This study shows that paroxysmal hemoglobinuria clones can be found in many patients with low risk myelodysplastic syndromes. See related perspective article on page 3.
Keywords: paroxysmal nocturnal hemoglobinuria, myelodysplastic syndrome, glycosylphosphatidylinositol-anchored proteins, flow cytometry immunophenotyping, aerolysin
Abstract
Background
The presence of paroxysmal nocturnal hemoglobinuria clones in the setting of aplastic anemia or myelodysplastic syndrome has been shown to have prognostic and therapeutic implications. However, the status of paroxysmal nocturnal hemoglobinuria clones in various categories of myelodysplastic syndrome and in other bone marrow disorders is not well-studied.
Design and Methods
By using multiparameter flow cytometry immunophenotypic analysis with antibodies specific for four glycosylphosphatidylinositol-anchored proteins (CD55, CD59, CD16, CD66b) and performing an aerolysin lysis confirmatory test in representative cases, we assessed the paroxysmal nocturnal hemoglobinuria-phenotype granulocytes in 110 patients with myelodysplastic syndrome, 15 with myelodysplastic/myeloproliferative disease, 5 with idiopathic myelofibrosis and 6 with acute myeloid leukemia.
Results
Paroxysmal nocturnal hemoglobinuria-phenotype granulocytes were detected in nine patients with low grade myelodysplastic syndrome who showed clinicopathological features of bone marrow failure, similar to aplastic anemia. All paroxysmal nocturnal hemoglobinuria-positive cases demonstrated loss of the four glycosylphosphatidylinositol-anchored proteins, with CD16−CD66b− clones being larger than those of CD55−CD59− (p<0.05). Altered glycosylphosphatidylinositol-anchored protein expression secondary to granulocytic hypogranulation, immaturity, and/or immunophenotypic abnormalities was present in a substantial number of cases and diagnostically challenging.
Conclusions
These results show that routine screening for paroxysmal nocturnal hemoglobinuria clones in patients with an intrinsic bone marrow disease who show no clinical evidence of hemolysis has an appreciable yield in patients with low grade myelodysplastic syndromes. The recognition of diagnostic caveats and pitfalls associated with the underlying intrinsic bone marrow disease is essential in interpreting paroxysmal nocturnal hemoglobinuria testing correctly. In our experience, the CD16/CD66b antibody combination is superior to CD55/CD59 in screening for subclinical paroxysmal nocturnal hemoglobinuria because it detects a large clone size and is less subject to analytical interference.
Introduction
Classic paroxysmal nocturnal hemoglobinuria (PNH) is an acquired hemolytic anemia characterized by an increased number of cells with deficiency of glycosylphosphatidylinositol (GPI)-anchored membrane proteins as a result hematopoietic progenitor cells with a phosphatidlyinositol glycan complementation group A (PIG-A) gene mutation.1 A small population of blood cells deficient in GPI-anchored proteins can be present in patients with bone marrow failure who show no clinical or laboratory signs of hemolysis.2,3 The presence of such small populations of cells deficient in GPI-anchored proteins in the setting of aplastic anemia or myelodysplastic syndromes (MDS) has been shown to have important prognostic and therapeutic implications,4–7 and the International PNH Group8 has, therefore, defined the association of PNH clones in aplastic anemia and MDS as subclinical PNH.
In classic PNH, CD55 and CD59 are the most relevant GPI-anchored proteins, since their deficiency leads to an increased sensitivity of PNH red cells to complement-mediated intravascular hemolysis.1,9 In subclinical PNH, however, even in the presence of a somatic mutation of the PIG-A gene in some of the hematopoietic stem cells and their progeny,10,11 the intrinsic abnormality is not conferred to the PIG-A-mutated cells, but represents a clonal expansion of PNH-phenotype cells related to immune-mediated destruction of stem cells12 or a growth advantage in an unfavorable bone marrow microenvironment.13 In screening for PNH clones in subclinical PNH granulocytes are the preferred cell population for analysis because of their short half life and less interference from prior red-cell transfusions.5,8,14–16 In addition to CD55 and CD59, CD16, CD24, and/CD66b have also been found to be useful in analyzing granulocytes.6,17,18
Clinical studies of subclinical PNH in association with MDS have been conducted predominantly on patients diagnosed as having refractory anemia (RA) according to the French-British-American (FAB) classification of MDS. The presence of PNH clones in different categories of MDS has only been reported in a limited number of studies,5,19,20 and the conclusions have been contradictory. Iwanaga et al.20 and Wang et al.5 found that cells with a PNH-phenotype (PNH+) were only present in patients with RA (according to the FAB definition), but not in patients with other subtypes of MDS. Wang et al.5 also found that the RA patients with a detectable PNH+ clone had a more indolent clinical course as compared with PNH-negative RA patients. In contrast, Kaiafa et al.19 recently reported that PNH+ cells were significantly more pronounced in high-grade MDS, such as refractory anemia with excess blasts (RAEB), RAEB in transformation (RAEB-t) and chronic myelomonocytic anemia (CMML). They concluded that the presence of a higher level of PNH+ cells in MDS predicted a poor clinical outcome.
In this study, we assessed the presence of PNH+ granulocytes in 136 patients diagnosed with various categories of MDS and related bone marrow intrinsic disorders by using a highly sensitive flow cytometry (FCM) immunophenotyping assay to test for the expression of multiple GPI-anchored proteins and by performing an aerolysin lysis confirmatory test in representative cases. We describe the clinicopathological characteristics of the subclinical PNH cases as well as the diagnostic caveats and pitfalls in screening for PNH clones in patients with MDS and other bone marrow disorders.
