This study emphasizes the wide heterogeneity of the course of cerebral vasculopathy in children with sickle-cell anemia on regular transfusion protocol.
Keywords: sickle-cell anemia, children, cerebral vasculopathy, stroke
Abstract
This retrospective study assessed the long-term effect of transfusional exchange therapy on MRA/MRI abnormalities in 24 homozygous sickle-cell anemia (HbSS) children presenting with abnormal brain MRA. The median time elapsed from baseline to last available MRA was 29 months. Follow-up MRAs showed improvement, stabilization or worsening of cerebrovascular lesions in 11, 6 and 7 patients respectively. Complete normalization of MRA was observed in 6 patients within a mean time of 1.4 years, but stenosis recurred at the same location in the 4 patients in whom transfusion therapy was discontinued. Baseline severe stenosis/occlusion of large cerebral arteries and occurrence of moyamoya syndrome were significantly associated with an absence of improvement of the cerebral vasculopathy. These data emphasize the heterogeneity of the course of cerebrovasculopathy in SS children receiving chronic transfusion. Further studies are needed to determine whether different therapeutic approaches have to be considered according to these different evolutive patterns in SS children.
Introduction
Cerebral vasculopathy is a devastating complication of sickle cell disease.1 Chronic transfusions are effective in preventing the short-term recurrence of infarctive stroke2 and the occurrence of a first cerebral infarction in HbSS children who have abnormally high velocities on transcranial Doppler ultrasonography (TCD).3 After acute stroke in sickle cell anemia patients, chronic transfusion therapy has been shown to nearly stop progression of stenosis in most children,4,5 but progressive large vessel disease has been evidenced in HbSS children presenting with moyamoya syndrome.6 The purpose of our study was to assess the long-term effect of repeated transfusion on brain magnetic resonance angiography (MRA) in 24 HbSS children presenting with narrowing of large cerebral arteries, regardless of the initial presentation of their cerebral vasculopathy.
Design and Methods
Patients
One thousand and seventy-five children with sickle cell disease were followed in our center between January 2000 and July 2007. HbSS and HbS-β0 children were screened yearly for cerebral vasculopathy by TCD. Patients with abnormally high velocity (>200 cm/sec) in at least one artery and those with a neurological event were imaged by MRI/MRA. Among them, we retrospectively reviewed the records of all patients who i) had cerebral vasculopathy on cerebral MRA; ii) received regular exchange transfusion (ET); iii) who were followed for a minimum of one year after the onset of transfusion therapy and iv) who had at least two available MRAs. Patients were assessed for indication for MRA, TCD imaging, brain MRA and MRI and mean hemoglobin S value (Hb S). Oral consent was obtained from the parents and, when possible, the patients, in keeping with French national recommendations. In accordance with French regulations, no ethical committee agreement was needed for this retrospective study.
Transfusion therapy
Patients who presented with an acute neurological event received one exchange transfusion equal to 1/2 of their blood volume within 24 hours following stroke. The remaining patients were put on a monthly transfusion program for three months as soon as abnormal velocities were identified on TCD. Subsequently all patients received exchange blood transfusions at 3–5 week intervals to maintain hemoglobin levels between 9 and 10 g/dL and HbS at less than 30%. Exchange transfusion was defined as either manual exchange transfusion or automated erythrocytopheresis. Reported HbS values where measured prior to each transfusional therapy by high performance liquid chromatography.
Transcranial Doppler
Duplex TCD imaging was performed using several ultrasound systems (Hitachi, Toshiba) as previously described.7
Brain magnetic resonance angiography and brain magnetic resonance imaging
Brain MRA/MRI was performed within 48 hours following an acute stroke, and within three months following the demonstration of an abnormal TCD screening. MRI/MRA was repeated every 1–2 years in each patient. Patients with moderate vascular disease had one more MRI/MRA six months after the first imaging. When MRA abnormalities resolved, a second MRA was performed within six months to confirm these features. MRI/MRA included brain parenchyma study by axial T1 and FLAIR sequences, coronal T2 turbo spin echo, and axial diffusion sequence, and a study of the Willis arterial circle by a 3D time-of-flight sequence.
