CD146+ bone marrow stromal cells have been recently recognized as clonogenic osteoprogenitors able to organize a complete hematopoietic microenvironment. This study shows their involvement in bone marrow stromal changes occurring in primary myelofibrosis.
Keywords: bone marrow osteoprogenitors, primary myelofibrosis
Abstract
CD146+ bone marrow stromal cells have been recently recognized as clonogenic osteoprogenitors able to organize a complete hematopoietic microenvironment. In this study we used immunohistochemical analysis to investigate the contribution of CD146+ bone marrow osteoprogenitors to the stromal remodeling occurring in the different stages of primary myelofibrosis. We found that CD146+ cells sited at the abluminal side of the bone marrow vessels and branching among hematopoietic cells significantly increased in the advanced stages of primary myelofibrosis (p<0.001), paralleling the extent of fibrosis (ρ=0.916, p<0.0001) and the microvascular density (r=0.883, p<0.0001). Coherently with a mural cell function, such cells also displayed smooth-muscle actin expression. Our data providing evidence of CD146+ cell involvement in bone marrow stromal changes occurring in primary myelofibrosis are consistent with the capability of these cells to participate in fiber deposition, angiogenesis, and bone formation. They could also represent rationale for new therapies targeting the bone marrow stroma in primary myelofibrosis.
Introduction
Primary myelofibrosis is Ph- hematopoietic stem cell (HSC) disorder characterized by the clonal proliferation of the myeloid and megakaryocytic populations and by the progressive development of reticulin and collagen fibrosis in the bone marrow (BM) parenchyma. 1 Due to its fibrogenic nature, leading to BM failure, primary myelofibrosis (PMF) has a poor prognosis and still lacks a valid therapeutic approach.
Besides the proliferation of the hematopoietic cell lineages, also the BM stromal reaction seems to paly a role in the pathogenesis of this disease. This is suggested by the increase in vessel neoformation and microvascular sprouting which can be observed throughout the different phases of the disease, and by the bone remodeling and formation (osteosclerosis) occurring in the advanced stages.2 The active role of the BM stroma is also supported by the increase in the microvessel density that can be observed in PMF paralleling the degree of BM fibrosis and retaining, along with fibrosis, a prognostic significance.3–5
Recently, the growing attention on the BM stromal niches and their cellular constituents has led to the identification of CD146 (MCAM)-expressing BM stromal cells as clonogenic BM osteoprogenitors, deriving from hematopoietic stem cells, able to induce bone formation and to organize a hematopoietic microenvironment.6,7 In the human BM in situ, such a BM stromal stem-cell (BM-SSC) phenotype has been shown to be shared by subendothelial (adventitial) reticular cells displaying mural-cell properties (e.g. smooth muscle actin expression, reticulin and collagen fiber deposition).6–8 Furthermore, CD146+ BM subendothelial cells have been shown to be able to sustain microvessel assembly and remodeling through the production of Angiopoietin-1 and its interaction with Tie-2 receptor on endothelial cells.6 On the basis of these observations, we hypothesized that such cells could play a role in the BM stromal changes occurring in PMF and wanted to assess the presence and distribution of CD146+ osteoprogenitors in the bone marrow of patients with different stages of PMF, namely early- (grade 0/1 fibrosis) and advanced-stages (grade 2/3 fibrosis), by the means of immunohistochemical analysis.
Design and Methods
This study was performed with the approval and permission of the Institutional Review Board of the University Hospital “Policlinico Paolo Giaccone” of Palermo. All the procedures followed were in accordance with the Helsinki Declaration.
Sample selection
The study was performed on bone marrow trephine biopsies obtained from 32 patients who were diagnosed with PMF according to the WHO criteria9 between January 2005 and December 2007 at the Department of Human Pathology of the University of Palermo. Only representative bone marrow trephine biopsies (i.e. non-tangential biopsies of more than 1.5 cm in length) collected at the time of diagnosis, before any treatment was started, were included in the study. Ten bone marrow biopsies from age-matched patients with Hodgkin’s lymphoma without BM involvement were used as controls. BM biopsies were fixed in formalin and paraffin embedded and 4 mm-thick sections were cut from paraffin blocks for histochemical and immunophenotypical evaluation. The following clinical data were retrospectively gathered from the patients’ medical records: age, gender, white blood cell count, platelet count, hemoglobin value, presence of one or more risk factors included in the Mayo prognostic score10 (Hb<10 g/dL, WBC<4 or >30×109/L, PLT<100×109/L, AMC >1×109/L), and JAK2 (V617F) mutational status.
