Abstract
The treatment of acute myeloid leukemia has improved in the last ten years, and in this perspective article Dr. Estey examines the recent progress in this field. See related articles on pages 54 and 102.
Ten years ago therapy of newly-diagnosed acute myeloid leukemia (AML) was largely invariant. Patients received daunorubicin or idarubicin for 3 days and cytarabine (ara-C) at a dose of 100 mg/m2 daily for 7 days as a continuous infusion, a regimen commonly known as “3+7”. Nowadays, however, guidelines, such as those in the paper by Morra et al.,1 recommend that many older patients be given investigational therapies at diagnosis. This change reflects the greater availability of new treatments, often thought to be targeted to specific abnormalities in AML blasts. The advent of a broader range of investigational therapies and increased knowledge about the molecular biology of AML has raised several questions, which I address here: (i) which patients are candidates for investigational therapy¿ (ii) should cytogenetic and molecular information be used to plan initial therapy¿ (iii) what is the current role of allogeneic hematopoietic stem cell transplant (HSCT)¿ (iv) regarding targeted therapy - are responses less than a complete response worthwhile, how long should therapy be continued before failure is declared, should combinations with chemotherapy or other targeted agents be explored sooner than is currently the case, and should these agents be reserved for a specific population or used more broadly¿ and (v) given the increasing recognition of the biological and prognostic heterogeneity of AML, should we depart from standard clinical trial methodology, which could lead to missing potentially important therapeutic advances as described in the paper by Schlenk et al.2
Which patients are candidates for investigational therapies?
All new agents brought to clinical trials have a plausible rationale. Many appear “encouraging” in early studies. However, such early results are often not confirmed. For example, only three of 37 drugs reported as “promising” in abstracts reported at the annual American Society of Hematology meetings between 1993 and 2001 subsequently gave positive results in randomized trials, and only one (gemtuzumab ozogamycin, GO) is being used in clinical practice.3 Hence the decision to administer investigational therapy must be based not on the promise of the new therapy but on the unsatisfactory results of standard therapy, and is dependent on an understanding of factors that govern prognosis following the use of such therapy.
Age is currently the prognostic factor most commonly used to assign untreated patients to new therapies. Typically, older patients are taken to be those aged 60 years or over. However, age behaves as a numerical (continuous) variable.4 Thus, on average, there is more difference in outcome between a 61-year old and a 68-year old (both considered elderly) than between a 59-year old (younger) and a 61-year old. Furthermore, age is not the major predictor for either treatment-related mortality (TRM) or resistance to therapy, the two causes of therapeutic failure in AML.5 Although it can be difficult to determine whether failure results from TRM or resistance, the factors associated with these outcomes are distinct. Performance status is the principal forecaster of TRM.6 Indeed, patients who are largely or completely bed-ridden are routinely ineligible for trials of new therapies because of the fear that they will die before response can be evaluated; thus such patients comprise a significant unmet need in AML therapeutics. Higher serum concentrations of creatinine, and bilirubin,6 and various co-morbidities,7 similar to those defined for HSCT, are other independent predictors of TRM. Rates of TRM after the 3+7 chemotherapy regimen range from less than 5% in fully ambulatory patients aged under 40 years old and with no co-morbidities to greater more than 50% in bedridden patients with other medical problems who are over 70 years old.6
Resistance to therapy, i.e. either failure to obtain a complete response despite living long enough to have done so or relapse from a complete response, is the most common cause of failure even in patients aged 70 years and above who are ambulatory.5 The main predictor of resistance is leukemia cell cytogenetics,8 which is typically divided into three categories ranging in prognosis from best to worst. Pericentric inversions of chromosome 16 (inv 16) or translocations between chromosomes 8 and 21(t(8;21) disrupt core binding factor (CBF), a critical normal regulator of gene transcription and subsequent differentiation.9 CBF AML accounts for 10–20% of all cases, and is particularly uncommon (less than 5% of cases) above the age of 