Mild anemia is a frequent laboratory finding in the elderly usually disregarded in clinical practice. This study shows that mild anemia is associated with increased risk of hospitalization and all-cause mortality in the elderly. See perspective article on page 1.
Keywords: mild anemia, elderly, mortality, hospitalization, hemoglobin
Abstract
Background
Mild anemia is a frequent laboratory finding in the elderly usually disregarded in everyday practice as an innocent bystander. The aim of the present population-based study was to prospectively investigate the association of mild grade anemia with hospitalization and mortality.
Design and Methods
A prospective population-based study of all 65 to 84 year old residents in Biella, Italy was performed between 2003 and 2007. Data from a total of 7,536 elderly with blood tests were available to estimate mortality; full health information available to evaluate health-related outcomes was available for 4,501 of these elderly subjects. Mild grade anemia was defined as a hemoglobin concentration between 10.0 and 11.9 g/dL in women and between 10.0 and 12.9 g/dL in men.
Results
The risk of hospitalization in the 3 years following recruitment was higher among the mildly anemic elderly subjects than among subjects who were not anemic (adjusted hazard ratio: 1.32; 95% confidence interval: 1.09–1.60). Mortality risk in the following 3.5 years was also higher among the mildly anemic elderly (adjusted hazard ratio: 1.86; 95% confidence interval: 1.34–2.53). Similar results were found when slightly elevating the lower limit of normal hemoglobin concentration to 12.2 g/dL in women and to 13.2 g/dL in men. The risk of mortality was significantly increased in mild anemia of chronic disease but not in that due to β-thalassemia minor.
Conclusions
After controlling for many potential confounders, mild grade anemia was found to be prospectively associated with clinically relevant outcomes such as increased risk of hospitalization and all-cause mortality. Whether raising hemoglobin concentrations can reduce the risks associated with mild anemia should be tested in controlled clinical trials.
Introduction
The prevalence and incidence of anemia tend to increase with advancing age (the Health and Anemia study, unpublished data and refs. 1 and 2). It has been estimated that almost three million elderly people are currently affected by anemia in the United States.3 Against a background of rapid and enduring population aging, the number of elderly anemic subjects in western countries will rise steeply in the near future. Relatively low hemoglobin concentrations are a common laboratory finding in the elderly, for the most part judged by physicians as a sign without clinical relevance or as a marker of an underlying chronic disease having no independent influence on health. Though data are still limited, in recent years a few studies have started to challenge the widespread and self-perpetuating perception of anemia as an innocent bystander, reporting increased disability, morbidity, and mortality in the anemic elderly (for an overview see references).4–8 As a result, anemia can have a relevant effect on healthcare requirements and substantially increase healthcare costs.9,10
The association between anemia and clinical outcomes is likely to be influenced by the poor prognosis of elderly with moderate to severe anemia. Recent studies have investigated the risks associated with specific intervals of hemoglobin concentrations.11–16 We have recently reported that mild grade anemia was cross-sectionally associated with worse selective attention and disease-specific quality of life measures.17
The primary objective of the present population-based study was to prospectively assess the association of mild anemia with clinically relevant outcomes such as hospitalization and all-cause mortality in the elderly. The secondary aim was to investigate the association of different types of mild anemia with mortality.
Design and Methods
Study population
Salute e Anemia (Health and Anemia) is a longitudinal population-based observational study of all 65- to 84-year old residents in the municipality of Biella, Piedmont, a town in the north-west of Italy. All registered individuals 65- to 84-year old residing in Biella on the prevalence day (May 12, 2003) were eligible for the study (n= 10,110). Age and residence were the only inclusion/exclusion criteria used. Case ascertainment was conducted between May 2003 and April 2004.
In consenting participants, arterial blood pressure (third reading), heart and respiratory rates, weight, and height were measured and blood samples were taken by trained, registered nurses at home (50%) or in an outpatient laboratory (49%) at the elderly person’s choice or, for institutionalized individuals, in nursing homes (1%). A questionnaire was also administered by the nurses in order to ascertain habits, present and past diseases, and hospital admissions and interventions in the preceding 5 years. An index of co-morbid disease severity was developed for the purposes of the study: based on the medical information collected by the nurses, co-morbid disease severity was graded by two physicians using a cumulative score with a 5-point scale. The definitions of rating points (from 1, no impairment, to 5, extremely severe) are very similar to those of the Cumulative Illness Rating Scale.18
In the sample of the present study population included in another part of the Health and Anemia Study devoted to investigating the effects of mild anemia on cognitive, mood, functional and quality of life variables, trained psychologists also collected, among other information, data on medical history.17 The information collected by the psychologists was blinded to that previously gathered by the nurses at the time of blood sampling and the two interviews were used here to control for the consistency of the medical histories reported by the participants. Agreement between comparable items of the medical histories taken by the nurses and by the psychologists was very high: Cohen’s κ was between 0.84 and 0.93. To control for the potential influence of a non-response bias, at the end of case ascertainment, a search was performed in the database of the Local Health Authority for hemoglobin tests done by non-participants (i.e. individuals not found, not in a condition to donate a blood sample, refusing to participate, or who died after the prevalence day but before contact) during the same period and in the same Biella Hospital Laboratory from which the data for the present study were collected. When more than one result was present, the most recent was considered. The output was an anonymous list of four data: sex (male/female), age (on test day), hemoglobin concentration, and number of days elapsed from the date of the blood test to death or end of follow-up. The study procedures were conducted in accordance with the principles outlined in the Declaration of Helsinki of 1964 and following amendments. The local research Ethics Committee of the Hospital Health Authority of Novara approved the study. Written informed consent was obtained from each participant prior to blood sampling.
