Abstract
In recent years, the tumor microenvironment has been increasingly studied. In this perspective article, Drs. Lejeune and Alvaro discuss the biologic and clinical relevance of microenvironmental patterns in follicular lymphoma. See related article on page 70.
Tumors cannot be considered as consisting simply of tumor cells. The intimate relationship between the tumor microenvironment and neoplastic cells implies a dynamic cross-talk in which tumor cells may give and receive instructions through a complex system with at least three important functional and structural components: the extracellular matrix, stromal cells and the immune response. In the last few years, it has been shown that there are specific patterns of immune response in lymphoproliferative syndromes.1 These patterns are regulated by microenvironmental effects on tumoral cells, appear related to the clinicopathological features of the disease and constitute prognostic and follow-up indicators. They represent the rationale for new therapies based on the action of the immune system and immunotherapies designed to induce sustained antitumoral immunity. In addition, indirect effects of the immune response, such as eliminating bacteria or viruses from the lymphoma environment, may stop tumor growth.
Follicular lymphoma (FL) is the prototype of indolent lymphoma, a slow growing lymphoma arising from follicular center B cells with a scarce tendency to invade to non-lymphoid tissues and a protracted clinical course. In physiological conditions, the stable formation of germinal centers requires the presence of functionally specialized T cells, dendritic and stromal cell subpopulations. FL is recognized as a disease of functional B cells in which T-cell co-stimulation is essential for the maintenance and ongoing development of B-cell secondary follicles. Evaluation of the large amount of genetic data concerning tumor cells of FL has focused increasing interest on the reactive microenvironment, whereas it seems that there are not different molecular signatures of tumor cells for the different histological grades of FL. In 70–90% of cases, the neoplastic B cells are characterized by the t(14;18) (q32;p21) translocation. As a result of this genomic event, the expression of the anti-apoptotic BCL2 gene is controlled by the immunoglobulin (Ig) enhancer leading to an overexpression of the anti-apoptotic BCL-2 protein. However, the acquisition of this genomic alteration has also been observed in more than 50% of normal individuals suggesting that there are other mechanisms involved in the development, growth and progression of the tumor.2 Interestingly, 15% of patients with FL have objective tumor regression in the absence of any antitumoral therapy. Data indicate that an effective immune response would involve both the tumor and the main histopathological and clinicobiological features.
Tumor transformation and development of unresponsiveness to standard chemotherapy or immunochemotherapy regimens in the course of FL represent the main causes of death in patients with this lymphoma. Anticancer immune responses may contribute to controlling the tumor after conventional chemotherapy. The importance of treatment as a prognostic variable in FL is currently masked by the fact that published studies include FL patients treated with markedly different regimens. Due to their specific targeting of some immune cells, chemotherapeutic agents may influence the prognostic impact of the different immune signatures present in FL, as suggested interestingly in the study by de Jong and colleagues published in this issue of the journal.3 In their study, de Jong et al. found that the tumor microenvironment, but not the tumor cells, was the fundamental key to choosing the most appropriate chemotherapeutic regimen.
Prominent immune components of the follicular lymphoma microenvironment
The simple histological observation of the existence of different subpopulations of immune/inflammatory cells infiltrated in the tumor transmits the idea that the local tumor microenvironment is an important modulator of ongoing tumor-specific immune responses. Tumor-infiltrating leukocytes may act against the tumor through their multiple cytokines, but can also facilitate tumor cell growth secreting growth factors, reactive oxygen species, proteases, prostaglandins and angiogenic growth factors. Macrophages may either exhibit antitumor cytotoxic activity or facilitate cell tumor growth and progression. Other cells of the myeloid lineage, such as mast cells, can promote growth, vascularization and dissemination of tumors.