Design and Methods
Patients and samples
Peripheral blood samples were collected over a 1-year period (December 2005 to December 2006) from patients who were referred to UMass Memorial Medical Center because of a recent diagnosis or differential diagnosis of MDS. The PNH test was requested by treating clinicians according to their clinical protocols. None of the patients had been previously treated with cytotoxic or other experimental drugs. All patients included in this study had a bone marrow aspirate and biopsy performed at UMass at the same time as the PNH tests were performed. The bone marrow samples were submitted for morphological evaluation, FCM immunophenotyping and cytogenetic analysis. Peripheral blood smears were obtained from all cases for review. All MDS patients enrolled at UMass were treated by the same group of hematologists. The treatment modalities included transfusion of blood products, administration of growth factors, administration of Food and Drug Administration (FDA)-approved drugs for MDS, such as azacitidine, decitabine, lenalidomide, and experimental protocols using a variety of agents such as thalidomide, arsenic trioxide, lintizumab, and tipifarnib. Immune suppressive therapy, such as antithymocyte globulin and cyclosporine, was not included in the protocols.
Peripheral blood samples obtained from 30 healthy adult donors were also analyzed for test validation, and one healthy control was included in each test run.
Morphological evaluation and disease classification
Morphological evaluation was performed independently by at least two hematopathologists in accordance with WHO criteria.21 For each individual case, routine hematoxylin and eosin stained histological sections of bone marrow biopsy and clot specimens and well-prepared Wright-Giemsa-stained aspirate smears were evaluated, and a differential count of 500 bone marrow nucleated cells was performed based on aspirate smears. Perls’ reaction for iron was performed on bone marrow aspirates and a silver impregnation stain for reticulin was performed on the bone marrow biopsy samples, if necessary. To define morphological dysplasia strictly, the features of dyserythropoiesis, dysgranulopoiesis and dysmegakaryopoiesis had to be present in at least 10% of cells of the respective lineage. The diagnosis of acute myeloid leukemia (AML) was made based on a blast count of greater or equal than 20% in the bone marrow and/or peripheral blood. Peripheral blood smears were reviewed in all cases and a manual differential count of 200 cells was performed when immature cells were present.
Four-color flow cytometric immunophenotypic analysis
For all specimens, four-color FCM was performed on Coulter FC-500 instruments with all antibodies purchased from Beckman Coulter (BC) (Hialeah, FL, USA). Red blood cells were lysed with buffered ammonium chloride and the remaining cells were washed once with phosphate-buffered saline-bovine serum albumin (PBS-BSA)-azide, and re-suspended to the desired cell concentration in PBS-BSA. One hundred microliters of the cell suspension (5×105 to 1×106 cells) were then incubated with appropriate amounts of titrated antibodies for 15 min at room temperature in the dark, washed once with PBS-BSA-azide, and resuspended in 0.1% paraformaldehyde. The instrument alignments, sensitivities, and spectral compensation were verified daily by standards, calibrators, procedural controls and normal peripheral blood samples prior to processing the patients’ samples.
Bone marrow samples
Bone marrow aspirate samples were analyzed using antibody panels designed to diagnose MDS and related bone marrow intrinsic diseases and to rule out a lympho-proliferative process, as published previously.22 In brief, the bone marrow samples were analyzed for aberrant antigenic expression or altered expression in blasts, differentiating myeloid cells and monocytes according to our previously published protocol and analytic approaches.22 The FCM results were correlated with the morphological and cytogenetic findings to render a final diagnosis and disease classification. In cases of AML, expression of myeloperoxidase, Tdt, cytoplasmic CD22, and cytoplasmic CD3 was assessed. None of the cases included in this study showed monoclonal B cells or abnormal T cells indicative of a lymphoproliferative process.
Paroxysmal nocturnal hemoglobinuria assay
Peripheral blood granulocytes were the primary cell population chosen for the detection of cells with a PNH+ phenotype. The combinations of monoclonal antibodies used were as follows: FITC-CD59(clone P282E)/PE-CD55(clone JS11KSC2.3)/ECD-CD45/PC5-CD15 (clone 80H5); and FITC-CD66b(clone 80H3)/PE-CD16 (clone 3G8)/ECD-CD45/PC5-CD15(clone 80H5). It should be noted that, CD16 (3G8) reacts to both FcgammaRIII A and B isoforms.
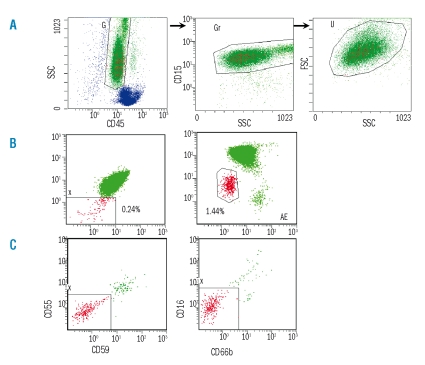
A total of 100,000 granulocytes were collected. The gating strategy is shown in Figure 1A. In brief, the granulocytes were initially identified on the basis of the CD45/SSC plot (Figure 1A-left), further defined by CD15/SSC (Figure 1A-middle) followed by FSC/SSC (Figure 1A-right). The granulocytes from the three combined analysis regions (G, Gr and U) were examined for CD55/CD59 and CD16/CD66b expression. Thirtyadult healthy donor blood samples were also analyzed similarly. PNH+ cells were defined by a loss of CD55/CD59 (Figure 1B-left) and/or CD16/CD66b (Figure 1B-right). For this study we required at least ten cells in a cluster to define a positive clone. The sensivity of the FCM assay was, therefore, 0.01%.
Figure 1.