Definitions of anemia and mild grade anemia
Anemia was defined by the World Health Organization (WHO) criteria as a hemoglobin concentration lower than 12 g/dL in women and 13 g/dL in men.19 In accordance with most grading classification systems,20,21 mild grade anemia was defined as a hemoglobin concentration between 10.0 and 11.9 g/dL in women and between 10.0 and 12.9 g/dL in men.
Laboratory methods
Venous blood samples were collected from participants in a sitting position by venipuncture. The complete blood count (CBC) was determined using a SIS-MEX SE-2100 electronic counter (Sysmex Corporation Kobe, Japan) in the Laboratory of Biella Hospital. Whenever the hemoglobin concentration was below WHO reference criteria for anemia, further laboratory investigations of serum iron, transferrin, ferritin, folic acid and vitamin B12 were performed.
Statistical analyses
The follow-up period in which death from any cause could be ascertained (data from the Registry Office and the Local Health Authority) ranged from 1 day to 3.5 years after blood collection. When the cumulative curve of number of deaths was visually inspected, two different slopes could be seen: from day 0 to approximately 2 months and from this time point to 3.5 years later. Since anonymous data on CBC were also collected in hospitalized individuals (n = 391), the first steeper slope was very likely due to the death of seriously ill patients. To avoid this unwanted effect, survival time was calculated for the interval between 60 days and 3.5 years after blood collection. Kaplan-Meier curves were constructed for mildly anemic and non-anemic men and women, and differences in survival were tested using log-rank tests. Age- and sex-corrected hazard ratios (HR) of death in the mildly anemic group relative to that in the non-anemic group were calculated using Cox proportional hazard regression models. When the analysis was restricted to participants, HR could be further adjusted for the potential confounding effect of education, smoking history, body mass index (BMI), hospital admission, and co-morbid diseases (diabetes, hypertension, myocardial infarction, heart failure, respiratory failure, renal failure, neurological diseases, and cancer) or co-morbid disease severity. Age, education, BMI, and co-morbid disease severity were processed as continuous variables. Following the observations of Borgna-Pignatti et al.22 and Kristal-Boneh et al.23 of lower concentrations of hemoglobin during the summer season (June, July, and August), we also added the variable being sampled in summer to the fully adjusted models to control for the potential confounding effect of seasonal variation of hemoglobin values. To examine whether the effect of mild anemia was similar over time, two survival analyses were set up: the first from 60 days after blood collection to 2 years, and the second from 2 to 3.5 years.
Subgroup analyses were performed to explore the potential risks of mortality associated with different types of anemia.
Kaplan-Meier curves and Cox models were also used to study the association of mild anemia with time to first hospitalization in the 3 years following blood sampling (data from the County Registry of the Local Health Authority).
Proportionality assumption of the models was checked inspecting the log(-log(survival)) versus log(survival time) graphs: no departure from parallelism was found.
To investigate whether WHO criteria may have affected the estimated effect of mild anemia on outcomes, we next used recently proposed lower limits of normal hemoglobin concentration to define anemia in white adults (lower than 12.2 g/dL in women and lower than 13.2 g/dL in men),24 which are slightly higher than those of WHO criteria and then re-evaluated the association of mild anemia with hospitalization and mortality. Following Beutler and Waalen’s methodology,24 we also tried to determine in the present study population the lower limit of normal hemoglobin concentration, calculated as the actual 5th percentile in the data set. All p values were two tailed. Data were analyzed using JMP v. 6.0.3 and SAS software, version 8.2 (both SAS Institute Inc., Cary, NC, USA).
Results
Study population
Of the 10,110 residents in Biella (6,146 women and 3,964 men) who were 65 to 84 years old on the prevalence day, 1,131 could not be reached by phone, 80 died before being contacted, 4,398 refused to or could not donate a blood sample, and 4,501 agreed to participate. Participants (mean age 73.6 years, standard deviation [SD] = 5.2) were on average approximately 1 year younger than non-participants (mean age 74.8 years, SD = 5.5). Among non-participants, 3,035 individuals had had a CBC done in the same period and hospital laboratory of the current survey. Thus 7,536 (4,501 + 3,035) individuals with a CBC could be included in the longitudinal study of mortality. The mean age of the remaining 2,574 elderly without a hemoglobin test available (73.9 years) was comparable to that of the 4,501 participants, the 7,536 individuals with a CBC available, and the entire population (mean ages between 73.6 and 74.3 years). The proportion of women was similar in the total population and all the above groups (between 60.2 and 61.6%).