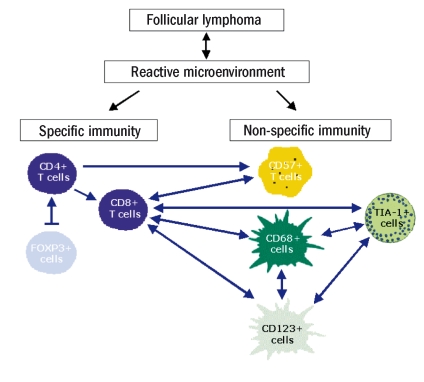
There are positive relationships between most of the cell types present in the reactive microenvironment of FL.4 The level of CD4+ T lymphocytes appears to be corelated with the level of CD8+ T lymphocytes, forkhead box protein 3 (FOXP3+) regulatory T cells, and CD57+ cells. The number of infiltrated CD8+ T lymphocytes has been correlated with the number of CD57+ cells, CD68+ macrophages, and CD123+ plasmacytoid cells. The quantity of TIA-1+ cells was correlated with the principal infiltrated cells with cytotoxic activity, such as CD8+ T lymphocytes, CD68+ macrophages, and CD123+ plasmacytoid cells (Figure 1). These positive r elationships between the different immune subpopulations underline a positive balance between specific (CD4+ and CD8+ T lymphocytes) and non-specific (CD57+ and CD68+ cells) immunity, showing that the entire immune response constitutes a coordinated and global mechanism in each tumor and each patient.
Figure 1.
Positive relationship between specific and non-specific cell-mediated immunity in FL. The broken arrow (|→) indicates an association with an inhibitory effect, while single-headed arrows (←) show a positive correlation, and double-headed arrows (↔) show a positive mutual correlation (reprinted with the permission of the Journal of Clinical Oncology).4
In FL, previous original data from gene expression profiling analyses have been confirmed by immunohistochemical and flow cytometry studies, showing the in loco presence of the main components of the anti-tumor immune response in FL, such as specific CD4+ T-helper lymphocytes5–7 and cytotoxic cells including CD8+ T lymphocytes4,8 and CD68+ macrophages.9,10 Others studies have also highlighted the presence of specific CD57+ T-helper cells,4,5 signal transducer and activator of transcription 1 (STAT-1)-positive tumor-associated macrophages10 and FOXP3+ regulatory T cells.6,7 A high level of CD4+ T lymphocytes was significantly associated with overall survival in some cases,6 but others studies found no effect.7 The number of CD8+ T lymphocytes was found to be associated with overall survival and disease-free survival4,8 but with discrepancies between the studies. FOXP3+ expression pattern has been suggested to be associated with poor survival,11 but also with a favorable outcome.6,7 The presence of CD68+ lymphoma-associated macrophages was associated with an adverse overall survival and/or progression-free survival in a selected group of FL patients.9,11 A subset of these macrophages expressing STAT-1 was further characterized as being predominantly associated with poor survival.10 Despite some contradictory results, the histological distribution and the level of these immune response cells appear to be significantly associated with the outcome of FL patients.
The explanation of some of the contradictions probably lies in the differences in the methodological approaches used as well as the cell populations and their location relative to the malignant follicle. However, the different therapies used in various cohorts of FL patients in these studies may also have had different impacts on cells within the tumor microenvironment so that all these studies will need to be revisited in the current era of therapy.12,13 The study by de Jong and colleagues supports this idea and shows that the specific microenvironment in FL is so important that in determines different responses to the different chemotherapeutic agents received by the patients.
Regulatory and dysfunctional immune patterns in follicular lymphoma
Although an immune response in the tumor may prevent tumor outgrowth, cancer cells can escape both innate and adaptive immune responses by selection of non-immunogenic tumor cell variants (immuno-editing) or by active suppression of the immune response (immuno-suppression).