Imaging data were reviewed by two pediatric radiologists (MEB, SV) and included the presence or absence of vascular lesions, their locations and severity, the presence or absence of moyamoya syndrome, and the presence or absence of parenchymal lesions; a score was assigned to each lesion. Degree of vessel stenosis (stenosis score) was assessed by the reviewers for each artery by a code number: 4-occluded vessel, 3-severely stenosed (75%), 2-moderately stenosed (50%), 1-mildly stenosed (25%), 0-normal vessel; moyamoya syndrome was defined as absent, moderate or severe (codes of 0, 1, 2 respectively). MRA score was obtained by summarizing the codes of each lesion, and ranged from 0 to 35. Brain lesions were classified as absent, white matter lesions, jonctional infarct, territory infarct (codes of 0, 1, 2, 3 respectively); cerebral atrophy was classified as absent, mild, moderate or severe (codes of 0, 1, 2, 3 respectively). MRI score was obtained by summarizing the codes of each lesion, and ranged from 0 to 21. Mild and moderate stenoses were grouped under the term “moderate abnormality”.
Statistical analysis
Discrete data were reported as frequencies. Non-normally distributed data are reported as median (first, third quartile). Discrete data were compared by the Fischer test. Continuous variables were compared by the Wilcoxon-Mann Whitney test. Monthly Hb S values were entered as time-dependent covariate in a Cox model. All tests were bilateral and alpha level of 5% was considered as statistically significant. Statistical analysis was performed using the SAS 9.1 (SAS Inc, Cary, NC, USA) software package.
Results and Discussion
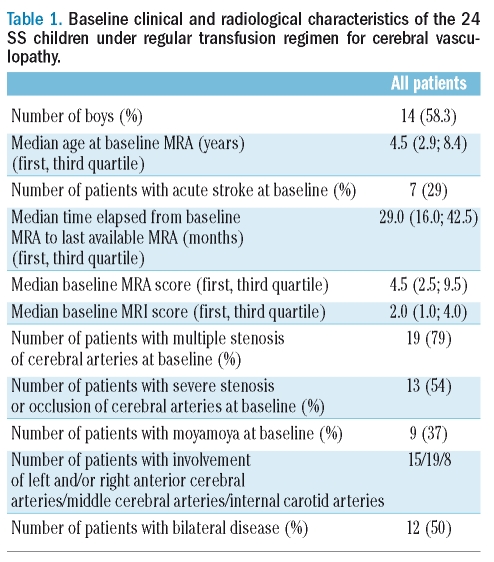
Twenty-four HbSS patients (14 males, 10 females) were included. Indication for MRA/MRI was abnormally high velocities on TCD screening in 17 patients or occurrence of an acute neurological event in 7 patients. Acute stroke occurred at a median age of 2.1 years (range, 1–6.6), and abnormally high velocities on TCD screening was detected at a median age of 8.5 years (range, 2.1–13.7) in the 17 remaining patients. The main baseline clinical and radiological characteristics of the patients are listed in Table 1. Baseline MRI was normal in 6 patients, and revealed territory infarct, jonctional infarct and white matter lesions in 12, 4 and 4 patients respectively.
Table 1.
Baseline clinical and radiological characteristics of the 24 SS children under regular transfusion regimen for cerebral vasculopathy.
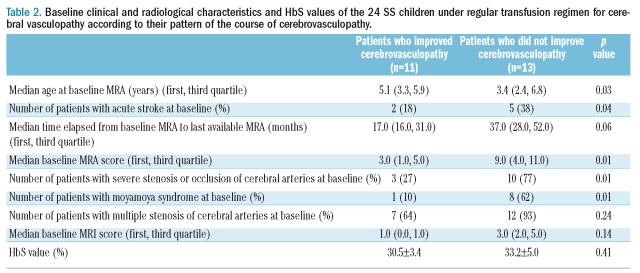
The median time elapsed from baseline to last available MRA was 29 months. Three evolutive patterns were observed. Cerebral vasculopathy improved in 11 patients with a decrease of median MRA score from 3 (range, 1–9) to 0 (range, 0–6). It remained stable in 6 patients (median MRA 5, range, 2–13). It worsened in 7 patients with an increase of median MRA score from 10 (range, 2–16) to 14 (range, 4–20), including the single patient who experienced a recurrent stroke. These three patterns were observed in the patients who presented with stroke at baseline (2/7, 2/7 and 3/7 patients respectively), as well as in the remaining patients who underwent a systematic screening (9/17, 4/17 and 4/17 patients respectively). The new stenoses involved mostly anterior cerebral arteries (5 stenoses) and less frequently internal carotid arteries (2 stenoses). Complete normalization of MRA was observed in 6 patients within a mean time of 1.4 years (range, 1–1.8 years); TCD was informative in 3 of these patients in whom normal cerebral velocities were evidenced. Transfusion therapy was stopped in 4/6 patients, but mild or moderate stenosis recurred at the same location, 10–16 months after the last exchange transfusion; cerebral velocities returned to conditional (2 patients) or abnormal values (2 patient). Baseline median MRI score was 2 (range, 0–8). None of the patients developed new or more extensive brain lesions, except for cerebral atrophy in 5 patients. Baseline severe stenosis/occlusion of large cerebral arteries and occurrence of moyamoya syndrome were significantly associated with an absence of improvement of the cerebral vasculopathy (Table 2). Conversely, MRI score, number of cerebral stenosis, median time elapsed from baseline to last available MRA and HbS value did not differ significantly between the patients who experienced an improvement in cerebral vasculopathy and those who did not (Table 2). Patients who presented with stroke where less likely to show an improvement in their cerebral vasculopathy than those who were diagnosed through systematic TCD screening, but the number of patients was low, and the difference did not reach significance.