60. CBF AML is associated with extreme sensitivity to ara-C, GO and possibly fludarabine and granulocyte colony-stimulating factor (G-CSF). In a highly influential study, the American Cancer and Leukemia Group (CALGB) found that 78% of patients under 60 years old with CBF AML given 3+7 induction, which will practically uniformly produce a complete response in CBF AML, remained in complete remission at 5 years if given four courses of ara-C at a dose of 3 g/m2 on days 1, 3, and 5 (so-called high-dose ara-C, HiDAC) followed by four courses of reduced amounts of the 3+7 regimen.10 In contrast, 5-year complete response rates were 57% and 16% if the four ara-C post-remission courses were given at a dose of 400 mg/m2 daily for 5 days and 100 mg/m2 daily for 5 days respectively.10 Results from the British Medical Council Research (MRC) studies suggest that an intermediate ara-C dose of 1 g/m2 is as effective as 3 g/m2. 11 More recently the MRC randomized patients under the age of 60 to the 3+7 regimen with or without GO and, separately, to a higher dose ara-C regimen also with or without GO.12 In both cases the addition of GO had no effect on the complete remission rate but dramatically improved survival (e.g. to 80–90%) in patients with CBF AML, rendering prognostically insignificant the presence of mutations in CKIT, which had been found to increase relapse rates in CBF AML.9 Data from the M.D. Anderson Cancer Center suggest that the use of fludarabine + G-CSF in addition to ara-C at 1–2 g/m2 daily provides superior results to those produced by similar doses of ara-C + idarubicin but without fludarabine + G-CSF.13
At the other extreme of the prognostic spectrum are complex karyotypes, defined by the presence of three to five distinct abnormalities14 or, as recently identified in patients age under 60 years old, a monosomal karyotype,15 defined by the presence of at least two monosomies or one monosomy plus one structural abnormality. Complex karyotypes, and almost certainly a monosomal karyotype, are more common in patients with secondary AML (a history of abnormal blood counts or myelodysplasia, or of chemotherapy for cancer) and in older patients, comprising 40–60% of such cases. However, even if under 60 years old and with de novo AML, patients with complex or monosomal karyotypes have complete remission rates of less than 50% with the 3+7 regimen.16 The complete remissions typically last less than 1 year, with potential cure (specified once a complete remission has continued for 3 years, after which the likelihood of relapse declines sharply to below 10%)17 essentially impossible even with the use of GO, HiDAC or HSCT.
Patients in the intermediate cytogenetic group have the most variable prognoses. The most common finding in this group, and the most common cytogenetic finding in AML, is a normal karyotype. In the past 5–10 years, 75% of patients in this group (at least when aged less than 60 and with de novo AML) have been shown to have various molecular abnormalities that are highly prognostic of outcome after the 3+7 induction and post-remission therapy with ara-C at various doses including HiDAC.18 Thus patients with a normal karyotype with a mutation in the NPM1 gene (NPM+) and no aberration in the FLT3 gene (FLT3−) have a prognosis similar to that of patients with CBF AML, while patients with a normal karyotype with an internal tandem duplication (ITD) of the FLT3 gene have a prognosis qualitatively similar to that of patients with complex karyotypes.5, 18 Most karyotypically normal patients do not have mutations in either NPM1 or FLT3; however if they have a mutation in the CEBPA gene or have increased expression of the BAALC gene and no partial tandem duplication in the MLL gene their prognosis becomes qualitatively similar to that of patients who are NPM+, FLT3−.18
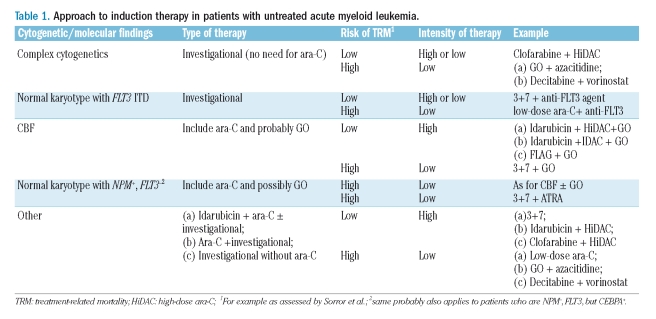
Given the prime importance of cytogenetics (and, in karyotypically normal patients, of molecular data) in determining outcome following the 3+7 regimen (as well as HiDAC, GO, and HSCT) and, conversely, the secondary prognostic role of age (even in forecasting TRM) it seems logical to assign patients to receive standard or investigational therapy based primarily on cytogenetic/molecular information rather than, as currently the case, on age. Age, together with other factors discussed above that influence TRM, would modify the type of investigational therapy received (Table 1). For example, the outcome with standard therapy in patients with complex cytogenetics is so poor that such patients should receive investigational therapy regardless of age. If they are under 60 years old, completely ambulatory, and without comorbidities, such therapy might be more intense, for example clofarabine + HiDAC.