Dysfunctional immune profiles observed in the tumor microenvironment of FL have been described to be related to the presence of some specific subsets of immune cells. Indeed, there is an increasing body of evidence pointing towards a subset of CD57+ T cells as being an indicator of general immune dysfunction. CD57, expressed on a subset of T cells known as natural killer (NK) cells, is a marker of one of the major effector cell populations in cellular cytotoxicity achieved through cytotoxic T-lymphocytes. In FL, CD57+ cells could diminish specific T-cell responses and, therefore, lead to more aggressive tumor behavior.4
The population of “naturally occurring” FOXP3+ CD4+CD25+ regulatory T cells seems to be able to reduce tumor-driven immune suppression with clinical benefit for these patients.7 The favorable clinical impact of a high number of these regulatory T cells may be due to a direct inhibitory effect on neoplastic B cells and/or an indirect effect on the reactive immune subpopulations. The function of these cells could differ fundamentally between lymphoid malignancies and epithelial carcinomas.14 Autologous FOXP3+ regulatory T cells derived from the lymph nodes of patients with FL may induce hyporesponsiveness of CD8+ T effector cells present in FL tumors. These FL tumor B cells can stimulate T-cell receptors of CD4+ T cells present in the microenvironment and induce these conventional T cells to express FOXP3 and to acquire regulatory function.15 So, in FL, not only does the immune system affect the tumor cells but the tumor cells may also affect the immune system, promoting immune escape.
Dysfunctional immune profiles in the tumor microenvironment of FL can also lead to reprogrammed immune cells such as tumor-associated macrophages. The dual nature of the polarized M1 and M2 macrophages implies differences that may be exhibited as either antitumor cytotoxic activity or facilitated tumor growth and progression.16 The evaluation of specific subsets of activated tumor-associated macrophages (STAT-1-positive) demonstrated an adverse impact of these cells on the global survival of FL patients.10 These tumor-associated macrophages have been considered as prototypic M2 polarized macrophages reprogrammed to induce in situ immune suppression of host defenses, through T-cell deletion in the tumor site dependent on STAT-1 signaling. Recently, the adverse prognostic effect of tumor-associated macrophages in FL patients was demonstrated to be circumvented by the addition of rituximab to treatment.12,13 Finally, follicular dendritic cells are able to avoid the spontaneous apoptosis of FL tumoral B cells preventing the activation of different caspases.17
Implications of microenvironmental patterns in the clinicobiological behavior of follicular lymphoma
As demonstrated in various studies, the clinicobiological course of FL is influenced by interactions between the tumor cells and the functional composition of the immune microenvironment.
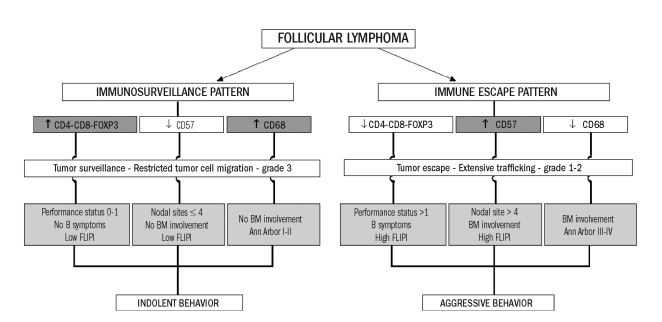
Patients with a very low number of regulatory T cells had a high-risk Follicular Lymphoma International Prognostic Index (FLIPI) score and also more frequently had refractory disease (failure of first-line treatment).7 In a large and representative cohort of FL patients, significant differences were found in host immune response between patients whose disease was indolent and those whose lymphoma had an aggressive clinical course.4 The group of FL patients with indolent disease had an immunosurveillance pattern of immune response with more infiltrated T lymphocytes and macrophages in the microenvironment of the tumor, a low performance status, absence of B symptoms, absence of bone marrow involvement, low number of nodal sites (≤4), low Ann Arbor stage and low-risk FLIPI score (Figure 2). The invasive potential of FL also appeared to be related to the number of certain components of the immune infiltrate such as CD68+ macrophages and CD57+ cells.4 Although the histological grade was not significantly associated with the FL patients’ survival, this study revealed a statistically significant lesser infiltration of CD68+ macrophages but also a greater infiltration of CD57+ cells in patients with low-grade (1–2) FL as compared to in patients with grade 3 FL. The apparently paradoxical observation that patients with low-grade FL have more extensive tumor cell trafficking among FL follicles during clonal expansion18,19 appears in agreement with these results, since patients with grade 1 and 2 FL show a significantly higher percentage of B symptoms and bone marrow involvement than those with grade 3.4
Figure 2.