Bone marrow histochemical and immunophenotypical analysis
On histopathological evaluation, all the previous diagnoses of PMF were confirmed. Assessment of the BM fibrosis was performed on Gomori’s silver stained sections on the basis of the European Myelofibrosis Network (EUMNET) consensus grading and scored accordingly from grade 0 to 3.11 All the cases were independently assessed by four of the authors (UG, EB, CT, AMF) and interobserver agreement on BM fibrosis was reached by re-examining discordant cases on a multi-headed microscope. Immunohistochemistry was performed on BM sections by means of the streptavidinbiotin-peroxidase complex with rhodamine-red amino-ethyl carbazole as chromogen. The following primary antibodies were used: mouse monoclonal anti-CD34 (clone QBEnd/10, Novocastra, UK), mouse monoclonal anti-CD146 (clone N1238, Novocastra, UK), mouse monoclonal anti-SMA (clone 1A4, Dako, Denmark). The microvascular density was assessed by counting the absolute number of CD34+ vessels out of 10 high-power (400×) microscopical fields (HPF) and was expressed as the mean value. CD146 was similarly evaluated by counting the number of positive cells out of 10 HPF and was expressed as the mean value.
Statistical analysis
The Mann Whitney U-test was performed to compare two independent groups of data. Bivariate correlation was tested by the means of Spearman’s rho test. Results of the statistical analysis were considered significant for p values <0.05. All calculations and plots were performed using the SPSS 13.0 statistical software package.
Results and Discussion
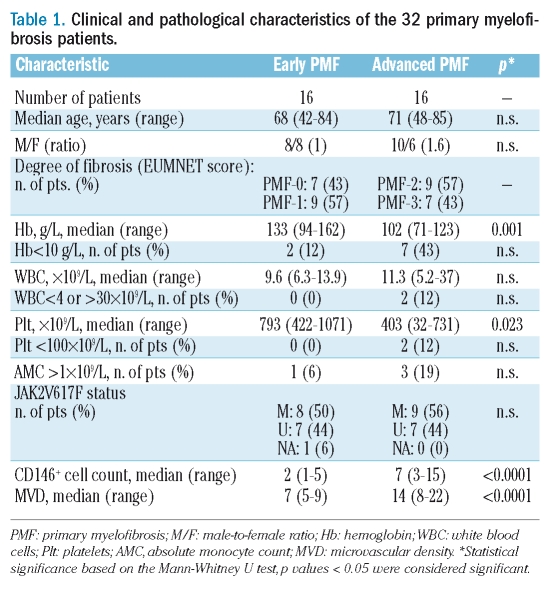
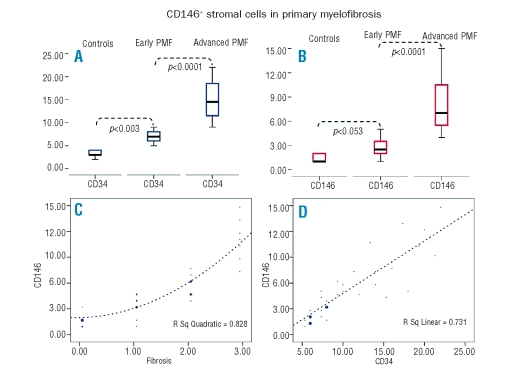
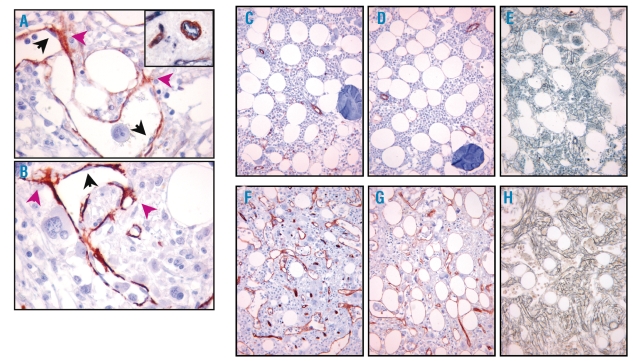
Clinical, histological, and immunophenotypical features of early- and advanced-PMF cases are summarized in Table 1. The assessment of CD34 immunostaining revealed a noticeable increase in the MVD in PMF cases as compared to control cases (p=0.003) and, consistently with previous observations, in PMF cases the MVD paralleled the increase in fiber content (Spearman’s ρ=0.880, p<0.001) (Figure 1A).3,4 CD146 proved to be expressed by reticular cells sited at the abluminal side of the BM vasculature extending dendritic processes among hematopoietic cells (Figure 2A). These cells with adventitial position and reticular morphology also expressed SMA, which confirmed their mural cell phenotype (Figure 2B). In control samples very few CD146+ cells were detected. No labeling for CD146 was detected in hematopoietic cells or in other BM stromal cell types (adipocytes, macrophages, osteoblasts and osteocytes, endosteal cells) in controls and PMF cases. In the early stages of PMF (grade 0/1 cases), CD146 expression was similar to that observed in control samples. A marked increase in CD146 expression was found in the advanced phases of PMF, with CD146+ subendothelial meshwork being more extended and denser than that of early phases (p<0.001) (Figure 1B, Figure 2C–H). Furthermore, CD146 proved to be significantly associated with the degree of fibrosis (Spearman’s rho=0.916, p<0.0001) (Figure 1C) as well as with the MVD (Spearman’s ρ=0.883, p<0.0001) (Figure 1D). However, the slight increase in the microvascular density which was observed in the early phases of the disease (grade 0/1) was not paralleled by a corresponding increase in CD146+ cells (Figure 2C–D). This finding further supports the evidence that the increase in microvascular density occurs as an early, though variably intense, phenomenon in the PMF pathogenesis,12 and suggests a prevalent contribution of CD146+ cells to the advanced stages of the disease.
