Abstract
Variable proportions of hematopoietic cells that are heterozygous or homozygous for the JAK2 (V617) mutation can be found in patients with myeloproliferative neoplasms. In this perspective article, Dr. Passamonti and Rumi discuss the biologic and clinical significance of the mutant allele burden. See related paper on page 38.
The identification of a gain-of-function mutation in the Janus kinase 2 gene, named JAK2 (V617F), opened a new era in the understanding of Philadelphia-negative myeloproliferative neoplasms,1,2 including polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). These entities share some clinical features such as a high risk of developing thrombosis,3 evolution into secondary myelofibrosis (for PV and ET) and transformation into leukemia.4
The most intriguing question that arose after the discovery of the mutation is how a single mutation might give rise to at least three different diseases. This question remains unanswered, but clinical, biological and pathological data have led to three potential hypotheses. One, called the gene-dosage hypothesis, postulates a correlation between disease phenotype and the proportion of JAK2 (V617F) mutant alleles introducing the concept of allele burden, that is, the ratio between mutant and wild type JAK2 in hematopoietic cells. Experiments on transgenic mice expressing variable levels of JAK2 (V617F) support this hypothesis.5 In fact low levels of JAK2 (V617F) load induce an ET-like phenotype dominated by thrombocytosis, whereas higher levels of mutant alleles lead to a PV-like phenotype. A critical role of gene dosage effect is also indicated by studies on erythroid colonies. Homozygous JAK2 (V617F) erythroid colonies are present in most patients with PV, but occur rarely in those with ET.6
A second hypothesis advocates the existence of a pre-JAK2 phase in which additional somatic mutations or inherited predisposing alleles establish clonal hematopoiesis before the acquisition of JAK2 (V617F). Thus, mutations other than JAK2 may determine disease phenotype directly or by co-operating with JAK2 mutations. Analysis of the X-chromosome inactivation pattern of clonality in familial cases of myeloproliferative neoplasm7 has provided support for this hypothesis.
Finally, host genetic factors may contribute to phenotypic diversity among myeloproliferative neoplasms. This was documented in patients with PV and ET tested for genetic variation within JAK2, MPL, EPOR, and GCSFR genes using single nucleotide polymorphisms.8 In addition, strain-specific differences in phenotype have been observed in mice transplanted with JAK2 (V617F) transfected cells: Balb/c mice demonstrated markedly higher leukocyte counts, splenomegaly, and bone marrow reticulin fibrosis compared with C57Bl/6 mice.9
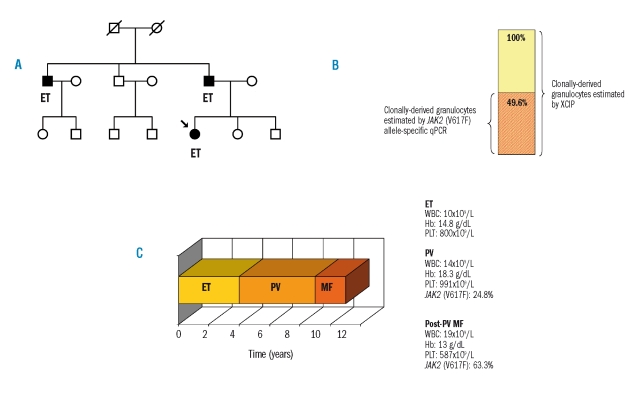
Likely, the three hypotheses, although explanatory individually, are not mutually exclusive. This is true for the patient whose clinical history is illustrated in Figure 1. This is the case of a 23-year old female with familial myeloproliferative neoplasm, whose father and uncle had ET (Figure 1a). The young girl, after an initial diagnosis of ET, developed PV with a JAK2 (V617F) allele burden of 24.8% and clonal hematopoiesis, demonstrated through X-chromosome inactivation patterns (Figure 1b). She had erythrocytosis, thrombocytosis and did not display mobilization of CD34-positive cells. A few years later she developed myelofibrosis with an increase of the mutant allele burden to 63.3%, and an increase of circulating CD34-positive cells (Figure 1c). This case is in favor of a critical gene-dosage effect of JAK2 (V617F) on disease evolution, as the increase of allele burden corresponded with the myelofibrotic transformation. However, the case also supports the role of additional pre-existing mutations inherited in a genetically predisposed individual. In fact, this is a case of familial myeloproliferative neoplasm and a low allele burden exists within a milieu of clonal hematopoiesis.
Figure 1.