Representation of the two immune patterns observed in FL patients significantly associated with their clinicobiological features. The immunosurveillance pattern (predominantly T lymphocytes and macrophages) is associated with grade 3 FL and an indolent clinical behavior. The immune-escape pattern (predominantly CD57+ T cells) is associated with low-grade FL and with an aggressive clinical behavior (reprinted with the permission of the Journal of Clinical Oncology).4
Considering the results obtained in these different immunohistochemical studies, the host response in FL could be represented by two immune patterns significantly associated with the clinicobiological features: an immunosurveillance pattern associated with an indolent clinical behavior and an immune escape pattern associated with an aggressive clinical behavior. In these patterns, the non-specific inflammatory infiltrate seems to be mainly involved in the control of growth and expansion of tumor cells whereas the specific immune infiltrate seems to be principally involved in the host immune response against the tumor and the main clinical features. Both systems seem to be directly associated with the capacity to disseminate tumoral cells.
Prognostic implications of the follicular lymphoma microenvironment
The outcome in FL can be explained by the different immune patterns of the host microenvironment, which suppress or stimulate FL tumor cells. The significant positive relationship between the different immune cell subpopulations4 implies that FL is an immunologically functional disease. Specific patterns including a dense, reactive infiltrate of T cells, regulatory T cells and macrophages predict a favorable outcome whereas patterns formed from CD57+ NK cells and activated macrophages are associated with the opposite outcome.
This pattern could be indicative of some functional alterations, so not only the quantitative but also the qualitative and functional aspects of these cells must be considered. The immune switch between macrophages and a T-cell-dominant response could be the signal predictive of outcome in FL patients.20 Thus, an emerging focus of attention is the relationship between the anti-cancer immune response and therapeutic success. The results obtained by de Jong and colleagues demonstrate that the therapeutic management can have an impact on prognosis in FL depending on the specific treatment protocols chosen according to the type of tumor microenvironment. This study represents an important step forward in the understanding of the contribution of antitumor immune responses to the therapeutic management of FL. Furthermore, if these findings are confirmed, the knowledge of specific markers would not only have prognostic significance but would also predict the success or lack of response to some treatments. In addition, these findings probably explain the very conflicting results in the scientific literature on immunohistochemical markers and their prognostic values.
Chemotherapeutic regimens and the tumor microenvironment in follicular lymphoma
The immune system can have a protective role (tumor-inhibiting) but also a tumor-promoting role in which therapy can make the difference. The efficacy of the immune system in combatting tumors does not depend only on the presence or absence of an immune reaction since some non-protective immune reactions not only result in the absence of protection, but may also exacerbate tumor growth. Subtle changes in the balance can be amplifies, so that a minimal stimulus in the initial phase of the response can induce very different results.21
In the search for new modalities of cancer treatment, the induction of a potent and specific immune response by the innate and/or acquired immune system has been described as a logical and reasonable strategy for controlling tumor evolution. Various, different approaches to induce such a response in FL have been evaluated in clinical trials. These strategies included providing antigen to the immune system of FL patient by vaccination, providing co-stimulatory signals on tumor cells, inducing cell death through cytototoxic drugs, sustaining immune effectors with NK cells, NK T cells or dendritic cell adjuvant, and improving the efficiency of cross-priming.22 Data from pre-clinical and early clinical vaccination studies provided insight into the mechanisms of interactions between effectors and tumor cells, raising some optimism regarding the treatment of FL.23 Successful anti-idiotypic or DNA/viral vaccines immunity requires the function of both the humoral and cellular arms of the immune response. The wide range of other treatments able to produce tumor regression in FL includes cytokines such as interferon-α; monoclonal antibodies directed against cell surface molecules such as Ig and CD20; and targeted therapies that interfere with cell functions such as antisense RNA to BCL-2 and the proteosome inhibitor bortezomib.