Flow cytometry analysis (FCM) of granulocytes with a paroxysmal nocturnal hemoglobinuria (PNH)+ phenotype. Granulocytes were initially identified on the basis of CD45/SSC plot (A-left), further defined by CD15/SSC (A-middle) followed by FSC/SSC (A-right). The granulocytes from the three combined analysis regions (G, Gr and U) were examined for CD55/CD59 (B-left) and CD16/CD66b (B-right) expression. The PNH FCM assay was repeated on the same blood sample after 1 h of incubation with pre-aerolysin at 37°C: PNH+ granulocytes were resistant to aerolysin lysis while the non-PNH granulocytes were nearly all lysed (C-left and right).
Aerolysin assay
Aerolysin, a toxin produced by Aeromonas hydrophilia which induces cell death by binding to GPI-anchored proteins in the cell membrane, is a product of pre-aerolysin (Protox Biotech, Victoria, Canada) after trypsin digestion.23 To verify the PNH+ cells detected by FCM, peripheral blood samples were incubated with pre-aerolysin (10−8 M) for 1 hour at 37°C after lysing erythrocytes with ammonium chloride. Following incubation cells were washed, centrifuged, resuspended in PBS-BSA and stained for antibodies to GPI-anchored proteins following the same PNH FCM testing protocol. Live cells were separated from dead cells by SSC/CD45, SSC/CD15 and SSC/FSC gating. True PNH+ cells are resistant to aerolysin lysis because of their lack of GPI-anchored proteins (Figure 1C-left and -right).
Cytogenetic analysis
Conventional cytogenetic analysis was performed by G-banding on all bone marrow aspirate specimens cultured overnight and for 24 hours. At least 20 or all available metaphases were analyzed. The criteria defined by the International System for Human Cytogenetic Nomenclature were used for the identification and reporting of clonal abnormalities.
Statistical analysis
The Mann-Whitney test was used for numerical comparisons between two groups. Survival data were calculated using the Kaplan-Meier method. The follow-up time was calculated from the time of diagnosis until death or the patients’ last visit. Data were considered statistically significant when the p value was lower or equal than 0.05 in a two-tailed t test.
Results
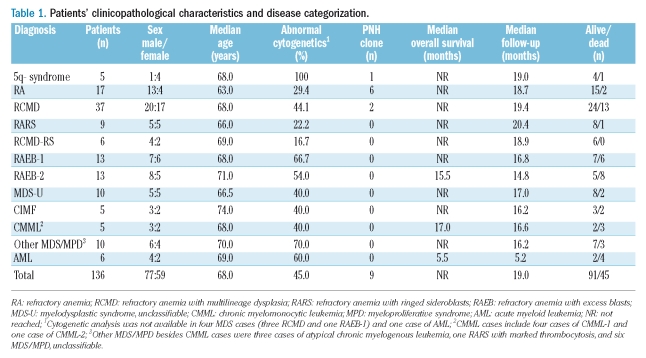
Patients’ characteristics and disease categorization
During 1-year period, FCM PNH analysis was performed on peripheral blood samples collected from a total of 136 patients with a clinically suspected diagnosis of MDS. The patients’ clinical and cytogenetic data according to disease classification are shown in Table 1. The final diagnosis of the 136 patients was MDS (n=110), myelodyspastic/myeloproliferative disease (MDS/MPD) (n=15), chronic idiopathic myelofibrosis (CIMF) (n=5), and AML (n=6). None of the MDS patients had a prior history of chemotherapy or radiation treatment and they were all considered to have primary MDS. Seventy-four MDS patients (67%) had lower than 5% bone marrow blasts and were classified as having low-grade disease; 26 patients had greater or equal than 5% blasts and were classified as having RAEB (13 RAEB-1 and 13 RAEB-2). The MDS/MPD group included five cases of CMML (4 CMML-1 and 1 CMML-2), three cases of atypical chronic myelogenous leukemia (CML), one RARS with marked thrombocytosis, and six cases of MDS/MPD-unclassifiable. Five CIMF and six AML patients were also tested for PNH because of a clinical differential diagnosis of MDS. Four of the AML cases were considered as AML with multilineage dysplasia, possibly evolved from a pre-exisiting MDS, one was a case of erythroleukemia, and one was a case of AML with differentiation. The AML patients showed 23 to 81% blasts in the bone marrow and one patient had blasts in the peripheral blood.
Table 1.
Patients’ clinicopathological characteristics and disease categorization
Detection of cells with a paroxysmal nocturnal hemoglobinuria phenotype
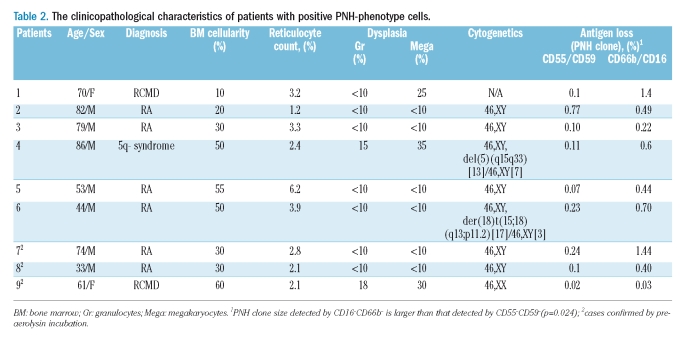
None of the peripheral blood samples obtained from 30 healthy donors showed ten cells in a cluster that was double-negative for CD55/CD59 or CD16/CD66b cells by the FCM assay, and all of these donors were, therefore, considered to be negative for PNH-cells. PNH+ granulocytes were detected in nine patients, all of whom had low-grade MDS with less than 5% blasts; these PNH+ cases comprised 1/5 (20%) patients with 5q- syndrome, 6/17 (35%) patients with RA, and 2/37 (5%) patients with refractory cytopenia with multilineage dysplasia (RCMD) (Table 2). All nine cases demonstrated loss of all four GPI-anchored protein antigens. The mean proportions of PNH+ granulocytes were 0.19% (range, 0.02–0.77%) according to CD15+CD55−CD59− clustering (Figure 1B-left) and 0.64% (range, 0.03–1.44%) according to CD15+CD16−CD66b− clustering (Figure 1B-right). The percentage of PNH+ granulocytes detected by CD16−CD66b− was significantly higher than that detected by CD55–CD59– (p=0.025). Peripheral blood specimens obtained from cases 7, 8, and 9 (Table 2) were incubated with preaerolysin, and the PNH+ cells, identified either as CD15+CD55−CD59− or CD15+CD16−CD66b− cells, showed resistance to aerolysin lysis (Figure1-C-left and C-right), and were proven to be true PNH+ cells.