Among the 4,501 participants 31 had moderate-severe anemia, thus the total number of mildly anemic (n=313) and non-anemic (n=4,157) participants included in the analyses was 4,470. Among the 7,536 elderly with a CBC available, 130 had moderate-severe anemia, thus the total number of mildly anemic (n=716) and non-anemic (n = 6,690) elderly with a CBC available included in the analyses was 7,406. The lower limits of normal hemoglobin concentration in the present study population were 12.0 g/dL in women and 12.8 g/dL in men.
Risk of hospitalization in the 4,470 participants
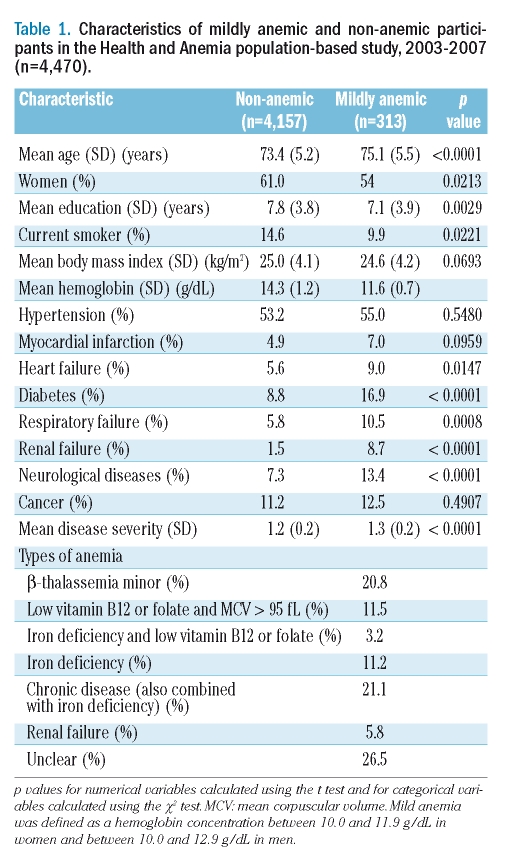
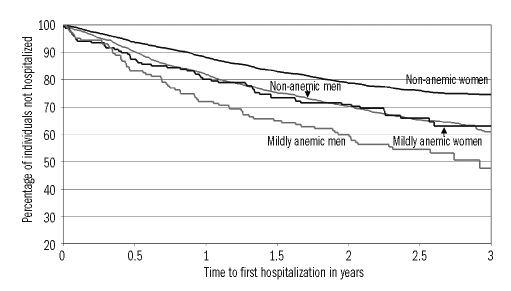
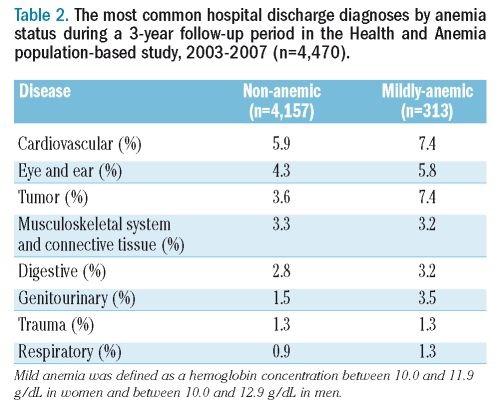
Mild anemia was cross-sectionally associated with a higher prevalence of several pathological conditions (Table 1). During a 3-year follow-up period, 123 out of 313 mildly anemic (39.3%) and 1,173 out of 4,157 non-anemic (28.2%) participants were hospitalized. Figure 1 shows Kaplan-Meier curves by mild anemic status and sex: unadjusted hospitalization risk was significantly higher both in mildly anemic women (p=0.0013) and men (p=0.0019). The risk of being hospitalized in the 3 years following blood sampling was significantly higher in the mildly anemic than in non-anemic participants (age- and sex-adjusted HR: 1.44; 95% CI: 1.19–1.73), also when adjusted for education, smoking history, BMI, co-morbid diseases or co-morbid disease severity (both adjusted HR: 1.32; 95% CI: 1.09–1.60). An identical HR was obtained also when the summer sampling variable was added to the model. When mild anemia was defined as a hemoglobin concentration between 10.0 and 12.1 g/dL in women and between 10.0 and 13.1 g/dL in men, results were very similar (the lowest adjusted HR: 1.29; 95% CI: 1.09–1.53). Using the lower limits of normal hemoglobin concentration found in the present study population, the fully adjusted HR was 1.45 (95% CI: 1.19–1.76). Table 2 shows the most common hospital discharge diagnoses by anemia status.