Currently, both passive and active immunotherapies are being tested, either individually or, more frequently, in combination with standard or high-dose chemotherapy. The need to re-evaluate the immunosuppressive side effects of massive chemotherapy and the modulation of the prognostic impact of potential prognostic markers by chemotherapy is now well-established. Prospective trials evaluating local immune responses would be very important to determine whether the anticancer immune response dictates long-term therapeutic success.
In this issue of the Journal, de Jong and colleagues present the results of an immunohistochemical study in which they aimed to evaluate the prognostic significance of the tumor microenvironment in 61 FL patients, comparing those treated with fludarabine to those treated with cyclophosphamide, vincristine and prednisone (CVP). FL has a widely variable clinical course and the prognostic role of immunohistochemical markers of the microenviroment is highly conflicting. De Jong and colleagues found that identical immune patterns had different prognostic significances depending on the chemotherapy regimen received by the patients: FL with a complete, dense microenvironment with many macrophages, FOXP3+ regulatory T cells and CD23+ follicular dendritic cells had a more favorable outcome after CVP treatment, while the same microenvironmental characteristics were associated with a poor outcome after treatment with fludarabine. The authors highlighted the direct action of some chemotherapeutic drugs on immune cells to explain these differences.
Although the great majority of anticancer drugs are directed only against tumor cells, many have additional effects on the immune system which may contribute to their therapeutic efficacy. Some agents, such as cyclophosphamide and fludarabine, can at least partially deplete or transiently inactivate tumor-protective regulatory T cells.22 Moreover, metronomic cyclophosphamide has been demonstrated to restore the proliferative capacity of effector T cells as well as the cytotoxicity of NK cells.24 Some anticancer therapeutic agents, such as anthracyclines (e.g. doxorubicin), can also stress tumor cells in such a way that these cells become immunogenic and sensitive to lysis.25 On the other hand, data from a large cohort of patients treated homogenously with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) indicated that the addition of rituximab (R-CHOP) to this chemotherapy regimen reverses the negative prognostic effect of a high content of tumor-associated macrophages.12,13
The impact of conventional chemotherapy could also differ depending on the precise moment of the administration during the evolution of the tumor. Therapy applied during the tumor escape phase not only affects the tumor but also modulates the intrinsic relationship between tumor cells and the immune system, inhibiting suppressive mechanisms of tumor-induced tolerance, boosting T and/or B cells, or stressings tumor cells in such a way that they become immunogenic and sensitive to lysis.22 The simple reduction of tumor mass by chemotherapy may also reduce the tumor’s immunosuppressive properties, reversing tumor-induced immune tolerance and restoring the antibody- and cell-mediated immune responses.26 Furthermore, by enforcing the selection of chemotherapy-resistant tumor cells and by causing additional mutations, therapy can induce the expression of new tumor antigens.
Conclusions
The characteristics of the microenvironment in FL determine the responses to different chemotherapeutic regimens. The same patterns of immune response appear to be related to different outcomes in FL patients treated with CVP or fludarabine. Interestingly, these immune cells (macrophages and regulatory T cells) are specific targets of chemotherapeutic drugs such as cyclophosphamide and fludarabine. Chemotherapy can interact with the immune system via a direct effect on immune effectors or regulatory mechanisms or indirectly, by causing lymphopenia. The subsequent homeostatic proliferation of immune effectors may reset the relationship between the tumor and the immune system to an active anticancer response. In these conditions, alterations of immune system function may be implicated in the etiology, the response to treatment and the evolution of FL.