Table 1.
Approach to induction therapy in patients with untreated acute myeloid leukemia.
The same type of therapy might be used even for patients aged 60–70, again provided they are completely ambulatory and without comorbidities. In contrast, for patients over 60 years old, or those under 60 years old but with a poor performance status or comorbidities, therapy would need to be less intense (Table 1). Investigational drugs that inhibit the abnormal tyrosine kinase formed as a consequence of the FLT3 ITD are being added the to 3+7 induction in karyotypically normal patients who are aged less than 60-year old and FLT3+. A similar approach in FLT3+ patients at higher risk of TRM might replace 3+7 with low-dose ara-C. Ara-C produces sufficiently good outcomes in patients with CBF AML or karyotypically normal AML who are NPM+, FLT3− (and probably NPM−, FLT3−, but CEBPA+) that such patients should always receive this drug. Given the MRC data,12 GO, which is relatively non-toxic at the 3 mg/m2 dose used by the MRC, should probably be added. GO might be particularly useful in patients at high-risk of TRM in whom ara-C dose reductions are contemplated. Based on the results of Schlenk et al.,2 ATRA might be similarly useful in patients who are NPM+ FLT3− but at high risk of TRM. Patients in the other, i.e. intermediate, group in Table 1 have complete response rates of 60–75% but potential cure rates of only 20–30%. These intermediate outcomes make choosing between investigational and more standard therapy difficult, thus explaining the range of therapies in Table 1. In particular, it is plausible that some patients in this group might prefer standard therapy feeling that investigational therapy might worsen outcome, while others might choose a combination of an investigational or a standard therapy, or only an investigational therapy either used once a complete remission has been obtained, or, for the reason noted in the next section, at initial diagnosis.
Should cytogenetic and molecular information be used to plan initial therapy?
Cytogenetic and molecular results often take several days to become available. Because AML is typically viewed, for example in major American medical textbooks, as an indication for rapid initiation of treatment these results tend to influence post-remission therapy, e.g. the decision to proceed to HSCT, rather than induction therapy. However, in some cases, such as those with complex cytogenetics, complete remission rates with standard therapy are less than 50%, thus making a case for delaying initial treatment until cytogenetic results are known. Furthermore, in cases such as those in the intermediate group referred to above, in which the complete remission rate is over 50%, beginning investigational treatment at diagnosis may lengthen the duration of the transient complete remissions seen with standard therapy, although improvements in complete remission rate may be more difficult to detect. An example of this phenomenon came from the trial of 3+7 therapy with or without GO mentioned earlier.12 A current trial in which patients under the age of 60 with FLT3+ AML are randomized to receive 3+7 with or without the anti-FLT3 agent midostaurin is an example of a trial that is using molecular information to assign initial therapy.