Table 1.
Characteristics of mildly anemic and non-anemic participants in the Health and Anemia population-based study, 2003–2007 (n=4,470).
Figure 1.
Time to first hospitalization by sex and mild anemia status in the Health and Anemia population-based study (2003–2007). Kaplan-Meier curves for individuals aged 65–84 years (n = 4,470) over 3 years after blood sampling. Mild anemia was defined as a hemoglobin concentration between 10.0 and 11.9 g/dL in women and between 10.0 and 12.9 g/dL in men.
Table 2.
The most common hospital discharge diagnoses by anemia status during a 3-year follow-up period in the Health and Anemia population-based study, 2003–2007 (n=4,470).
Risk of mortality
Mortality risk in the 7,536 elderly with CBC available
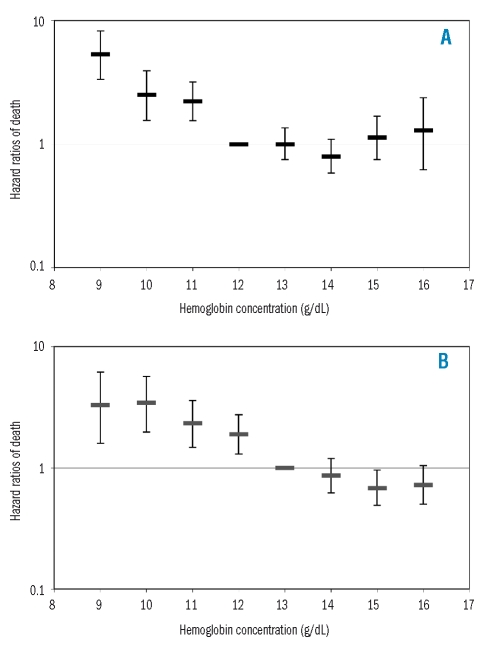
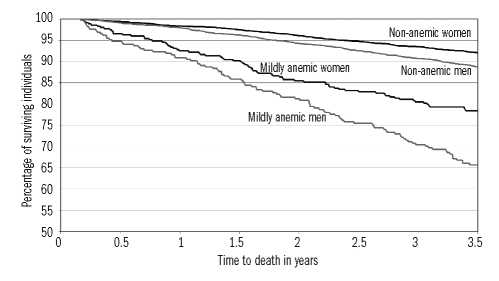
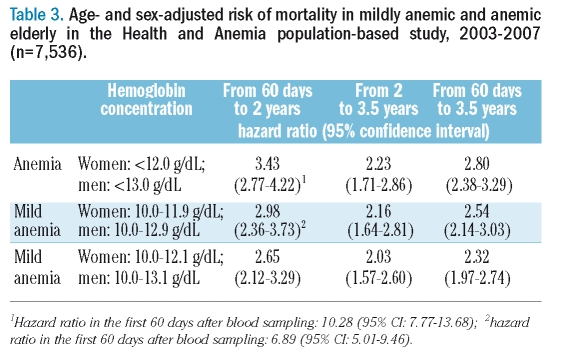
Over a 3.5-year follow-up starting from 60 days after blood sampling, 36 moderately to severely anemic (45.0%), 170 mildly anemic (26.6%), and 598 non-anemic (9.1%) individuals died. Figure 2A and B show the age-adjusted HR of death in women and men by categorical hemoglobin concentrations (both p<0.0001). Risks of mortality were not significantly different among the hemoglobin classes above the WHO criteria for anemia in either women (p=0.2902) or men (p=0.1239). Figure 3 shows Kaplan-Meier curves by mild anemic status and sex over this 3.5-year period: the unadjusted mortality risk was significantly higher (p<0.0001) in both mildly anemic women and men than in their non-anemic counterparts. The age- and sex-adjusted HR for mortality were significantly higher among mildly anemic versus non-anemic individuals using either the WHO criteria or newly proposed lower limits of normal hemoglobin concentration and, as expected, the HR increased also when the moderately to severely anemic elderly were included in the analysis (Table 3).
Figure 2.
(A) Age-adjusted hazard ratios of death (95% CI) by hemoglobin concentration in women aged 65–84 years (n = 4,561) from 60 days to 3.5 years after blood sampling in the Health and Anemia population-based study (2003–2007). (B) Age-adjusted hazard ratios of death (95% CI) by hemoglobin concentration in men aged 65–84 years (n = 2,975) from 60 days to 3.5 years after blood sampling in the Health and Anemia population-based study (2003–2007).
Figure 3.
Time to death by sex and mild anemia status in the Health and Anemia population-based study (2003–2007). Kaplan-Meier curves for individuals aged 65–84 years (n = 7,406) from 60 days to 3.5 years after blood sampling. Mild anemia was defined as a hemoglobin concentration between 10.0 and 11.9 g/dL in women and between 10.0 and 12.9 g/dL in men.
