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arzt M, Young T, Finn L, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166:1716–22. doi: 10.1001/archinte.166.16.1716. [DOI] [PubMed] [Google Scholar]
- 35.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–33. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- 36.Gleeson K, Zwillich CW, White DP. The influence of increasing ventilatory effort on arousal from sleep. Am Rev Respir Dis. 1990;142:295–300. doi: 10.1164/ajrccm/142.2.295. [DOI] [PubMed] [Google Scholar]
- 37.Bowes G, Townsend ER, Kozar LF, Bromley SM, Phillipson EA. Effect of carotid body denervation on arousal response to hypoxia in sleeping dogs. J Appl Physiol. 1981;51:40–5. doi: 10.1152/jappl.1981.51.1.40. [DOI] [PubMed] [Google Scholar]
- 38.Hedemark LL, Kronenberg RS. Ventilatory and heart rate responses to hypoxia and hypercapnia during sleep in adults. J Appl Physiol. 1982;53:307–12. doi: 10.1152/jappl.1982.53.2.307. [DOI] [PubMed] [Google Scholar]
- 39.Hlavac MC, Catcheside PG, McDonald R, Eckert DJ, Windler S, McEvoy RD. Hypoxia impairs the arousal response to external resistive loading and airway occlusion during sleep. Sleep. 2006;29:624–31. [PubMed] [Google Scholar]
- 40.Kimoff RJ, Makino H, Horner RL, et al. Canine model of obstructive sleep apnea: model description and preliminary application. J Appl Physiol. 1994;76:1810–7. doi: 10.1152/jappl.1994.76.4.1810. [DOI] [PubMed] [Google Scholar]
- 41.Sin DD, Fitzgerald F, Parker JD, et al. Relationship of systolic BP to obstructive sleep apnea in patients with heart failure. Chest. 2003;123:1536–43. doi: 10.1378/chest.123.5.1536. [DOI] [PubMed] [Google Scholar]
- 42.Bradley TD, Floras JS. Sleep apnea and heart failure: Part II: central sleep apnea. Circulation. 2003;107:1822–6. doi: 10.1161/01.CIR.0000061758.05044.64. [DOI] [PubMed] [Google Scholar]
- 43.Bonnet MH, Dexter JR, Arand DL. The effect of triazolam on arousal and respiration in central sleep apnea patients. Sleep. 1990;13:31–41. doi: 10.1093/sleep/13.1.31. [DOI] [PubMed] [Google Scholar]
- 44.Biberdorf DJ, Steens R, Millar TW, Kryger MH. Benzodiazepines in congestive heart failure: effects of temazepam on arousability and Cheyne-Stokes respiration. Sleep. 1993;16:529–38. doi: 10.1093/sleep/16.6.529. [DOI] [PubMed] [Google Scholar]
- 45.Andreas S, Weidel K, Hagenah G, Heindl S. Treatment of Cheyne-Stokes respiration with nasal oxygen and carbon dioxide. Eur Respir J. 1998;12:414–9. doi: 10.1183/09031936.98.12020414. [DOI] [PubMed] [Google Scholar]
- 46.Xie A, Rankin F, Rutherford R, Bradley TD. Effects of inhaled CO2 and added dead space on idiopathic central sleep apnea. J Appl Physiol. 1997;82:918–26. doi: 10.1152/jappl.1997.82.3.918. [DOI] [PubMed] [Google Scholar]