Considering chemotherapy as an example of immunotherapy and the possible influence of treatment on the predictive value of the immune microenvironment strengthen and confirm the rationale of clinical trials evaluating and possibly increasing the contribution of antitumor immune responses to the chemotherapeutic management of FL. It is not the molecular anomalies but the specific patterns of immune response that have predictive values, as showed by genetic and immuno-histochemical data. While immunosurveillance patterns have been related to the main clinicobiological features of FL, immune escape patterns would be principally involved in the control of growth and expansion of tumor cells. Results of several lines of investigations have highlighted that FL is an immunologically functional disease in which an active interaction between tumor cells and the functional composition of the microenvironment determines the clinical behavior, prognosis and response to specific treatment protocols.
Acknowledgments
Dr. Tomás Álvaro was supported by grant from the Ministerio de Sanidad y Consumo, Spain (FIS 05/0474).
References
- 1.Alvaro T, Lejeune M, Escrivá P, Pons L, Bosch R, Jaén J, et al. Appraisal of immune response in lymphoproliferative syndromes: a systematic review. Crit Rev Oncol Hematol. 2008 doi: 10.1016/j.critrevonc.2008.09.013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.de Jong D. Molecular pathogenesis of follicular lymphoma: a cross talk of genetic and immunologic factors. J Clin Oncol. 2005;23:6358–63. doi: 10.1200/JCO.2005.26.856. [DOI] [PubMed] [Google Scholar]
- 3.de Jong D, Koster A, Hagenbeek A, Raemaekers J, Veldhuizen D, Heisterkamp S, et al. Impact of the tumor microenvironment on prognosis in follicular lymphoma is dependent on specific treatment protocols. Haematologica. 2009;94:70–7. doi: 10.3324/haematol.13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvaro T, Lejeune M, Salvado MT, Lopez C, Jaen J, Bosch R, et al. Immunohistochemical patterns of reactive microenvironment are associated with clinicobiologic behavior in follicular lymphoma patients. J Clin Oncol. 2006;24:5350–7. doi: 10.1200/JCO.2006.06.4766. [DOI] [PubMed] [Google Scholar]
- 5.Glas AM, Knoops L, Delahaye L, Kersten MJ, Kibbelaar RE, Wessels LA, et al. Gene-expression and immunohistochemical study of specific T-cell subsets and accessory cell types in the transformation and prognosis of follicular lymphoma. J Clin Oncol. 2007;25:390–8. doi: 10.1200/JCO.2006.06.1648. [DOI] [PubMed] [Google Scholar]
- 6.Lee AM, Clear AJ, Calaminici M, Davies AJ, Jordan S, MacDougall F, et al. Number of CD4+ cells and location of forkhead box protein P3-positive cells in diagnostic follicular lymphoma tissue microarrays correlates with outcome. J Clin Oncol. 2006;24:5052–9. doi: 10.1200/JCO.2006.06.4642. [DOI] [PubMed] [Google Scholar]
- 7.Carreras J, Lopez-Guillermo A, Fox BC, Colomo L, Martinez A, Roncador G, et al. High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood. 2006;108:2957–64. doi: 10.1182/blood-2006-04-018218. [DOI] [PubMed] [Google Scholar]
- 8.Wahlin BE, Sander B, Christensson B, Kimby E. CD8+ T-cell content in diagnostic lymph nodes measured by flow cytometry is a predictor of survival in follicular lymphoma. Clin Cancer Res. 2007;13:388–97. doi: 10.1158/1078-0432.CCR-06-1734. [DOI] [PubMed] [Google Scholar]
- 9.Farinha P, Masoudi H, Skinnider BF, Shumansky K, Spinelli JJ, Gill K, et al. Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL) Blood. 2005;106:2169–74. doi: 10.1182/blood-2005-04-1565. [DOI] [PubMed] [Google Scholar]