In an investigation of the effect of time from diagnosis to initiation of ara-C-containing therapy on complete remission and survival in previously untreated patients with AML presenting with a white cell count lower than 50×109/L, Sekeres et al. found, that after accounting for performance status, white cell count, cytogenetics, and de novo vs. secondary disease, time from diagnosis to treatment had no influence on outcome in patients who were over 60 years old.19 This finding provides a further rationale for the use of cytogenetic/molecular information in planning initial treatment in such patients. In contrast, longer time from diagnosis to treatment adversely affected complete remission and survival rates in younger patients. However, it remains unclear whether this effect might eventually be more than counterbalanced if patients with complex, or monosomal, karyotypes were spared the morbidity, and occasional mortality, following use of therapies such as 3+7, which are known to be ineffective against these types of AML.
What is the role of allogeneic hematopoietic stem cell transplant?
Typically myleoablative allogeneic HSCT has been used primarily in patients in first complete remission. It is now generally accepted that outcomes of patients with CBF AML treated as described above are such that the risk of TRM associated with myeloablative allogeneic HSCT is difficult to justify. The same is presumably true for patients with NPM+ FLT3− AML. In contrast, meta-analyses using data from four European cooperative groups indicated that among AML patients with other karyotypes, including a normal karyotype, the risk of TRM or death from relapse after myeloablative allogeneic HSCT in patients with HLA-identical sibling donors (n = 748) was 85% (95% confidence interval 0.74–0.95) that seen in patients (n = 1372) without such donors; 82% of those with donors received a myeloablative HSCT.19 However the improvement in survival was limited to patients under 35-year old.20 The risk of death in such younger patients with donors was 73% of that in younger patients without donors. While this result is highly statistically significant (95% CI 0.62–0.85) its medical significance may be less. For example, a hazard ratio of 0.73 translates into a 1.36-fold improvement in survival. Thus, patients with a median survival of 12 months without allogeneic HSCT would expect an improvement in survival to 16.4 months with the procedure. Under the circumstances it is understandable that, while some patients might prefer to receive a standard allogeneic HSCT, others might prefer investigational therapies. These of course could include investigational approaches to reduce TRM or relapse after allogeneic HSCT, the latter being the principal cause of failure after the procedure. Current attempts to prevent relapse involve use of radiolabeled antiCD45 antibodies in the preparative regimen, or of azacitidine, decitabine, or lenalidomide after allogeneic HSCT. Allogeneic HSCT has been revolutionized by the development of reduced intensity transplants and the use of stem cells from non HLA-identical siblings, matched unrelated donors, haploidentical donors, and umbilical cord blood.20 Reduced intensity allogeneic HSCT relies on a graft-versus-AML effect, thus allowing the preparative regimen to be non-myeloablative, which reduces TRM, and permits patients up to 70–75-year old without co-morbidities to undergo the transplant procedure. Reduced intensity allogeneic HSCT in first complete remission was reported to prolong survival in patients over 50-year old with cytogenetic abnormalities other than CBF.21 However, only 14% of patients in first complete remission underwent reduced intensity allogeneic HSCT, casting doubt on the relevance of the procedure and introducing the possibility of selection bias.21 The use of alternative donors also increases the number of patients who can undergo allogeneic HSCT. Current data suggest that outcomes are similar in recipients of grafts from HLA-sibling and matched unrelated donors. Obviously the use of allogeneic HSCT from alternative donors falls into the category of investigational therapy, increasing the likelihood of graft-vs.-host and graft-vs.-AML (GVL) effects, and thus highlighting the importance of separating these two effects. Finally, the development of sensitive and specific techniques, e.g. flow cytometry, for monitoring minimal residual disease in patients in complete remission may reduce risk by allowing HSCT to be done only in patients with evidence of residual disease.22
Issues raised by the use of targeted therapies
Responses less than a complete response
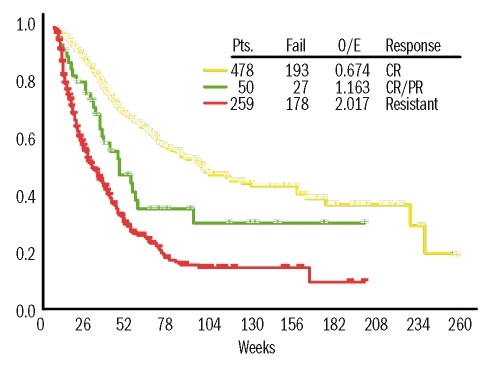