Table 2.
The clinicopathological characteristics of patients with positive PNH-phenotype cells
None of the other cases, including those with refractory anemia with ringed-sideroblasts (RARS), RCMD-RS, RAEB-1, RAEB-2, MDS/MPD, CIMF and AML, showed the presence of an authentic PNH+ clone as defined by either CD15+CD55−CD59− or CD15+CD16−CD66b− clustering (Table 1).
Clinicopathological characteristics of myelodysplastic syndromes patients with a positive paroxysmal nocturnal hemoglobinuria clone in comparison with those of myelodysplastic syndromes patients without a paroxysmal nocturnal hemoglobinuria clone
PNH+ clones were identified exclusively in patients with low-grade MDS (blasts lower than 5%). The detailed clinicopathological features are listed in Table 2. None of these patients showed clinical or laboratory evidence of clinical hemolysis. None of the patients progressed to develop AML during a median follow-up of 19 months. Immunosuppressive therapy was not included in the MDS treatment protocol at our institution. Only one patient (Table 2, patient 7) received antithymocyte globulin and rituximab (anti-CD20) late in the course of disease but died of gastrointestinal bleeding 1 week after initiating treatment. We could not, therefore, compare a possible different response to immunosuppressants between PNH+ and PNH− MDS patients in our cohort.
We compared the clinical data and patients’ demographic information of the PNH+ cases to those of their appropriate counterparts, the MDS cases with lower than 5% blasts and without PNH+ cells (Table 3). Demographically, both groups of patients were of comparable age and demonstrated a slight male predominance. Clinically, the hematologic indices, including absolute neutrophil count, platelet count, mean corpuscular volume and reticulocyte count, showed no statistically significant differences between these two groups. However, the disease classification differed (p=0.009). The PNH+ MDS patients comprised 35% of the cases of RA, 5% of RCMD and 20% of 5q- syndrome, but none of the cases of RARS, RCMD-RS or MDS-U. Furthermore, the PNH+ MDS patients demonstrated a significantly lower bone marrow cellularity (mean 37% vs. 59%, p=0.017), and a lower number of bone marrow blasts (mean 0.7% vs. 1.5%, p=0.039). Cytogenetic abnormalities were detected in 2/9 patients (22%) in the PNH+ group and in 28/64 (44%) PNH− MDS patients (p=0.292, not significant probably due to the small sample size). With a median follow-up of 19 months, 2/9 (22%) patients with PNH+ MDS and 9/65 (14%) patients with PNH− MDS had died, and the median survivals of each group had not been reached.
Table 3.
Clinical and laboratory comparison of patients with low grade myelodysplastic syndrome with or without a PNH clone
GPI-anchored protein alteration related to the underlying intrinsic bone marrow diseases: the diagnostic pitfalls and caveats
Difficulty in separating granulocytes from monocytes
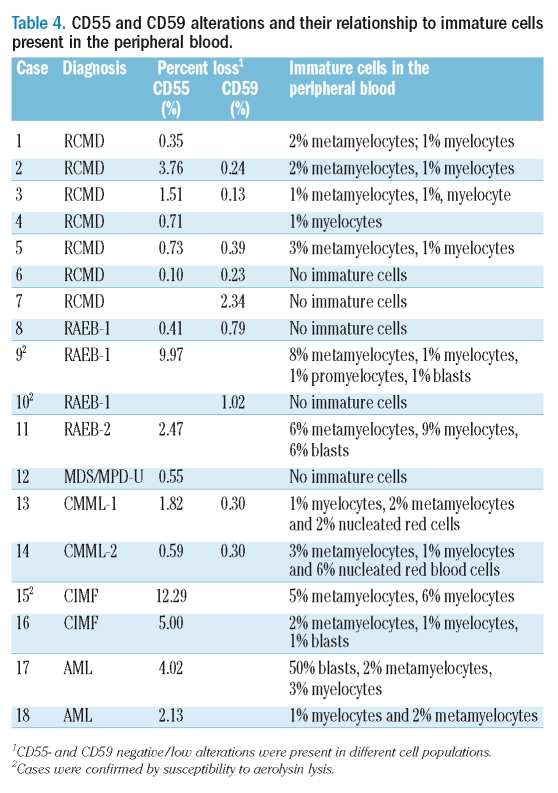
Decreased SSC of granulocytes (hypogranulation) or decreased CD45 expression of monocytes or both were observed in 32/136 (24%) cases, resulting in significant overlap between granulocytes and monocytes on the CD45/SSC plot (Figure 2A3). Decreased CD15 expression on granulocytes was seen in 34/136 (25%) patients, while increased CD15 expression on monocytes was observed in 41/136 (30%) cases. As a result, it became difficult to separate granulocytes from monocytes even by using a three-step gating strategy including CD45/SSC, CD15/SSC, and FS/SSC (Figure 2A3 and 2A4). We observed a CD15+CD16−CD66b− cell population (Figure 2A2) in nine (7%) cases, which showed a CD16 expression level differing from that of true PNH+ cells (compare Figure 1B-right and Figure 2A2). CD59 single antigen loss/low expression (mean: 0.67%; range, 0.13% to 2.34%) without concomitant CD55 or CD16/CD66b loss (Figure 2A1) was observed in nine patients (7%), in whom the altered CD59 expression was not observed in red blood cells (data not shown). In some cases, the CD59 low/negative cells were traced back to the junction of monocytes and granulocytes (Figure 2A3). These CD15+CD16−CD66b− cells and CD15+CD59− cells were susceptible to aerolysin lysis (a total of six cases tested); the results are presented in Table 4 (cases 9, 10, and 15) and (Figure 2C1 and 2C2). Decreased CD16 and/or an increased CD66b expression on granulocytes was observed in 18 (13%) and 11 (8%) cases, respectively; these cells did not, however, form a distinct CD16−CD66b− population (Figure 2B2).