Table 1.
Clinical and pathological characteristics of the 32 primary myelofibrosis patients.
Figure 1.
(A) Comparison between microvascular density in controls, early and advanced stage of primary myelofibrosis. Microvascular density shows a remarkable increase in primary myelofibrosis cases. (B) Comparison between CD146 expression in controls, early and advanced primary myelofibrosis cases. Advanced primary myelofibrosis cases display a noticeable increase in CD146 positive cells compared to early stage and controls, while the differences between early stage cases and controls are less remarkable. (C) The graph shows the significant association between the number of CD146 positive cells and the degree of fibrosis, graded according to the EUMNET consensus criteria. Larger dots represent a higher number of cases. (D) The graph shows a significant association between number of CD146 positive cells and the microvascular density in different phases of primary myelofibrosis. Larger dots represent a higher number of cases.
Figure 2.
A–B. Sections from a case of advanced (grade-2) primary myelofibrosis showing that subendothelial cells, branching into the surrounding hematopoietic parenchyma (violet arrows), stain positive either for CD146 (A) or smooth muscle actin (B), while endothelial cells are negative (black arrows) for both antibodies. Inset: the distinction between CD34 positive endothelial cells (blue) and CD146 positive adventitial cells (red) is highlighted by double immunostaining. (Strept-ABC, original magnification 400x).
C–H. Differences in microvascular density and in the number of CD146 positive cells betwen an early (C, D, E; grade-1 fibrosis) and an advanced primary myelofibrosis (F, G, H; grade-2 fibrosis). CD146 positive subendothelial cells form a wider and denser meshwork in the advanced stage (G) of primary myelofibrosis than in the early subendothelial stage (D). The slight increase in microvascular density observed in the early stage (C) is not associated with a remarkable increase in CD146 expression (D) (C, F: CD34 immunostaining; D, G: CD146 immunostaining; E, H: Gomori’s staining; original magnification 200×).
The strong association we found between the CD146 cell count and the degree of fibrosis prompted us to test for possible associations occurring between these two variables and the clinical data of the 32 PMF patients (listed in Table 1). Both the CD146 cell count and the degree of fibrosis proved to be significantly associated with a lower Hb value (ρ=− 0.745, p=0.001 and ρ= −0.798 p<0.0001, respectively) and PLT count (ρ= −0.630, p=0.009 and ρ= −0.657, p=0.006 respectively) on diagnosis. These data are in keeping with those from previous observations indicating that the progression of the BM stromal reaction is often matched by the decline of hematopoietic function.5,13
Interestingly, the CD146 cell count also correlated with the presence of risk factors on diagnosis (ρ=0.634, p=0.008), while the association of this latter with the extent of fibrosis did not reach statistical significance. No associations were found with the remaining presenting features, including the JAK2 mutational status. However, the analysis of the JAK2V617F allele burden, which was not performed in this study, could possibly lead to different results. Moreover, due to the descriptive design of this study and the small number of cases included, the results coming from such a clinicopathological analysis should be considered with caution.
Overall, our results suggest the involvement of CD146+ subendothelial reticular cells the stromal remodeling that occurs in the advanced stages of PMF. In this setting, CD146+ cells might play a role mainly through their recently reported function of clonogenic osteoprogenitor cells responsible for new bone formation6 and through the direct production and deposition of reticulin and collagen fibers. CD146+ cells might also support BM neoangiogenesis through the angiopoietin-1/Tie-2 pathway, complementary to that of VEGF which has been recently described in Ph- CMPDs.14
In conclusion, our data on the participation of CD146+ subendothelial osteoprogenitors in PMF stromal changes could represent a rationale for new studies focusing on these cells as targets for new therapies affecting the BM stroma. To this purpose, along with CD146, other recently identified BM-SSC markers like CD200, and CD73 could possibly be taken into consideration.15
Further studies are also needed to investigate the clonal nature of CD146+ BM cells in PMF and their possible prognostic relevance.