Genetic predisposition and gene-dosage effect of JAK2 (V617F) in a single patient with familial essential thrombocythemia (ET), who progressed to polycythemia vera (PV) and post-PV myelofibrosis (post-PV MF). (A) Pedigree of the family. The proband (indicated by an arrow) had ET, as did her father and uncle. The familial cluster supports the hypothesis of an inherited genetic predisposition. (B) Comparison of the proportion of clonally derived granulocytes determined by X-chromosome inactivation pattern (XCIP) (yellow bar) and by JAK2 (V617F) allele-specific quantitative polymerase chain reaction (qPCR) (red striped bar) at evolution into PV. The 24.8% mutant allele burden translated into 49.6% of granulocytes being heterozygous for the JAK2 (V617F), or 24.8% of granulocytes being homozygous for the mutation. This supports the existence of a pre-JAK2 phase as a low allele burden exists within a milieu of clonal hematopoiesis. (C) Scheme of the clinical course of the disease over time. Representation of evolution from ET to PV and to post-PV MF. The right side of the panel reports hematologic (white blood cell count, WBC; hemoglobin concentration, Hb: platelet count, PLT) and molecular data. The critical role of a gene dosage effect on the progression of the disease is highlighted.
The distribution of the JAK2 (V617F) mutation among PV, ET and PMF seems heterogeneous, as almost all patients with PV and with post-PV myelofibrosis and about half of those with ET and PMF carry the mutation. There is now a growing interest in JAK2 (V617F) allele burden and its potential influence on disease phenotype, disease complications and evolution. The starting point for studying the clinical significance of allele burden is its correct assessment by quantitative assays. In this regard, the paper by Lippert et al. in this issue of the journal is of major interest.10 Lippert et al. report the concordance of 11 different techniques, carried out in 16 laboratories, using various instruments to quantify JAK2 (V617F) allele burden. The study indicates the importance of using appropriate standards for calibration of JAK2 (V617F) quantitative assays and of using a single mode of expression of results: the percentage of JAK2 (V617F) on total JAK2. The caveat raised by the authors is that none of the assays tested can guarantee accurate quantification of mutant alleles for all patients: any unexpected mutation occurring within the sequence of primers can potentially reduce or prevent the amplification.
The current knowledge on the clinical relevance of mutant allele burden enables tentative explanations of both correlation with disease presentation and correlation with disease-related symptoms or complications. Concerning the correlation between allele burden and disease presentation, we first described that the distribution of allele burden was different within myeloproliferative neoplasms.11 Patients with ET have the lowest allele burden, those with PV and PMF an intermediate one and those with post-PV myelofibrosis the highest burden. This pivotal concept was further validated by other investigators.12,13 Given the wide spread of allele burden, PV represents the ideal model for studying clinical correlations of mutant allele load. A higher burden of JAK2 (V617F) unequivocally induces enhanced myelopoiesis, with patients developing leukocytosis. Concerning erythropoiesis, a linear relationship between allele burden and hemoglobin concentration has been documented in some studies,14 but not in others.15 In this regard, it is interesting to note that patients with PV that has evolved into myelofibrosis have the highest allele burden and almost all have anemia.16
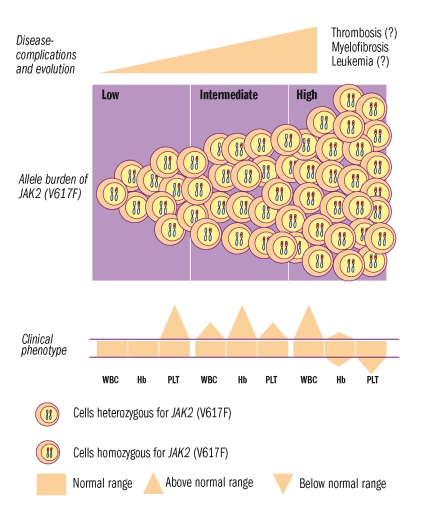
Thrombopoiesis is particularly stimulated by low allele burden, as an inverse relationship between allele burden and platelet count has been reported. This is in keeping with the low level of mutant alleles found in ET patients, whose clinical phenotype is dominated by thrombocytosis.12,13 Finally, all studies reported a correlation between allele burden and spleen size, confirming the role of mutant allele burden in stimulating myelopoiesis. Allele burden correlates linearly with leukocyte count and spleen size also in patients with ET and PMF.13,17–19 The bottom of Figure 2 illustrates the tentative correlations between allele burden and leukocyte count, hemoglobin level and platelet count. Disease-related complications and evolution basically include thrombosis, myelofibrosis and leukemia. Thrombosis is the most frequent event during follow-up, but many factors may interfere with its occurrence: patient-related factors, such as age and prior thrombosis, and disease-related factors such as leukocyte count and the JAK2 (V617F) mutation.
Figure 2.
Schematic representation of JAK2 (V617F) allele burden (middle panel) and its relationship with clinical phenotype (bottom panel), and disease complications (top panel). At low levels of mutant allele the clinical phenotype is dominated by thrombocytosis, at intermediate levels by erythrocytosis, and at higher levels by leukocytosis. Among complications, current evidence indicates a relationship between allele burden and evolution into myelofibrosis.