Figure 2.
Examples of immunophenotypic alterations associated with the underlying bone marrow disease: diagnostic pitfalls and caveats. Case 10-refractory anemia with excess blasts-1: 1.02% CD59 negative (A1) and 2.03% CD16-negative/CD66b-negative (A2) cells were detected, which were back-traced to the monocyte region on the CD45/SSC plot (A3) and showed a low level of CD15 expression (A4). Case 15-chronic idiopathic myelofibrosis: 12.29% CD55 low/negative cells were detected (B1) which were CD16 low (B2) and CD45 low (B3), corresponding to myeloid cells of intermediate maturation. In all cases illustrated above, the granulocytes were susceptible to aerolysin lysis and were not true PNH+ cells (C1 and C2).
Table 4.
CD55 and CD59 alterations and their relationship to immature cells present in the peripheral blood
Alteration of GPI-anchored protein expression is related to myeloid immaturity
Peripheral blood smears from 61 of the 136 (44.9%) patients showed the presence of immature myeloid forms (metamyelocytes, myelocytes, promyelocytes, blasts) (Table 4). On FCM analysis, CD55 loss/low expression (mean 2.90%; range, 0.10% to 12.29%) without concomitant CD59 and/or CD16/CD66b loss was observed in 16 (12%) patients, 14 (88%) of whom had immature myeloid cells in the peripheral blood (Table 4). The presence of CD55− cells detected by FCM was significantly correlated with the presence of immature myeloid cells (p<0.0001). Furthermore, we demonstrated that CD55 low/negative granulocytes were CD16 low (Figure 2B1) and CD45 low (Figure 2B3) further indicating immaturity. These CD55− cells were also susceptible to aerolysin lysis (Figure 2C1). It is noteworthy that the detection of CD59 low/negative cells did not correlate with the presence of peripheral immature myeloid cells (p=0.73).
Discussion
In this study, we utilized a highly sensitive FCM assay to assess for PNH clones in 136 patients with various bone marrow diseases, including MDS (n=110), MDS/MPD (n=15), CIMF (n=5) and AML (n=6). We compared the clinicopathological characteristics of PNH+ cases with their PNH-negative counterparts. We compared the sensitivity and specificity of CD15+CD55−CD59− and CD15+CD16−CD66b− in detecting cells with a PNH phenotype. We also illustrated in detail the diagnostic caveats and pitfalls in screening for PNH clones in the setting of an intrinsic bone marrow disease.
We detected a PNH+ clone in nine patients who represented 8% of the 110 MDS cases and 12% (9/75) of the MDS cases with lower than 5% blasts. The PNH+ MDS cases comprised six patients with RA, two with RCMD and one with 5q- syndrome. As compared to PNH-negative MDS cases with lower than 5% blasts, the PNH+ MDS cases showed a different distribution of WHO MDS classification in that most of the cases were RA and there were no cases of RARS, RCMD-RS, or MDS-U. These results are in agreement with those reported by Iwanaga et al. and Wang et al.5,20 who found that PNH+ clones were present exclusively in RA patients. Although in these earlier studies RA defined by FAB criteria probably included several different WHO categories, Wang et al.5 reported significantly less granulocytic dysplasia in their PNH+ MDS patients. In addition, we also observed that the bone marrow cellularity and blast percentage in PNH+ MDS patients were significantly lower than those in the PNH-negative MDS patients. With a median follow-up of 19 months, two patients had died as a result of bone marrow failure, and none of the patients had undergone AML transformation. The above findings indicate that the presence of PNH+ cells in MDS patients may not be related to the preleukemic nature of MDS, but more to bone marrow failure, similar to aplastic anemia. It is known that in some cases of moderate aplastic anemia, the distribution of the bone marrow hematopoietic elements may be heterogeneous, and a biopsy taken from residual hematopoietic nests can reveal a normal or even a hypercellular bone marrow.24 Nakao et al.25 considered most of these PNH+ RA cases as moderate aplastic anemia misclassified as MDS because of normocellularity or hypercellularity. In our series, the six PNH+ MDS cases showed only erythroid dysplasia that may have been a non-specific/secondary alteration. However, three patients did show morphological dysplasia beyond the erythroid lineage. One of these cases had the 5q- syndrome, a well-defined MDS entity. Therefore, although we acknowledge some similarity between PNH+ MDS and aplastic anemia, our findings also indicate the existence of a real association between a PNH clone and MDS.
In this study we utilized four GPI-anchored proteins, CD55, CD59, CD16 and CD66b, to screen for granulocytes with a PNH phenotype. While the combination of CD55/CD59 has been used most frequently by others,5,15,19,20 the combination of CD16/CD66b was also shown by Dunn et al.6 to effectively identify patients with subclinical PNH (aplastic anemia and RA) with a better response to immunosuppressive therapy. In our study, although the combinations of CD15 with CD55/CD59 and CD15 with CD66b/CD16 were both effective in detecting PNH+ granulocytes, CD16/CD66b detected a larger and more distinctive PNH+ granulocytic population, with the added advantage of clearly separating neutrophils from eosinophils.