For many years response in AML meant a complete remission. The importance of this complete response was pointed out by Freireich et al., who noted that patients who entered complete remission lived longer than those who did not.23 The difference was entirely accounted for by time spent in complete remission, suggesting that better survival did not result from an inherently better prognosis but from achievement of complete remission. Beginning with GO24 investigators have defined new response categories, such as complete response with incomplete platelet recovery, marrow complete response with no requirement for the blood count recovery, or hematologic improvement as defined for myelodysplastic syndromes.25 The criteria for these categories are uniformly less stringent than those for a complete response. A recent combined analysis by ECOG and the team at MD Anderson suggested that essentially all patients who lived 5 years after beginning ara-C-containing therapy achieved a complete remission.26 Whether the same is true with targeted therapy remains to be seen. It does appear that, although conveying shorter survival than a complete response, complete response with incomplete platelet recovery is independently associated with longer survival than that seen in patients who lived long enough to obtain such responses but did not27 (Figure 1). Given the paramount importance of survival to patients, it seems worthwhile, rather than uncritically accepting new response criteria, to examine whether these responses, while denoting activity, prolong survival more than might a total absence of response.
Figure 1.
Effect of response on survival. The analysis was limited to patients who lived at least 60 days from the start of induction therapy by which time more than 90% of patients who achieved a complete response and 75% of those who achieved a complete response with incomplete platelet recovery had done so. Results were similar if patients who died before day 35 (median time to complete response), day 90, etc. were excluded.
How long should therapy be continued until failure is declared¿
Patients not in complete remission after two courses of standard therapy, e.g. the 3+7 regimen, are typically considered to have failed treatment. Their chance of achieving complete remission with a third course is considerably less than the chance with a second course, and even patients who require two courses to enter complete remission have shorter remissions than patients in complete remission after only one course.5 However, data from trials of decitabine and azacitidine in myelodysplastic syndromes have raised the possibility that response to targeted agents requires more time than response to cytotoxic therapy.28, 29 This requirement may be a general reflection of the use of low-dose therapy since Burnett et al. reported that the median number of courses to achieve complete remission with low-dose ara-C is three, similar to what has been observed with decitabine or azacitidine.30
At any rate investigators have to avoid both discontinuing a therapy prematurely leading to a false negative conclusion or continuing a therapy so long that little time remains to administer other therapies. Under the circumstances it seems worthwhile to examine whether surrogate indicators of eventual response, for example bone marrow findings after three courses of therapy, can be identified.
Combinations with chemotherapy or other targeted agents
Farnesyl transferase inhibitors (e.g. tipifarnib), hypomethylating agents (decitabine and azacitidine), FLT3 inhibitors (lestaurinib and midostaurin), and anti-CD33 antibodies (GO and HuM195) are targeted agents that have undergone extensive investigation as single agents.31 Tipifarnib has been largely abandoned, while the other drugs are being combined with cytotoxic therapy, or with other targeted agents, e.g. inhibitors of his-tone decetylase. Given the time required for phase 1, phase 2 and perhaps phase 3 testing of each new drug or combination, would it be preferable to begin with combinations rather than only proceeding to them once the single agent trial is completed¿ Furthermore, given the number of plausible combinations and the need for clinical, rather than pre-clinical data, to know which is best, it seems advisable to conduct smaller trials allowing more therapies to be investigated.32 Such studies may be underpowered. However, the false negative rates of 20% (power = 80%) built into larger studies are nominal and ignore the false negative cases that may result when one drug or combination is selected for trial while others are not.32 The view that the worst false negative rate may result when a treatment is not investigated has led the MRC to conduct small randomized trials in older patients with untreated AML, with the goal of selecting the best new drug/combination for comparison with more standard therapy.
