In recent years, chemoimmunotherapies that combine cytotoxic agents and monoclonal antibodies have been studied intensively for the treatment of B-cell chronic lymphocytic leukemia (CLL). Of particular interest are fludarabine-based combination regimens such as FluCam (fludarabine and alemtuzumab), FCR (fludarabine, cyclophosphamide, and rituximab), and FCCam (fludara-bine, cyclophosphamide, and alemtuzumab).1–4 Alemtuzumab (Campath), a humanized anti-CD52 monoclonal antibody, is currently approved in the United States as first-line, single-agent treatment of CLL and in the European Union as first-line treatment of CLL when fludarabine combination chemotherapy is not appropriate. When administered at the standard dosing schedule [30 mg intravenously (IV) 3 times a week (TIW) for up to 12 weeks], alemtuzumab demonstrated an overall response rate (ORR) of 33–50% in fludarabine-refractory patients [complete response (CR) rate, 0–4%]5–7 and 83–87% (CR rate, 19–24%) in previously untreated patients.8,9 At this time, only limited data are available on the pharmacokinetics (PK) of alemtuzumab, and the approved dosing schedule of alemtuzumab monotherapy was developed empirically in the absence of detailed PK studies.
Recently, our group reported on the results of a phase II study that evaluated concomitant use of IV fludarabine and alemtuzumab administered with a novel schedule (FluCam regimen) in patients with relapsed/refractory CLL.1 We conducted the present study to investigate the PK of alemtuzumab in patients who received the FluCam regimen. Fourteen patients with relapsed/refractory CLL were enrolled, all of whom gave written informed consent prior to study entry in accordance with the Declaration of Helsinki. Patient eligibility criteria have been previously described.1 The study protocol was approved by the institutional review board. Following alemtuzumab dose escalation (3 to 10 to 30 mg over three days), fludarabine 30 mg/m2 followed by alemtuzumab 30 mg was given IV on days 1–3 of a 28-day cycle for up to 6 cycles. Patients were managed as previously described.1 In 5 patients, treatment cycles were extended to up to 42 days because of critical neutropenia. Response was assessed on the first day of cycle 4 and 1–3 months after the last cycle of therapy according to the 1996 National Cancer Institute Working Group criteria. Bone marrow aspiration and biopsy were performed two months after demonstration of CR by clinical and laboratory assessments. Patient serum samples were collected on day 0 (the day before start of cycle) and on days 1 (before drug administration), 4, 7, 14, 21, and 28 (and days 35 and 42 in patients with extended cycles). Samples were stored at −70°C until analysis. Plasma concentrations of alemtuzumab were determined using the previously described method.10 Mean plasma concentration on a defined day (e.g., day 1) of a treatment cycle was calculated as the mean value across all treatment cycles. Correlation coefficient was calculated using Spearman’s rank method. Patient serum samples were not assessed for antiglobulin response.
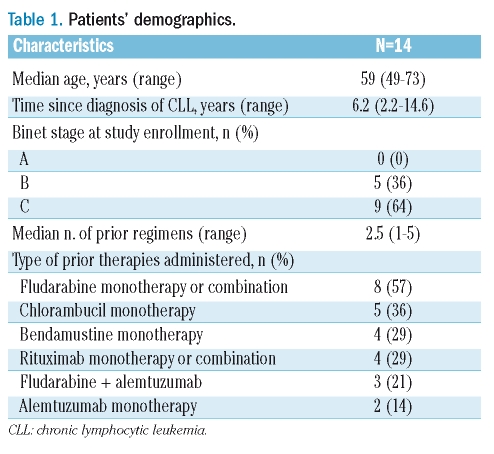
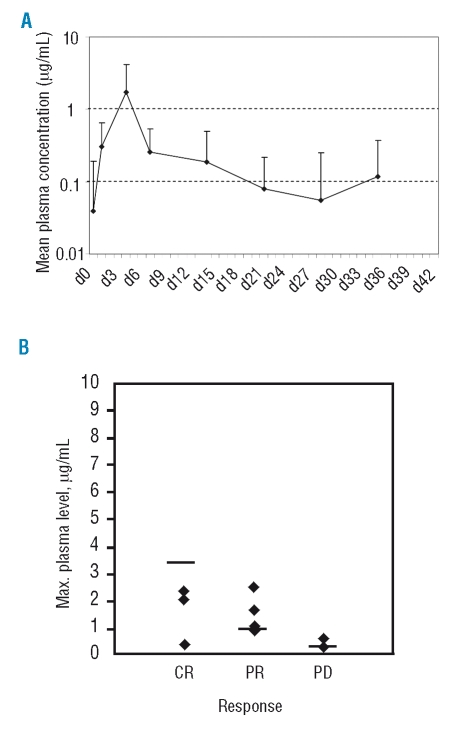