Table 2.
Baseline clinical and radiological characteristics and HbS values of the 24 SS children under regular transfusion regimen for cerebral vasculopathy according to their pattern of the course of cerebrovasculopathy.
The present study emphasizes the wide heterogeneity of the course of cerebral vasculopathy in HbSS children on regular transfusion protocol. Vessel abnormalities have been reversed in one quarter of the patients, while MRA evidenced progressive large vessel disease in one other quarter. The pathophysiology of SCD cerebrovasculopathy remains poorly understood, and might be determined by a genetic factor, such as VCAM polymorphisms, which alters stroke risk.8 Different therapeutic approaches might be considered according to these different patterns of evolution. Hydroxyurea (HU) has been proposed as an alternative to transfusion therapy in children with cerebrovascular disease.9 Multiple properties may explain the beneficial effects of HU: decrease in expression of red cell and endothelial-cell adhesion molecules, decrease in neutrophil and reticulocyte counts, inhibition of erythrocyte sickling by increasing fetal hemoglobin and decrease in the plasma-free hemoglobin by giving nitric-oxyde.10 HU decreased elevated TCD velocities,11,12 or allowed transfusion to be stopped in some patients who had normalized high velocities upon transfusion therapy.7 However, in all these studies, a subset of patients did not respond to HU, presenting with a higher rate of stroke recurrence than expected with transfusion therapy,11,12 or redeveloping high velocities when off transfusion.7 Multicenter trials are needed to identify a selected SS children population who might be switched from transfusion regimen to HU therapy. In our series, recurrence of arterial stenosis at the same location was observed in 4 children after discontinuation of transfusion therapy, as previously reported in 2 children.4 These features suggest the persistence of endothelial dysfunction as the result of intimal hypertrophy, as evidenced in Kawasaki disease after regression of coronary aneurysms.13 Thus, treatment must not be disrupted even after disappearance of cerebral stenosis on MRA imaging. The respective indications for regular transfusion regimen and HU therapy in these patients remain to be determined. Conversely, progressive large vessel changes were observed in one quarter of patients and was associated with high baseline stenosis and moyamoya scores, reflecting a severe endothelial involvement. Moyamoya syndrome had been previously shown to be associated with progressive vessel narrowing in children who received transfusion therapy.6 Risk of recurrence of stroke is decreased from 25.6/100 patient-years in non-transfused children to 4.2/100 patient-years in children receiving chronic transfusion,2 but not completely suppressed, as evidenced in our series. Thus, new therapeutic approaches are needed in the subset of patients presenting with progressive cerebral vascular disease leading to recurrent strokes despite transfusion therapy.
Finally, our data emphasize the heterogeneity of the course of cerebrovasculopathy in SS children receiving chronic transfusion. Further studies are needed to determine if different therapeutic approaches have to be considered according to these different patterns of evolution.
Acknowledgments
we thank Mrs Priscilla Armoogum for her help with statistical analysis.
Footnotes
Authorship and Disclosures
BB-M, SV, ME-B, GI, FS, SF, FM, RD, AB, MB have participated in the conception of the study, in the analysis of the data and in writing the manuscript. CA, IZ have participated in the analysis and statistical interpretation of data.