It is well known that in MDS bone marrow, myeloid cells frequently show an altered immunophenotype, and the detection of these immunophenotypic aberrancies by FCM has been utilized in both the diagnosis and risk stratification of MDS.22,26–28 It is also known that the peripheral blood of MDS patients often contains hypogranular neutrophils and/or immature myeloid cells. Although the immunophenotypic aberrancies in myeloid cells may be less pronounced in the peripheral blood than in the bone marrow, Cheiran et al.29,30 were able to demonstrate significant immunophenotypic alterations in MDS peripheral blood granulocytes, as compared to healthy controls. In our study, we observed a number of changes related to the underlying intrinsic bone marrow diseases, which could produce potential pitfalls and necessitate caveats when screening patients with subclinical PNH. First, we show that the altered SSC characteristics of the hypogranular neutrophils and decreased CD45 expression of immature monocytes can lead to significant overlap between granulocytes and monocytes on the CD45/SSC plot. This problem may be further complicated by decreased CD15 expression on granulocytes and substantially increased CD15 expression on mononcytes, an alteration often observed in MDS bone marrow cells.22 Although our stringent three-step gating strategy showed greater utility in separating granulocytes from monocytes as compared to the CD15/SSC and FSC/SSC two-step gating recommended by others,8 in some cases, especially the cases with severe granulocytic dysplasia and/or CMML, a small number of monocytes were inseparable from granulocytes and were included in the granulocyte gate. Monocytes have a CD16−CD66b− immunophenotype and can be mistaken for cells with a PNH+ phenotype. We observed, however, that these monocytes showed a different level of expression of CD16, and could be differentiated from PNH+ cells by FCM back-tracking and confirmed by aerolysin lysis (performed, in our study, in a total of six cases). Another potential problem with granulocyte analysis is the presence of immature myeloid forms in MDS or other intrinsic bone marrow diseases. It has been shown that the expression of GPI-anchored proteins varies throughout neutrophil and monocyte maturation.17,31,32 The level of CD55 expression is low in early myeloid precursors until the intermediate stages of maturation, and increases in bands and granulocytes. The expression of CD16 is detectable on metamyelocytes, reaching highest levels on bands/mature neutrophils. Conversely, the expression of CD66b reaches its highest level on myelocytes, but decreases thereafter with maturation. We observed that CD55 loss/low expression alone was significantly correlated with immature myeloid cells in the peripheral blood, confirmed by FCM back-tracking and these cells’ susceptibility to aerolysin lysis (cases 9 and 15). Decreased CD16 and increased CD66b expression was also observed in a substantial number of cases, similar to that described by Cherian et al.30 Because of the paradoxical alterations of CD16 and CD66b expression levels, the CD16−CD66b− combination did not cause diagnostic difficulties and, instead, showed its advantage over CD55/CD59 in such a setting. We also observed some cells with CD59 single antigen loss/low expression in the granulocyte gate that were susceptible to aerolysin lysis (case 10). The presence of cells with CD59 loss/low expression was not related to immature myeloid cells in the peripheral blood, a finding in keeping with the observation by Hernandez-Campo et al. that there is no significant change in CD59 expression throughout myeloid maturation.31 We back-traced the cells with CD59 loss/low expression and found, at least in some cases, that these cells clustered in the junction between monocytes and granulocytes (Figure 2A3). It is noteworthy that monocytes often exhibit significantly dimmer expression of CD59 than granulocytes,17 and the expression level decreases further with monocyte immaturity. Although the nature of these cells with CD59 loss/low expression needs further investigation, especially to rule out an isolated inherited deficiency, in at least some cases, we speculate that these cells likely represent immature/dysplastic monocytes, which are difficult to separate from granulocytes in the cases with marked granulocytic dysplasia or increased immature monocytes. Kaiafa et al. found that PNH+ cells, defined by either CD55− or CD59−, were more prominent in CMML or RAEB in their series19 and concluded that the detection of large numbers of PNH+ cells was associated with a poor prognosis. Although the observation that significant alterations of CD55 or CD59 may be linked to a poor prognosis in MDS/CMML is an interesting finding, it is also possible that the dysplastic/immaturity-related findings were misinterpreted as PNH+ cells. Laboratories performing PNH testing in the setting of an intrinsic bone marrow disease must be aware of these issues. The development of the GPI anchor protein-specific reagent fluorescent aerolysin (FLAER)33 is promising, as it is reportedly less influenced by the maturation-dependent levels of expression of some of the individual GPI-linked antigens; however, its clinical validity in detecting subclinical PNH needs to be studied and verified.
In summary, subclinical PNH in association with MDS and other bone marrow diseases is infrequent. In this study, subclinical PNH cases were identified exclusively in the low-grade MDS category (but not including RARS or RCMD-RS), with many features of a bone marrow failure similar to that of aplastic anemia. Alterations of GPI-anchored proteins can be associated with granulocytic/monocytic dysplasia and immaturity, and should not be misinterpreted as cells with a true PNH phenotype. We agree with the recommendation of the International PNH Group that screening for subclinical PNH requires quantification of at least two GPI-anchored proteins. Laboratories that perform PNH testing in MDS patients should be cognizant of the diagnostic pitfalls and caveats associated with dysplasia and immaturity in granulocytes and monocytes to ensure diagnostic accuracy.
Acknowledgments
we thank the technical staff of the flow cytometry laboratory at the Department of Pathology, UMass Memorial Medical Center for their dedicated and innovative work.