Patients’ demographics are shown in Table 1. Half of the patients received 3 or more prior lines of therapy. All patients had extensive bone marrow infiltration at baseline, and the majority had lymphadenopathy and/or splenomegaly. Patients received a median of 4 cycles of FluCam (range, 1–6). There were 5 patients with CR, 7 patients with partial response (PR), and 2 patients with progressive disease (PD) (ORR 86% and CR rate 36%). Three patients had received 4 cycles of FluCam as their last treatment prior to study enrollment, and in this study they received fludarabine 25 mg/m2, cyclophosphamide 200 mg/m2, and alemtuzumab 30 mg on the same schedule, with 1 CR and 2 PR achieved. For PK analysis, a total of 158 patient serum samples were collected, of which 120 were tested. Within a treatment cycle, the mean plasma concentration of alemtuzumab increased from days 1 to 4, reaching 1.97 μg/mL on day 4, then decreased to 0.28 μg/mL on day 7. By day 21, plasma concentration of alemtuzumab decreased to undetectable levels (Figure 1A). There was a trend toward a higher plasma alemtuzumab level in patients with a better response [correlation coefficient r=0.527, p=0.078 (two-sided)], although it did not reach a statistically significant level, probably because of the small patient cohort. The highest plasma level achieved in a given patient in all treatment cycles averaged 3.35 μg/mL in patients with CR, 0.98 μg/mL in patients with PR, and 0.32 μg/mL in patients with PD (Figure 1B). We did not observe a clear correlation between initial white blood cell count and plasma alemtuzumab level (data not shown). No significant correlation was observed between plasma alemtuzumab level and CD4+ T-cell level or incidence of infection (data not shown).
Table 1.
Patients’ demographics.
Figure 1.
( A ) Mean plasma concentration of alemtuzumab in patients receiving the FluCam regimen. Data are plotted in log-scale. Error bars indicate standard deviation. (B) Relationship between maximal plasma level of alemtuzumab and clinical response. Plasma concentration data are available for 4 patients with complete response (CR), 6 patients with partial response (PR), and 2 patients with progressive disease (PD). Horizontal bars denote the mean values of individual patient groups.
At present, PK data on alemtuzumab are limited. PK of alemtuzumab was characterized by a two-compartment model with nonlinear elimination. Interpatient variability was large, probably reflecting differences in tumor burden and disease activity among patients.11 Positive correlations between plasma alemtuzumab level and clinical response have been demonstrated by Hale et al. and Montillo et al.10,12 Our data further confirm such positive correlations. Based on in vitro results, a concentration of 1.0 μg/mL has been previously suggested as the minimum alemtuzumab concentration required for lympholytic activity.10 However, no in vivo data are available to define the plasma alemtuzumab concentration required for clinical activity. Considering the high response rate achieved with the FluCam regimen in this study, we propose that a plasma alemtuzumab level lower than 1.0 mg/mL can still be clinically effective in combination therapy, possibly because of potential synergy between fludarabine and alemtuzumab.
Based on the positive results of the phase II FluCam trial, a large, international phase III trial (CAM314) comparing the FluCam regimen with fludarabine alone has been initiated. Because plasma drug level positively correlates with response to alemtuzumab-containing therapy, future clinical studies of alemtuzumab-containing therapy should ideally include measurement of plasma drug concentration to evaluate the feasibility of PK-directed dosing, with the aim of improving patient response and minimizing toxicity.
Acknowledgments
we thank Wei Jiang, PhD, for assistance in preparing and editing this manuscript.