Table 3.
Age- and sex-adjusted risk of mortality in mildly anemic and anemic elderly in the Health and Anemia population-based study, 2003–2007 (n=7,536).
Mortality risk in the 4,470 participants
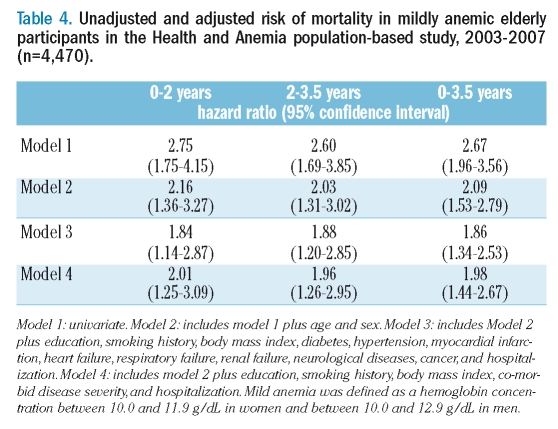
When the analysis was restricted to the 4,470 mildly anemic and non-anemic participants, the age- and sex-adjusted HR for mortality could be further adjusted for the possible confounding effect of education, smoking history, BMI, hospital admission, co-morbid diseases or co-morbid disease severity. Over the 3.5 year follow-up 52 mildly anemic (16.6%), and 274 non-anemic (6.6%) individuals died. As shown in Table 4, the mortality risks were significantly higher in the group with mild anemia, with similar HR in the first 2 years and in the following 1.5 years. An almost identical HR was also obtained when the summer sampling variable was added to model 4. Comparable results were found if mild anemia was defined as a hemoglobin concentration between 10.0 and 12.1 g/dL in women and between 10.0 and 13.1 g/dL in men (adjusted HR over 3.5 years: 1.65; 95% CI: 1.23–2.21). Using the lower limits of normal hemoglobin concentration found in the present study population, the fully adjusted HR was 2.06 (95% CI: 1.48–2.80).
Table 4.
Unadjusted and adjusted risk of mortality in mildly anemic elderly participants in the Health and Anemia population-based study, 2003–2007 (n=4,470).
We performed a subgroup analysis by anemia type to further investigate the association between mild anemia and mortality over 3.5 years. Except for β-thalassemia minor which was not associated with an increased risk of mortality (fully adjusted HR: 0.54; 95% CI: 0.09–1.69), results in the other types of anemia tended, to various degrees, to confirm the findings in the overall population of mildly anemic subjects. However, the number of cases or deaths was too small to obtain reliable results in iron deficiency, renal failure, and folate or vitamin B12 deficiency anemias, while the risk of mortality was significantly increased in subjects with mild anemia of chronic disease (fully adjusted HR: 5.44; 95% CI: 3.53–8.06). When the elderly with β-thalassemia minor were excluded from the analyses, the association between mild anemia and mortality over 3.5 years was slightly more pronounced (fully adjusted HR: 2.18; 95% CI: 1.56–2.99).
Discussion
This is a prospective population-based study specifically aimed at thoroughly investigating the impact of mild grade anemia in the elderly. Blood samples were collected for the purposes of the study from an unselected population. Risks of mortality and hospitalization were significantly higher among mildly anemic elderly subjects compared with non-anemic ones, also when adjusted for a large number of potential confounders. Mortality hazard ratios over the first 2 and the following 1.5 years were significantly increased and very similar. The risk of mortality was found to be associated with mild anemia of chronic disease but not with the anemia of β-thalassemia minor.
A few retrospective1,11 and prospective12,15 studies have reported an increased risk of hospitalization among the elderly with all grades of anemia and, in the Established Populations for Epidemiologic Studies of the Elderly (EPESE) study,12 there was a significant trend towards an increased risk of hospitalization with decreasing categorical hemoglobin concentrations. In the current study we show a higher rate of hospitalization in the 3 years following blood sampling, independently of many potential confounders, also among mildly anemic elderly subjects.
A possible excess mortality in elderly women with a low hematocrit had already been observed a few decades ago.25 Recent community-based studies have further investigated this association.11–14,16,26,27 In the EPESE and Leiden 85-plus studies an increased risk of mortality over 4 and 10 years, respectively, was associated with decreasing categorical hemoglobin concentrations.12,13 In the Duke EPESE study a significantly higher risk of mortality 8 years after blood collection was found in elderly women but not in elderly men with mild anemia at baseline.11 In the Women’s Health and Aging Study I, disabled older women with mildly low concentrations of hemoglobin had a greater mortality risk over 5 years.14 In the Health, Aging, and Body Composition Study an increased mortality risk over 6 years was found in well-functioning older white men and women with hemoglobin concentrations lower than 12.0 g/dL, but not in black women or men, or in white men with hemoglobin concentrations between 12.0 and 12.9 g/dL.16 In the current study we found evidence of an independent effect of a mild grade anemia on survival, such that the risk of dying over 2 and 3.5 years among subjects with mild anemia was almost twice that among non-anemic peers. Estimates of mortality risk over longer periods of time are already planned; however, when the classification of a person into the anemic or non-anemic group is based on just one measurement of hemoglobin concentration over time, as is consistently done, the longer the follow-up, the higher the probability of an intervening change in the anemic/non-anemic status of a person.