Should only ‘target-positive’ patients receive given targeted therapy¿
This approach is being utilized in the American Intergroup trial of the 3+7 regimen with or without midostaurin, for which only patients with FLT3+ AML are eligible. Because it is unlikely that results will be positive in target-negative patients but negative in target-positive patients this approach allows more therapies to be explored than if all patients were given the targeted therapy. While rational and preferable, the approach carries the risk of possible overestimation of our knowledge of the target of the targeted therapy. For example, although azacitidine and decitabine are commonly considered demethylating agents, there have been only inconstant correlations between response to these drugs and either pretreatment methylation status (globally or with respect to specific genes such as p15) or drug-induced demethylation (globally or involving particular genes).31
Failure of current clinical trial methodology to address heterogeneity in acute myeloid leukemia
An all encompassing theme of this paper is the heterogeneous nature of AML as most recently exemplified by identification of various molecularly-defined subgroups. The number of such subgroups will certainly increase, and each may receive a unique therapy, e.g. chemotherapy + ATRA, as administered by Schlenk et al. for NPM+ FLT3− AML.2 The number of patients in the various subgroups will inevitably be so small that it will be impossible to meet the current requirement for 80–90% power to detect relatively small differences with a false positive rate of 5%. Clinical trial methodology will have to adapt to these new realities by accepting as of interest, only larger, perhaps more medically (as opposed to statistically) significant differences, or by accepting higher false positive rates (p>0.05) or higher false negative rates (power lower than 80%).
I would suggest that, in particular, the p<0.05 criterion be revisited. It seems illogical to demand more protection (95% reflecting a p value of 0.05) against a false positive result than against a false negative result (80% corresponding to a power of 80%). Such an approach seems more appropriate for a disease for which current therapy is satisfactory than it does for AML. In the former case it is paramount to avoid replacing the current standard with a therapy that is incorrectly felt to be superior. However, satisfactory therapies exist for only a minority of patients with AML and the consequences of false positive results are, therefore, much less.
The neglect of heterogeneity is also problematic when examining the effect of a single treatment in groups with varying prognoses. Thus, despite the known effect of cytogenetics on outcome after a range of therapies, standard methodology continues to regard patients as essentially interchangeable regardless of cytogenetics. For example, a trial of a new drug in patients who are over 60 years old with untreated AML might target a complete response rate of 60% vs. an expected rate of 40% with standard treatment and wish to have a false positive rate less than 5% and a false negative rate less than 20%. Using a standard Simon two-stage optimum design 16 patients would be treated in the first stage and the drug rejected if fewer than eight of the 16 patients entered complete remission. But, if by chance, a disproportionate number of these first 16 had complex cytogenetics the stopping criterion might be met, and a falsely negative conclusion drawn, simply because the expected complete remission rate in these patients was in fact 25%, not 40%. The opposite extreme, i.e. conducting separate trials of the same drug in various cytogenetically defined subsets has the disadvantages of being time-consuming and not allowing data from a trial in one subgroup to adaptively affect the conduct of the same trial in other subgroups. Alternative phase 2 designs that, unlike the Simon design, account for prognostic heterogeneity have been proposed.33 Regardless of this specific issue, it may be fair to ask whether current statistical methodology best serves our needs and note that the statistical literature is replete with new designs that address the many problems inherent in standard methodology, which has remained unchanged for 30 years.