The authors reported no potential conficts of interest.
References
- 1.Wang WC, Kovnar EH, Tonkin IL, Mulhern RK, Langston JW, Day SW, et al. High risk of recurrent stroke after discontinuance of five to twelve years of transfusion therapy in patients with sickle cell disease. J Pediatr. 1991;118:377–82. doi: 10.1016/s0022-3476(05)82150-x. [DOI] [PubMed] [Google Scholar]
- 2.Pegelow CH, Adams RJ, McKie V, Abboud M, Berman B, Miller ST, et al. Risk of recurrent stroke in patients with sickle cell disease treated with erythrocyte transfusions. J Pediatr. 1995;126:896–9. doi: 10.1016/s0022-3476(95)70204-0. [DOI] [PubMed] [Google Scholar]
- 3.Adams RJ, McKie VC, Hsu L, Files B, Vichinsky E, Pegelow C, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 4.Russell MO, Goldberg HI, Hodson A, Kim HC, Halus J, Reivich M, et al. Effect of transfusion therapy on arteriographic abnormalities and on recurrence of stroke in sickle cell disease. Blood. 1984;63:162–9. [PubMed] [Google Scholar]
- 5.Abboud MR, Cure J, Granger S, Gallagher D, Hsu L, Wang W, et al. STOP study. Magnetic resonance angiography in children with sickle cell disease and abnormal transcranial Doppler ultrasonography findings enrolled in the STOP study. Blood. 2004;103:2822–6. doi: 10.1182/blood-2003-06-1972. [DOI] [PubMed] [Google Scholar]
- 6.Dobson SR, Holden KR, Nietert PJ, Cure JK, Laver JH, Disco D, et al. Moyamoya syndrome in childhood sickle cell disease: a predictive factor for recurrent cerebrovascular events. Blood. 2002;99:3144–50. doi: 10.1182/blood.v99.9.3144. [DOI] [PubMed] [Google Scholar]
- 7.Bernaudin F, Verlhac S, Coic L, Lesprit E, Brugieres P, Reinert P. Long-term follow-up of pediatric sickle cell disease patients with abnormal high velocities on transcranial Doppler. Pediatr Radiol. 2005;35:242–8. doi: 10.1007/s00247-005-1419-5. [DOI] [PubMed] [Google Scholar]
- 8.Taylor JG, 6th, Tang DC, Savage SA, Leitman SF, Heller SI, Serjeant GR, et al. Variants in the VCAM1 gene and risk for symptomatic stroke in sickle cell disease. Blood. 2002;100:4303–9. doi: 10.1182/blood-2001-12-0306. [DOI] [PubMed] [Google Scholar]
- 9.Switzer JA, Hess DC, Nichols FT, Adams RJ. Pathophysiology and treatment of stroke in sickle-cell disease: present and future. Lancet Neurol. 2006;5:501–12. doi: 10.1016/S1474-4422(06)70469-0. [DOI] [PubMed] [Google Scholar]
- 10.Gladwin MT, Shelhamer JH, Ognibene FP, Pease-Fye ME, Nichols JS, Link B, et al. Nitric oxide donor properties of hydroxyurea in patients with sickle-cell disease. Br J Haematol. 2002;116:436–44. doi: 10.1046/j.1365-2141.2002.03274.x. [DOI] [PubMed] [Google Scholar]
- 11.Gulbis B, Haberman D, Dufour D, Christophe C, Vermylen C, Kagambega F, et al. Sickle cell disease in children and for prevention of cerebrovascular events: the Belgian experience. Blood. 2005;105:2685–90. doi: 10.1182/blood-2004-07-2704. [DOI] [PubMed] [Google Scholar]
- 12.Zimmerman SA, Schultz WH, Burgett S, Mortier NA, Ware RE. Hydroxyurea therapy lowers transcranial Doppler flow velocities in children with sickle cell anemia. Blood. 2007;110:1043–7. doi: 10.1182/blood-2006-11-057893. [DOI] [PubMed] [Google Scholar]
- 13.Furuyama H, Odagawa Y, Katoh C, Iwado Y, Ito Y, Noriyasu K, et al. Altered myocardial flow reserve and endothelial function late after Kawasaki disease. J Pediatr. 2003;142:149–54. doi: 10.1067/mpd.2003.46. [DOI] [PubMed] [Google Scholar]