The definition of anemia suggested by the WHO has been repeatedly questioned and attempts have been made to propose new lower limits of normal of hemoglobin concentration according to the individual’s ethnic origin, sex, and age.24 It is beyond the scope of the present study to discuss the predictive validity of the various cut-offs of hemoglobin concentration suggested to define anemia in the elderly, but it is interesting to note that in our study when anemia was defined by hemoglobin concentrations just slightly higher (lower than 12.2 g/dL in women and lower than 13.2 in men)24 than those of the WHO criteria, the risk of hospitalization and mortality remained significantly higher in the mildly anemic group. Thus, the somewhat arbitrarily chosen lower limits of normal hemoglobin concentration used did not bias the estimated effect of mild anemia.
Consistent with the results of Penninx et al.12 we did not find a significant association between highest hemoglobin concentrations and increased risk of hospitalization. At variance with Elwood et al. (in a study of women with a hematocrit above 45%),25 Zakai et al.,26 Denny et al. (in women),11 and Culleton et al. (in women seeking medical care),15 and in agreement with Gagnon et al. (in elderly women and men with hematocrit of 46% to 65% and 49% to 70%, respectively),28 Izaks et al. (individuals aged 85 years and older),13 Penninx et al.,12 Denny et al. (in men),11 Chaves et al. (in disabled women),14 Culleton et al. (in men seeking medical care),15 and Patel et al. (in well functioning adults aged 70 to 79 years)16 we did not find a significantly increased risk of all-cause mortality among elderly with the highest hemoglobin concentrations. Inspection of Figure 2A might suggest a possible trend towards an increased risk of mortality in women with high-normal hemoglobin concentrations, but larger samples are needed to reach any firm conclusion.
Except for a study of anemia in moderately-to-severely disabled older women,29 no population-based study that we know of has previously investigated the association of types of mild anemia with the risk of mortality. In the present study, at variance with the overall results seen in the entire group with mild anemia, individuals with β-thalassemia minor did not have a shorter survival than non-anemic elderly subjects.
The specific mechanisms by which anemia would adversely affect relevant health-related outcomes in the elderly are unknown. However, elderly individuals with an inherited, generally asymptomatic, lifelong condition such as β-thalassemia minor were not at increased risk of mortality, while those with an acquired disorder such as mild anemia of chronic disease, showed a five-fold increase in mortality risk. This seems to suggest that, when developed later in life, mild anemia could significantly contribute to health vulnerability and poor clinical outcomes in the elderly.
Some potential limitations of this study deserve discussion. In general, findings in a specific geographical area, also being influenced by the homogeneous characteristics of the particular population under study, might not necessarily be replicated in other population-based surveys. Information on health conditions of participants was mainly based on self-reports. Previous studies have, however, shown that taking medical histories in elderly individuals participating in population-based surveys provides an accurate and complete clinical picture,30 with no age differences in response accuracy for health-related questions.31 Even mildly demented subjects were found to be no less likely than cognitively unimpaired elderly to report disorders of recent onset,32 and in the current study population only 0.8% of the participants showed a cognitive impairment. Furthermore, the reliability of the interview was very high and imprecise reporting would apply to both the mildly anemic and non-anemic groups. Although the possible influence of a non-response bias on the associations studied cannot be excluded, this seems rather improbable considering the condition examined (an anemia of mild grade of which most of the elderly were unaware) together with the nature of the outcomes studied. In any case, when more than half of the self-selected non-participants were added to the participants, the risk of mortality associated with mild anemia did not vary greatly; indeed sex- and age-adjusted hazard ratios increased from 2.09 to 2.54. Even though results were adjusted for quite a number of potential demographic and clinical confounders, others may not have been identified. Conversely, the large number of confounders entered in the multivariable analyses may have led to overadjustment and consequent underestimation of the strength of the associations. A further concern regards the intrinsic impossibility of ascertaining a causal relation in observational studies: association does not imply causation. However striking and suggestive the results, given the observational nature of the study it cannot be inferred from the findings that restoring normal hemoglobin concentrations would necessarily reduce the observed risks associated with mild grade anemia.
Mild anemia is common and frequently undiagnosed in the elderly. Our findings show that mild grade anemia is independently associated with increased risk of clinically relevant outcomes such as hospitalization and mortality. Observational studies cannot provide proof of causality, but the well-founded suspicion that mild anemia in the elderly might not be just an innocent bystander calls for controlled clinical trials to test whether raising hemoglobin concentrations can effectively reduce the risks associated with this condition.