References
- 1.Morra E, Barosi G, Bosi A, Ferrara F, Locatelli F, Marchetti M, et al. Clinical management of primary non-APL acute myeloid leukemia: practice Guidelines by the Italian Society of Hematology, the Italian Society of Experimental Hematology and the Italian Group for Bone Marrow Transplantation. Haematologica. 2009;94:102–12. doi: 10.3324/haematol.13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlenk RF, Dohner K, Kneba M, Gotze K, Hartmann F, Valle Fd, et al. Gene mutations and response to treatment with all-trans retinoic acid in elderly patients with acute myeloid leukemia-results from the AMLSG trial AML HD98B. Haematologica. 2009;94:54–60. doi: 10.3324/haematol.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estey EH, Bedikian SH, Witter DC, Pierce SA, Giles FJ. The predictive value of a “Positive” ASH abstract in AML therapeutics. Blood. 2006;108 abstract#1964. [Google Scholar]
- 4.Thall P, Estey E. Graphical methods for evaluating covariate effects in the Cox model. In: Crowley J, editor. Handbook of Statistics in Clinical Oncology. New York: Marcel-Dekker; 2001. pp. 411–33. [Google Scholar]
- 5.Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 6.Estey E, Smith TL, Keating MJ, McCredie KB, Gehan EA, Freireich EJ. Prediction of survival during induction therapy in patients with newly diagnosed acute myeloblastic leukemia. Leukemia. 1989;3:257–63. [PubMed] [Google Scholar]
- 7.Giles FJ, Borthakur G, Ravandi F, Faderl S, Verstovsek S, Thomas D, et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br J Haematol. 2007;136:624–7. doi: 10.1111/j.1365-2141.2006.06476.x. [DOI] [PubMed] [Google Scholar]
- 8.Grimwade D, Walker H, Harrison G, Oliver F, Chatters S, Harrison CJ, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1312–20. doi: 10.1182/blood.v98.5.1312. [DOI] [PubMed] [Google Scholar]
- 9.Paschka P. Core binding factor acute myeloid leukemia. Semin Oncol. 2008;35:410–7. doi: 10.1053/j.seminoncol.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Bloomfield CD, Lawrence D, Byrd JC, Carroll A, Pettenati MJ, Tantravahi R, et al. Frequency of prolonged remission duration after high-dose cytarabine intensification in acute myeloid leukemia varies by cytogenetic subtype. Cancer Res. 1998;58:4173–9. [PubMed] [Google Scholar]
- 11.Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92:2322–33. [PubMed] [Google Scholar]
- 12.Burnett AK, Kell WJ, Goldstone AH, Milligan D, Hunter A, Prentice AG, et al. The addition of gemtuzumab ozogamicin to induction chemotherapy for AML improves disease free survival without extra toxicity: preliminary anlysis of 1115 patients in the MRC AML15 trial. Blood. 2006;108 abstract #13. [Google Scholar]
- 13.Borthakur G, Kantarjian H, Wang X, Plunkett WK, Jr, Gandhi VV, Faderl S, et al. Treatment of core-binding-factor in acute myelogenous leukemia with fludarabine, cytarabine, and granulocyte colony-stimulating factor results in improved event-free survival. Cancer. 2008;113:3181–5. doi: 10.1002/cncr.23927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mrozek K. Cytogenetic, molecular genetic, and clinical characteristics of acute myeloid leukemia with a complex karyotype. Semin Oncol. 2008;35:365–77. doi: 10.1053/j.seminoncol.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breems DA, Van Putten WL, De Greef GE, Van Zelderen-Bhola SL, Gerssen-Schoorl KB, Mellink CH, et al. Monosomal karyotype in acute myeloid leukemia: a better indicator of poor prognosis than a complex karyotype. J Clin Oncol. 2008;26:4791–7. doi: 10.1200/JCO.2008.16.0259. [DOI] [PubMed] [Google Scholar]
- 16.Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–83. [PubMed] [Google Scholar]