Footnotes
Authorship and Disclosures
SAW: study design, data analysis, tables, figures, writing and revision of the manuscript; OP: data collection, analysis, tables, figures; JLJ: discussion, suggestions, and revision of the manuscript; LJM: discussion, suggestions and proof writing; DS: data collection; MA: data analysis and figures; AR: clinical information and patients’ follow-up; BAW: design, and proof writing.
The authors reported no potential conflicts of interest.
References
- 1.Takeda J, Miyata T, Kawagoe K, Iida Y, Endo Y, Fujita T, et al. Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell. 1993;73:703–11. doi: 10.1016/0092-8674(93)90250-t. [DOI] [PubMed] [Google Scholar]
- 2.Griscelli-Bennaceur A, Gluckman E, Scrobohaci ML, Jonveaux P, Vu T, Bazarbachi A, et al. Aplastic anemia and paroxysmal nocturnal hemoglobinuria: search for a pathogenetic link. Blood. 1995;85:1354–63. [PubMed] [Google Scholar]
- 3.Schubert J, Vogt HG, Zielinska-Skowronek M, Freund M, Kaltwasser JP, Hoelzer D, et al. Development of the glycosylphosphatidylinositol-anchoring defect characteristic for paroxysmal nocturnal hemoglobinuria in patients with aplastic anemia. Blood. 1994;83:2323–8. [PubMed] [Google Scholar]
- 4.Maciejewski JP, Rivera C, Kook H, Dunn D, Young NS. Relationship between bone marrow failure syndromes and the presence of glycophosphatidylinositol-anchored protein-deficient clones. Br J Haematol. 2001;115:1015–22. doi: 10.1046/j.1365-2141.2001.03191.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Chuhjo T, Yasue S, Omine M, Nakao S. Clinical significance of a minor population of paroxysmal nocturnal hemoglobinuria-type cells in bone marrow failure syndrome. Blood. 2002;100:3897–902. doi: 10.1182/blood-2002-03-0799. [DOI] [PubMed] [Google Scholar]
- 6.Dunn DE, Tanawattanacharoen P, Boccuni P, Nagakura S, Green SW, Kirby MR, et al. Paroxysmal nocturnal hemoglobinuria cells in patients with bone marrow failure syndromes. Ann Int Med. 1999;131:401–8. doi: 10.7326/0003-4819-131-6-199909210-00002. [DOI] [PubMed] [Google Scholar]
- 7.Schrezenmeier H, Hertenstein B, Wagner B, Raghavachar A, Heimpel H. A pathogenetic link between aplastic anemia and paroxysmal nocturnal hemoglobinuria is suggested by a high frequency of aplastic anemia patients with a deficiency of phosphatidylinositol glycan anchored proteins. Exp Hematol. 1995;23:81–7. [PubMed] [Google Scholar]
- 8.Parker C, Omine M, Richards S, Nishimura J, Bessler M, Ware R, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:3699–709. doi: 10.1182/blood-2005-04-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosse WF. New insights into paroxysmal nocturnal hemoglobinuria. Curr Opin Hematol. 2001;8:61–7. doi: 10.1097/00062752-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Risitano AM, Maciejewski JP, Selleri C, Rotoli B. Function and malfunction of hematopoietic stem cells in primary bone marrow failure syndromes. Curr Stem Cell Research. 2007;2:39–52. doi: 10.2174/157488807779316982. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto M, Shichishima T, Noji H, Ikeda K, Nakamura A, Akutsu K, et al. High frequency of several PIG-A mutations in patients with aplastic anemia and myelodysplastic syndrome. Leukemia. 2006;20:627–34. doi: 10.1038/sj.leu.2404135. [DOI] [PubMed] [Google Scholar]
- 12.Young NS. Pathophysiologic mechanisms in acquired aplastic anemia. Hematology Am Soc Hematol Educ Program. 2006:72–7. doi: 10.1182/asheducation-2006.1.72. [DOI] [PubMed] [Google Scholar]
- 13.Barcellini W, Fermo E, Guia Imperiali F, Zaninoni A, Bianchi P, Boschetti C, et al. Increased resistance of PIG-A- bone marrow progenitors to tumor necrosis factor α and interferon γ: possible implications for the in vivo dominance of paroxysmal nocturnal hemoglobinuria clones. Haematologica. 2004;89:651–6. [PubMed] [Google Scholar]
- 14.van der Schoot CE, Huizinga TW, van’t Veer-Korthof ET, Wijmans R, Pinkster J, von dem Borne AE. Deficiency of glycosyl-phosphatidy-linositol-linked membrane glyco-proteins of leukocytes in paroxysmal nocturnal hemoglobinuria, description of a new diagnostic cytofluorometric assay. Blood. 1990;76:1853–9. [PubMed] [Google Scholar]
- 15.Sugimori C, Chuhjo T, Feng X, Yamazaki H, Takami A, Teramura M, et al. Minor population of CD55-CD59- blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood. 2006;107:1308–14. doi: 10.1182/blood-2005-06-2485. [DOI] [PubMed] [Google Scholar]
- 16.Hall SE, Rosse WF. The use of monoclonal antibodies and flow cytometry in the diagnosis of paroxysmal nocturnal hemoglobinuria. Blood. 1996;87:5332–40. [PubMed] [Google Scholar]
- 17.Olteanu H, Karandikar NJ, McKenna RW, Xu Y. Differential usefulness of various markers in the flow cytometric detection of paroxysmal nocturnal hemoglobinuria in blood and bone marrow. Am J Clin Pathol. 2006;125:781–8. doi: 10.1309/AT9Y-6WR0-3PX1-K228. [DOI] [PubMed] [Google Scholar]