Acknowledgments
the authors are grateful to all the elderly participants of Biella who made this investigation possible and to the “Health and Anemia” Study Group: Elena Clivio, Tania Maierini, Luca Pasina, Anna Busillo, Antonia Gianaroli, Maria Orgiana, Patrizia Panfili, Simona Banino, Pamela Cinti, Francesca Giardini, Elena Grappolo, Paola Minacapelli, Luigi Savoia, Manuela Saviolo, the Registry Office and Local Health Authority (ASL) of Biella, Fondo Edo Tempia, Lega Italiana per la Lotta contro i Tumori, and Fondazione Clelio Angelino.
Footnotes
Funding: this study was supported by a research grant from Amgen Italy. The sponsor of the study had no role in the conception or design of the study; collection, management, analysis, or interpretation of data; preparation and writing of the report or in the decision to submit the manuscript for publication.
Authorship and Disclosures
ER, MT, PM, GA and UL conceived and designed the research. ER, MT, PM, GA, FG, AN, MVT, PD, AGi, MC, PT, AGu, GF, and UL performed data collection and management. MVT and PD performed laboratory analyses. MT performed statistical analyses. EM, MT, PM, GA, and UL analyzed and interpreted the data. UL drafted the manuscript. ER, MT, PM, GA, FG, AN, MVT, PD, AGi, MC, PT, AGu, GF, and UL revised the manuscript and approved the final version of it.
The authors reported no potential conficts of interest.
References
- 1.Salive ME, Cornoni-Huntley J, Guralnik JM, Phillips CL, Wallace RB, Ostfeld AM, et al. Anemia and hemoglobin levels in older persons: relationship with age, gender, and health status. J Am Geriatr Soc. 1992;40:489–96. doi: 10.1111/j.1532-5415.1992.tb02017.x. [DOI] [PubMed] [Google Scholar]
- 2.Anía BJ, Suman VJ, Fairbanks VF, Rademacher DM, Melton LJ., III Incidence of anemia in older people: an epidemiological study in a well defined population. J Am Geriatr Soc. 1997;45:825–31. doi: 10.1111/j.1532-5415.1997.tb01509.x. [DOI] [PubMed] [Google Scholar]
- 3.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–8. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 4.Carmel R. Anemia and aging: an overview of clinical, diagnostic and biological issues. Blood Rev. 2001;15:9–18. doi: 10.1054/blre.2001.0146. [DOI] [PubMed] [Google Scholar]
- 5.Nissenson AR, Goodnough LT. Anemia. Not just an innocent bystander? Arch Intern Med. 2003;163:1400–4. doi: 10.1001/archinte.163.12.1400. [DOI] [PubMed] [Google Scholar]
- 6.Lipschitz D. Medical and functional consequences of anemia in the elderly. J Am Geriatr Soc. 2003;51(3 suppl):S10–3. doi: 10.1046/j.1532-5415.51.3s.6.x. [DOI] [PubMed] [Google Scholar]
- 7.Woodman R, Ferrucci L, Guralnik J. Anemia in older adults. Curr Opin Hematol. 2005;12:123–8. doi: 10.1097/01.moh.0000154030.13020.85. [DOI] [PubMed] [Google Scholar]
- 8.Spivak JL. Anemia in the elderly. Time for new blood in old vessels? Arch Intern Med. 2005;165:2187–9. doi: 10.1001/archinte.165.19.2187. [DOI] [PubMed] [Google Scholar]
- 9.Robinson B. Cost of anemia in the elderly. J Am Geriatr Soc. 2003;51(3 suppl):S14–7. doi: 10.1046/j.1532-5415.51.3s.5.x. [DOI] [PubMed] [Google Scholar]
- 10.Ershler WB, Chen K, Reyes EB, Dubois R. Economic burden of patients with anemia in selected diseases. Value Health. 2005;8:629–38. doi: 10.1111/j.1524-4733.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 11.Denny SD, Kuchibhatla MN, Cohen HJ. Impact of anemia on mortality, cognition, and function in community-dwelling elderly. Am J Med. 2006;119:327–34. doi: 10.1016/j.amjmed.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 12.Penninx BWJH, Pahor M, Woodman RC, Guralnik JM. Anemia in old age is associated with increased mortality and hospitalization. J Gerontol A Biol Sci Med Sci. 2006;61:474–9. doi: 10.1093/gerona/61.5.474. [DOI] [PubMed] [Google Scholar]
- 13.Izaks GJ, Westendorp RGJ, Knook DL. The definition of anemia in older persons. JAMA. 1999;281:1714–7. doi: 10.1001/jama.281.18.1714. [DOI] [PubMed] [Google Scholar]
- 14.Chaves PHM, Xue Q-L, Guralnik JM, Ferrucci L, Volpato S, Fried LP. What constitutes normal hemoglobin concentration in community-dwelling disabled older women? J Am Geriatr Soc. 2004;52:1811–6. doi: 10.1111/j.1532-5415.2004.52502.x. [DOI] [PubMed] [Google Scholar]