Acknowledgments
The authors would like to acknowledge Menarini Diagnostics, Italy for kindly providing the anti-CD146 monoclonal antibody.
Footnotes
Authorship and Disclosures
CT and ADB designed the study; EI and GF selected the cases and contributed to drawing up the manuscript; UG, EB, and AMF performed the histopathological evaluation of the cases and scored the degree of fibrosis; CG, RP, and SI performed the immunohistochemical reactions; ADB and MPT evaluated the immunoistochemical data and performed the statistical analysis; A.M. Florena revised the manuscript. All the authors approved the final version of the manuscript.
The authors reported no potential conflicts of interest.
References
- 1.Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22:14–22. doi: 10.1038/sj.leu.2404955. [DOI] [PubMed] [Google Scholar]
- 2.Thiele J, Kvasnicka HM. Hemato-pathologic findings in chronic idiopathic myelofibrosis. Semin Oncol. 2005;32:380–94. doi: 10.1053/j.seminoncol.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Mesa RA, Hanson Ca, Rajkumar V, Schroeder G, Tefferi A. Evaluation and clinical correlations of bone marrow angiogenesis in myelofibrosis with myeloid metaplasia. Blood. 2000;96:3374–80. [PubMed] [Google Scholar]
- 4.Boveri E, Passamonti F, Rumi E, Pietra D, Elena C, Arcaini L, et al. Bone marrow microvessel density in chronic myeloproliferative disorders: a study of 115 patients with clinicopathological and molecular corelations. Br J Haematol. 2007;140:162–8. doi: 10.1111/j.1365-2141.2007.06885.x. [DOI] [PubMed] [Google Scholar]
- 5.Vener C, Fracchiolla NS, Gianelli U, Calori R, Radaelli F, Iurlo A, et al. Prognostic implications of the European consensus for grading of bone marrow fibrosis in chronic idiopathic myelofibrosis. Blood. 2008;111:1862–5. doi: 10.1182/blood-2007-09-112953. [DOI] [PubMed] [Google Scholar]
- 6.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–36. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 7.Sorrentino A, Ferracin M, Castelli G, Biffoni M, Tomaselli G, Baiocchi M, et al. Isolation and characterization of CD146+ multipotent mesenchymal stromal cells. Exp Hematol. 2008;36:1035–46. doi: 10.1016/j.exphem.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Cattoretti G, Schiró R, Orazi A, Soligo D, Colombo MP. Bone marrow stroma in humans: anti-nerve growth factor receptor antibodies selectively stain reticular cells in vivo and in vitro. Blood. 1993;81:1726–38. [PubMed] [Google Scholar]
- 9.Jaffe ES, Harris NL, Stein H, Vardiman JW. World Health Classification of Tumors of Haematopoietic and Lymphoid Tissue. Lyon, France; IARC Press: 2001. [Google Scholar]
- 10.Tefferi A, Huang J, Schwager S, Li CY, Wu W, Pardanani A, et al. Validation and comparison of contemporary prognostic models in primary myelofibrosis:analysis based on 334 patients from a single institution. Cancer. 2007;109:2083–88. doi: 10.1002/cncr.22630. [DOI] [PubMed] [Google Scholar]
- 11.Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica. 2005;99:1128–32. [PubMed] [Google Scholar]
- 12.Streuer M, Zoller H, Augustin F, Fong D, Heiss S, Strasser-Weippl K, et al. Increased angiogenesis in chronic idiopathic myelofibrosis: vascular endothelial growth factor as a prominent angiogenic factor. Hum Pathol. 2007;38:1057–64. doi: 10.1016/j.humpath.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Thiele J, Kvasnicka HM. Grade of bone marrow fibrosis is associated with relevant hematological findings – a clinicopathological study on 865 patients with chronic idiopathic myelofibrosis. Ann Hematol. 2006;85:226–32. doi: 10.1007/s00277-005-0042-8. [DOI] [PubMed] [Google Scholar]
- 14.Gianelli U, Vener C, Raviele PR, Savi F, Somalvico F, Calori R, et al. VEGF expression correlates with microvessel density in Philadelphia chromosome-negative chronic myeloproliferative disorders. Am J Clin Pathol. 2007;128:966–73. doi: 10.1309/FP0N3LC8MBJUFFA6. [DOI] [PubMed] [Google Scholar]
- 15.Delorme B, Ringe J, Gallay N, Le Vern Y, Kerboeuf D, Jorgensen C, et al. Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells. Blood. 2008;111:2631–5. doi: 10.1182/blood-2007-07-099622. [DOI] [PubMed] [Google Scholar]