- 18.Alfinito F, Del Vecchio L, Rocco S, Boccuni P, Musto P, Rotoli B. Blood cell flow cytometry in paroxysmal nocturnal hemoglobinuria: a tool for measuring the extent of the PNH clone. Leukemia. 1996;10:1326–30. [PubMed] [Google Scholar]
- 19.Kaiafa G, Papadopoulos A, Ntaios G, Saouli Z, Savopoulos C, Tsesmeli N, et al. Detection of CD55- and CD59-deficient granulocytic populations in patients with myelodysplastic syndrome. Ann Hematol. 2008;87:257–62. doi: 10.1007/s00277-007-0420-5. [DOI] [PubMed] [Google Scholar]
- 20.Iwanaga M, Furukawa K, Amenomori T, Mori H, Nakamura H, Fuchigami K, et al. Paroxysmal nocturnal haemoglobinuria clones in patients with myelodysplastic syndromes. Br J Haematol. 1998;102:465–74. doi: 10.1046/j.1365-2141.1998.00794.x. [DOI] [PubMed] [Google Scholar]
- 21.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November, 1997. Ann Oncol. 1999;10:1419–32. doi: 10.1023/a:1008375931236. [DOI] [PubMed] [Google Scholar]
- 22.Stachurski D, Smith BR, Pozdnyakova O, Andersen M, Xiao Z, Raza A, et al. Flow cytometric analysis of myelomonocytic cells by a pattern recognition approach is sensitive and specific in diagnosing myelodysplastic syndrome and related marrow diseases: emphasis on a global evaluation and recognition of diagnostic pitfalls. Leuk Res. 2008;32:215–24. doi: 10.1016/j.leukres.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Mukhina GL, Buckley JT, Barber JP, Jones RJ, Brodsky RA. Multilineage glycosylphosphatidylinositol anchor-deficient haematopoiesis in untreated aplastic anaemia. Br J Haematol. 2001;115:476–82. doi: 10.1046/j.1365-2141.2001.03127.x. [DOI] [PubMed] [Google Scholar]
- 24.Kouba M, Maaloufova J, Campr V, Belohlavek O, Drugova B. G-CSF stimulated islands of haematopoiesis mimicking disseminated malignancy on PET-CT and MRI scans in a patient with hypoplastic marrow disorder. Br J Haematol. 2005;130:807. doi: 10.1111/j.1365-2141.2005.05574.x. [DOI] [PubMed] [Google Scholar]
- 25.Nakao S, Sugimori C, Yamazaki H. Clinical significance of a small population of paroxysmal nocturnal hemoglobinuria-type cells in the management of bone marrow failure. Int J Hematol. 2006;84:118–22. doi: 10.1532/IJH97.06077. [DOI] [PubMed] [Google Scholar]
- 26.Kussick SJ, Fromm JR, Rossini A, Li Y, Chang A, Norwood TH, et al. Four-color flow cytometry shows strong concordance with bone marrow morphology and cytogenetics in the evaluation for myelodysplasia. Am J Clin Pathol. 2005;124:170–81. doi: 10.1309/6PBP-78G4-FBA1-FDG6. [DOI] [PubMed] [Google Scholar]
- 27.Stetler-Stevenson M, Arthur DC, Jabbour N, Xie XY, Molldrem J, Barrett AJ, et al. Diagnostic utility of flow cytometric immunophenotyping in myelodysplastic syndrome. Blood. 2001;98:979–87. doi: 10.1182/blood.v98.4.979. [DOI] [PubMed] [Google Scholar]
- 28.Wells DA, Benesch M, Loken MR, Vallejo C, Myerson D, Leisenring WM, et al. Myeloid and monocytic dyspoiesis as determined by flow cytometric scoring in myelodysplastic syndrome correlates with the IPSS and with outcome after hematopoietic stem cell transplantation. Blood. 2003;102:394–403. doi: 10.1182/blood-2002-09-2768. [DOI] [PubMed] [Google Scholar]
- 29.Cherian S, Moore J, Bantly A, Vergilio JA, Klein P, Luger S, et al. Peripheral blood MDS score: a new flow cytometric tool for the diagnosis of myelodysplastic syndromes. Cytometry. 2005;64:9–17. doi: 10.1002/cyto.b.20041. [DOI] [PubMed] [Google Scholar]
- 30.Cherian S, Moore J, Bantly A, Vergilio JA, Klein P, Luger S, et al. Flow-cytometric analysis of peripheral blood neutrophils: a simple, objective, independent and potentially clinically useful assay to facilitate the diagnosis of myelodysplastic syndromes. Am J Hematol. 2005;79:243–5. doi: 10.1002/ajh.20371. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez-Campo PM, Almeida J, Matarraz S, de Santiago M, Sanchez ML, Orfao A. Quantitative analysis of the expression of glycosylphosphatidylinositol-anchored proteins during the maturation of different hematopoietic cell compartments of normal bone marrow. Cytometry. 2007;72:34–42. doi: 10.1002/cyto.b.20143. [DOI] [PubMed] [Google Scholar]
- 32.Terstappen LW, Nguyen M, Lazarus HM, Medof ME. Expression of the DAF (CD55) and CD59 antigens during normal hematopoietic cell differentiation. J Leukoc Biol. 1992;52:652–60. doi: 10.1002/jlb.52.6.652. [DOI] [PubMed] [Google Scholar]
- 33.Brodsky RA, Mukhina GL, Li S, Nelson KL, Chiurazzi PL, Buckley JT, et al. Improved detection and characterization of paroxysmal nocturnal hemoglobinuria using fluorescent aerolysin. Am J Clin Pathol. 2000;114:459–66. doi: 10.1093/ajcp/114.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]