- 15.Culleton BF, Manns BJ, Zhang J, Tonelli M, Klarenbach S, Hemmel-garn BR. Impact of anemia on hospitalization and mortality in older adults. Blood. 2006;107:3841–6. doi: 10.1182/blood-2005-10-4308. [DOI] [PubMed] [Google Scholar]
- 16.Patel KV, Harris TB, Faulhaber M, Angleman SB, Connelly S, Bauer DC, et al. Racial variation in the relationship of anemia with mortality and mobility disability among older adults. Blood. 2007;109:4663–70. doi: 10.1182/blood-2006-10-055384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucca U, Tettamanti M, Mosconi P, Apolone G, Gandini F, Nobili A, et al. Association of mild anemia with cognitive, functional, mood and quality of life outcomes in the Elderly: The “Health and Anemia” study. PLoS ONE. 2008;3(4):e1920. doi: 10.1371/journal.pone.0001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parmelee PA, Thuras PD, Katz IR, Lawton MP. Validation of the Cumulative Illness Rating Scale in a geriatric residential population. J Am Geriatr Soc. 1995;43:130–7. doi: 10.1111/j.1532-5415.1995.tb06377.x. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Nutritional anaemias: Report of a WHO Scientific Group. World Health Organ Tech Rep Ser. 1968;405:5–37. [PubMed] [Google Scholar]
- 20.Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91:1616–34. doi: 10.1093/jnci/91.19.1616. [DOI] [PubMed] [Google Scholar]
- 21.Dallman PR, Yip R, Johnson C. Prevalence and causes of anemia in the United States, 1976 to 1980. Am J Clin Nutr. 1984;39:437–45. doi: 10.1093/ajcn/39.3.437. [DOI] [PubMed] [Google Scholar]
- 22.Borgna-Pignatti C, Ventola M, Friedman D, Cohen AR, Origa R, Galanello R, et al. Seasonal variation of pretransfusion hemoglobin levels in patients with thalassemia major. Blood. 2006;107:355–7. doi: 10.1182/blood-2005-03-1231. [DOI] [PubMed] [Google Scholar]
- 23.Kristal-Boneh E, Froom P, Harari G, Shapiro Y, Green MS. Seasonal changes in red blood cell parameters. Br J Haematol. 1993;85:603–7. doi: 10.1111/j.1365-2141.1993.tb03354.x. [DOI] [PubMed] [Google Scholar]
- 24.Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107:1747–50. doi: 10.1182/blood-2005-07-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elwood PC, Waters WE, Benjamin IT, Sweetnam PM. Mortality and anaemia in women. Lancet. 1974;1:891–4. doi: 10.1016/s0140-6736(74)90346-8. [DOI] [PubMed] [Google Scholar]
- 26.Zakai NA, Katz R, Hirsch C, Shlipak MG, Chaves PHM, Newman AB, et al. A prospective study of anemia status, hemoglobin concentration, and mortality in an elderly cohort. The Cardiovascular Health Study. Arch Intern Med. 2005;165:2214–20. doi: 10.1001/archinte.165.19.2214. [DOI] [PubMed] [Google Scholar]
- 27.Atti AR, Palmer K, Volpato S, Zuliani G, Winbland B, Fratiglioni L. Anaemia increases the risk of dementia in cognitively intact elderly. Neurobiol Aging. 2006;27:278–84. doi: 10.1016/j.neurobiolaging.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Gagnon DR, Zhang T-J, Brand FN, Kannel WB. Hematocrit and the risk of cardiovascular disease: the Framingham Study: a 34-year follow-up. Am Heart J. 1994;127:674–82. doi: 10.1016/0002-8703(94)90679-3. [DOI] [PubMed] [Google Scholar]
- 29.Semba RD, Ricks MO, Ferrucci L, Xue Q-L, Chaves P, Fried LP, et al. Types of anemia and mortality among older disabled women living in the community: the Women’s Health and Aging Study I. Aging Clin Exp Res. 2007;19:259–64. doi: 10.1007/bf03324699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lagaay AM, van der Meij JC, Hijmans W. Validation of medical history taking as part of a population based survey in subjects aged 85 and over. Br Med J. 1992;304:1091–2. doi: 10.1136/bmj.304.6834.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herzog AR, Dielman L. Age differences in response accuracy for factual survey questions. J Gerontol. 1985;40:350–7. doi: 10.1093/geronj/40.3.350. [DOI] [PubMed] [Google Scholar]
- 32.Davis PB, Robins LN. History-taking in the elderly with and without cognitive impairment. How useful is it? J Am Geriatr Soc. 1989;37:249–55. doi: 10.1111/j.1532-5415.1989.tb06815.x. [DOI] [PubMed] [Google Scholar]