- 17.de Lima M, Strom SS, Keating M, Kantarjian H, Pierce S, O’Brien S, et al. Implications of potential cure in acute myelogenous leukemia: development of subsequent cancer and return to work. Blood. 1997;90:4719–24. [PubMed] [Google Scholar]
- 18.Mrozek K, Marcucci G, Paschka P, Whitman SP, Bloomfield CD. Clinical relevance of mutations and gene-expression changes in adult acute myeloid leukemia with normal cytogenetics: are we ready for a prognostically prioritized molecular classification¿. Blood. 2007;109:431–48. doi: 10.1182/blood-2006-06-001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sekeres MA, Elson P, Kalaycio ME, Advani AS, Copelan EA, Faderl S, et al. Time from diagnosis to treatment initiation predicts survival in younger, but not older, acute myeloid leukemia patients. Blood. 2008 Sep 30; doi: 10.1182/blood-2008-05-157065. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornelissen JJ, van Putten WL, Verdonck LF, Theobald M, Jacky E, Daenen SM, et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom¿. Blood. 2007;109:3658–66. doi: 10.1182/blood-2006-06-025627. [DOI] [PubMed] [Google Scholar]
- 21.Estey E, de Lima M, Tibes R, Pierce S, Kantarjian H, Champlin R, et al. Prospective feasibility analysis of reduced-intensity conditioning (RIC) regimens for hematopoietic stem cell transplantation in elderly patients with acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood. 2007;109:1395–400. doi: 10.1182/blood-2006-05-021907. [DOI] [PubMed] [Google Scholar]
- 22.Freeman SD, Jovanovic JV, Grimwade D. Development of minimal residual disease-directed therapy in acute myeloid leukemia. Semin Oncol. 2008;35:388–400. doi: 10.1053/j.seminoncol.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Freireich EJ, Gehan EA, Sulman D, Boggs DR, Frei E., 3rd The effect of chemotherapy on acute leukemia in the human. J Chronic Dis. 1961;14:593–608. doi: 10.1016/0021-9681(61)90118-7. [DOI] [PubMed] [Google Scholar]
- 24.Sievers EL, Larson RA, Stadtmauer EA, Estey E, Lowenberg B, Dombret H, et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol. 2001;19:3244–54. doi: 10.1200/JCO.2001.19.13.3244. [DOI] [PubMed] [Google Scholar]
- 25.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 26.Estey E, Sun Z, Rowe J, Faderl S, Cassileth P, Sartiano G, et al. A 3,239-patient combined Eastern Cooperative Oncology Group (ECOG), M.D. Anderson Cancer Center (MDA) analysis of the effect of CR vs. responses<CR on long-term survival in newly-diagnosed AML treated with ara-C-containing regimens: implications of targeted drug development. Blood. 2007;110 abstract #298. [Google Scholar]
- 27.Estey EH, Garcia-Manero G, Giles FJ, Cortes J, O’Brien S, Kantarjian HM. Clinical relevance of CRp in untreated AML. Blood. 2005;106 abstract#541. [Google Scholar]
- 28.Silverman LR, McKenzie DR, Peterson BL, Holland JF, Backstrom JT, Beach CL, et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol. 2006;24:3895–903. doi: 10.1200/JCO.2005.05.4346. [DOI] [PubMed] [Google Scholar]
- 29.Kantarjian H, Oki Y, Garcia-Manero G, Huang X, O’Brien S, Cortes J, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109:52–7. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
- 30.Burnett AK, Milligan D, Prentice AG, Goldstone AH, McMullin MF, Hills RK, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109:1114–24. doi: 10.1002/cncr.22496. [DOI] [PubMed] [Google Scholar]
- 31.Estey E. New drugs in acute myeloid leukemia. Semin Oncol. 2008;35:439–48. doi: 10.1053/j.seminoncol.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Estey EH, Thall PF. New designs for phase 2 clinical trials. Blood. 2003;102:442–8. doi: 10.1182/blood-2002-09-2937. [DOI] [PubMed] [Google Scholar]
- 33.Wathen JK, Thall PF, Cook JD, Estey EH. Accounting for patient heterogeneity in phase II clinical trials. Stat Med. 2008;27:2802–15. doi: 10.1002/sim.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]