- 10.Alvaro T, Lejeune M, Camacho FI, Salvado MT, Sanchez L, Garcia JF, et al. The presence of STAT1- positive tumor-associated macrophages and their relation to outcome in patients with follicular lymphoma. Haematologica. 2006;91:1605–12. [PubMed] [Google Scholar]
- 11.Kelley T, Beck R, Absi A, Jin T, Pohlman B, Hsi E. Biologic predictors in follicular lymphoma: importance of markers of immune response. Leuk Lymphoma. 2007;48:2403–11. doi: 10.1080/10428190701665954. [DOI] [PubMed] [Google Scholar]
- 12.Canioni D, Salles G, Mounier N, Brousse N, Keuppens M, Morchhauser F, et al. High numbers of tumor-associated macrophages have an adverse prognostic value that can be circumvented by rituximab in patients with follicular lymphoma enrolled onto the GELA-GOELAMS FL-2000 trial. J Clin Oncol. 2008;26:440–6. doi: 10.1200/JCO.2007.12.8298. [DOI] [PubMed] [Google Scholar]
- 13.Taskinen M, Karjalainen-Lindsberg ML, Nyman H, Eerola LM, Leppa S. A high tumor-associated macrophage content predicts favorable outcome in follicular lymphoma patients treated with rituximab and cyclophosphamidedoxorubicin-vincristine-prednisone. Clin Cancer Res. 2007;13:5784–9. doi: 10.1158/1078-0432.CCR-07-0778. [DOI] [PubMed] [Google Scholar]
- 14.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–74. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ai WZ, Hou JZ, Zeiser R, Czerwinski D, Negrin RS, Levy R. Follicular lymphoma B cells induce the conversion of conventional CD4(+) T cells to T-regulatory cells. Int J Cancer. 2008;124:239–44. doi: 10.1002/ijc.23881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 17.Goval JJ, Thielen C, Bourguignon C, Greimers R, Dejardin E, Choi YS, et al. The prevention of spontaneous apoptosis of follicular lymphoma B cells by a follicular dendritic cell line: involvement of caspase-3, caspase-8 and c-FLIP. Haematologica. 2008;93:1169–77. doi: 10.3324/haematol.12127. [DOI] [PubMed] [Google Scholar]
- 18.Oeschger S, Brauninger A, Kuppers R, Hansmann ML. Tumor cell dissemination in follicular lymphoma. Blood. 2002;99:2192–8. doi: 10.1182/blood.v99.6.2192. [DOI] [PubMed] [Google Scholar]
- 19.Su W, Spencer J, Wotherspoon AC. Relative distribution of tumour cells and reactive cells in follicular lymphoma. J Pathol. 2001;193:498–504. doi: 10.1002/path.820. [DOI] [PubMed] [Google Scholar]
- 20.Byers RJ, Sakhinia E, Joseph P, Glennie C, Hoyland JA, Menasce LP, et al. Clinical quantitation of immune signature in follicular lymphoma by RT-PCR-based gene expression profiling. Blood. 2008;111:4764–70. doi: 10.1182/blood-2007-10-115915. [DOI] [PubMed] [Google Scholar]
- 21.Dalgleish A. The relevance of non-linear mathematics (chaos theory) to the treatment of cancer, the role of the immune response and the potential for vaccines. QJM. 1999;92:347–59. doi: 10.1093/qjmed/92.6.347. [DOI] [PubMed] [Google Scholar]
- 22.Zitvogel L, Apetoh L, Ghiringhelli F, Andre F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? J Clin Invest. 2008;118:1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dermime S, Aljurf MD. Current advances, problems and prospects for vaccine-based immunotherapy in follicular non-Hodgkin’s lymphoma. Leuk Lymphoma. 2005;46:497–507. doi: 10.1080/104281904000025104. [DOI] [PubMed] [Google Scholar]
- 24.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–8. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 26.Danna EA, Sinha P, Gilbert M, Clements VK, Pulaski BA, Ostrand-Rosenberg S. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004;64:2205–11. doi: 10.1158/0008-5472.can-03-2646. [DOI] [PubMed] [Google Scholar]