Footnotes
Funding: This work was supported in part by research funding from Bayer Schering AG. Thomas Elter received grant support from Bayer Schering AG for probe analysis. Julia Kilp, Peter Borchmann, Holger Schulz, Michael Hallek, and Andreas Engert have no conflicts of interest to disclose.
References
- 1.Elter T, Borchmann P, Schulz H, Reiser M, Trelle S, Schnell R, et al. Fludarabine in combination with alemtuzumab is effective and feasible in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: results of a phase II trial. J Clin Oncol. 2005;23:7024–31. doi: 10.1200/JCO.2005.01.9950. [DOI] [PubMed] [Google Scholar]
- 2.Elter T, James R, Wendtner CM, Stilgenbauer S, Winkler D, Ritgen M, et al. Treatment of patients with relapsed/refractory CLL using a combination of fludarabine, cyclophosphamide and alemtuzumab: first safety analysis of the CLL2L trial of the German CLL Study Group. J Clin Oncol. 2008;26 (Suppl):385s. [Abstract 7053] [Google Scholar]
- 3.Keating MJ, O’Brien S, Albitar M, Lerner S, Plunkett W, Giles F, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–88. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 4.Wierda W, O’Brien S, Wen S, Faderl S, Garcia-Manero G, Thomas D, et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4070–8. doi: 10.1200/JCO.2005.12.516. [DOI] [PubMed] [Google Scholar]
- 5.Keating MJ, Flinn I, Jain V, Binet JL, Hillmen P, Byrd J, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99:3554–61. doi: 10.1182/blood.v99.10.3554. [DOI] [PubMed] [Google Scholar]
- 6.Osterborg A, Dyer MJ, Bunjes D, Pangalis GA, Bastion Y, Catovsky D, et al. Phase II multicenter study of human CD52 antibody in previously treated chronic lymphocytic leukemia. European Study Group of CAMPATH-1H Treatment in Chronic Lymphocytic Leukemia. J Clin Oncol. 1997;15:1567–74. doi: 10.1200/JCO.1997.15.4.1567. [DOI] [PubMed] [Google Scholar]
- 7.Rai KR, Freter CE, Mercier RJ, Cooper MR, Mitchell BS, Stadtmauer EA, et al. Alemtuzumab in previously treated chronic lymphocytic leukemia patients who also had received fludarabine. J Clin Oncol. 2002;20:3891–7. doi: 10.1200/JCO.2002.06.119. [DOI] [PubMed] [Google Scholar]
- 8.Hillmen P, Skotnicki AB, Robak T, Jaksic B, Dmoszynska A, Wu J, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol. 2007;25:5616–23. doi: 10.1200/JCO.2007.12.9098. [DOI] [PubMed] [Google Scholar]
- 9.Lundin J, Kimby E, Bjorkholm M, Broliden PA, Celsing F, Hjalmar V, et al. Phase II trial of subcutaneous anti-CD52 monoclonal antibody alemtuzumab (Campath-1H) as first-line treatment for patients with B-cell chronic lymphocytic leukemia (B-CLL) Blood. 2002;100:768–73. doi: 10.1182/blood-2002-01-0159. [DOI] [PubMed] [Google Scholar]
- 10.Hale G, Rebello P, Brettman LR, Fegan C, Kennedy B, Kimby E, et al. Blood concentrations of alemtuzumab and antiglobulin responses in patients with chronic lymphocytic leukemia following intravenous or subcutaneous routes of administration. Blood. 2004;104:948–55. doi: 10.1182/blood-2004-02-0593. [DOI] [PubMed] [Google Scholar]
- 11.Mould DR, Baumann A, Kuhlmann J, Keating MJ, Weitman S, Hillmen P, et al. Population pharmacokinetics-pharmacodynamics of alemtuzumab (Campath) in patients with chronic lymphocytic leukaemia and its link to treatment response. Br J Clin Pharmacol. 2007;64:278–91. doi: 10.1111/j.1365-2125.2007.02914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montillo M, Tedeschi A, Miqueleiz S, Veronese S, Cairoli R, Intropido L, et al. Alemtuzumab as consolidation after a response to fludarabine is effective in purging residual disease in patients with chronic lymphocytic leukemia. J Clin Oncol. 2006;24:2337–42. doi: 10.1200/JCO.2005.04.6037. [DOI] [PubMed] [Google Scholar]