There is considerable debate on these latter potential risk factors, but broad consensus has not yet been reached. The mutation may affect leukocyte count, leukocyte and platelet activation,11 platelet-leukocyte interactions, as well as plasma hypercoagulation factors20 and, in turn, these activated factors may potentially influence thrombosis. Results from clinical studies aimed at defining the correlation between the mutation or its allele burden and the occurrence of vascular complications ae conflicting.21 The knowledge on this field seems premature and needs further validation: this accounts for the question marks we use in the top panel of Figure 2. Myelofibrosis is considered a bona fide natural evolution in both PV and ET. We reported that patients with myeloproliferative neoplasms who carry the JAK2 (V617F) mutation have a higher risk of post-PV myelofibrosis than those who do not carry the mutation.1 Two studies applying a semi-quantitative assay found that transformation into post-PV myelofibrosis occurred more frequently in homozygous PV patients than in heterozygous ones.22,23 In ET the much less frequent occurrence of myelofibrosis and the low allele burden did not allow any correlation to be found. The relationship between mutant allele burden and the occurrence of leukemia in PV and ET has not been defined so far. Concerning myelofibrosis, one study supports a critical role of allele burden in the progression of the disease, as the majority of patients with leukemia were homozygous for the mutation.19 However, another study reported opposite results, as the majority of patients with leukemia had a low allele burden or wild type JAK2.18 This suggests that a low allele burden may be overwhelmed by a more dominant JAK2 (V617F)-negative clone with a higher propensity to leukemic transformation.
In conclusion, current knowledge indicates that allele burden has a role in clinical phenotype and disease-evolution and suggests a potential relationship with vascular complications. Although these correlations have been consistently observed in clinical practice, it is premature to consider allele burden as a prognostic parameter to be applied for therapeutic interventions. In our opinion, we must now wait for the next lesson, which will come from the new agents with a potential to switch-off JAK2, named JAK2-inhibitors. Will these compounds be able to reduce allele burden without toxicity¿ Will the current knowledge be validated¿
References
- 1.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–90. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 2.Cazzola M, Skoda R. Gain of function, loss of control - a molecular basis for chronic myeloproliferative disorders. Haematologica. 2005;90:871–4. [PubMed] [Google Scholar]
- 3.De Stefano V, Za T, Rossi E, Vannucchi AM, Ruggeri M, Elli E, et al. Recurrent thrombosis in patients with polycythemia vera and essential thrombocythemia: incidence, risk factors, and effect of treatments. Haematologica. 2008;93:372–80. doi: 10.3324/haematol.12053. [DOI] [PubMed] [Google Scholar]
- 4.Passamonti F, Rumi E, Arcaini L, Boveri E, Elena C, Pietra D, et al. Prognostic factors for thrombosis, myelofibrosis, and leukemia in essential thrombocythemia: a study of 605 patients. Haematologica. 2008;93:1645–51. doi: 10.3324/haematol.13346. [DOI] [PubMed] [Google Scholar]
- 5.Tiedt R, Hao-Shen H, Sobas MA, Looser R, Dirnhofer S, Schwaller J, et al. Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood. 2008;111:3931–40. doi: 10.1182/blood-2007-08-107748. [DOI] [PubMed] [Google Scholar]
- 6.Scott LM, Scott MA, Campbell PJ, Green AR. Progenitors homozygous for the V617F mutation occur in most patients with polycythemia vera, but not essential thrombocythemia. Blood. 2006;108:2435–7. doi: 10.1182/blood-2006-04-018259. [DOI] [PubMed] [Google Scholar]
- 7.Rumi E, Passamonti F, Pietra D, Della Porta MG, Arcaini L, Boggi S, et al. JAK2 (V617F) as an acquired somatic mutation and a secondary genetic event associated with disease progression in familial myeloproliferative disorders. Cancer. 2006;107:2206–11. doi: 10.1002/cncr.22240. [DOI] [PubMed] [Google Scholar]
- 8.Pardanani A, Fridley BL, Lasho TL, Gilliland DG, Tefferi A. Host genetic variation contributes to phenotypic diversity in myeloproliferative disorders. Blood. 2008;111:2785–9. doi: 10.1182/blood-2007-06-095703. [DOI] [PubMed] [Google Scholar]
- 9.Wernig G, Mercher T, Okabe R, Levine RL, Lee BH, Gilliland DG. Expression of Jak2V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood. 2006;107:4274–81. doi: 10.1182/blood-2005-12-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lippert E, Girodon F, Hammond E, JJelinek J, Reading NS, Fehse B, et al. Concordance of assays designed for the quatification of JAKV61F. a multicenter study. Haematologica. 2009;94:38–45. doi: 10.3324/haematol.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Passamonti F, Rumi E, Pietra D, Della Porta MG, Boveri E, Pascutto C, et al. Relation between JAK2 (V617F) mutation status, granulocyte activation, and constitutive mobilization of CD34+ cells into peripheral blood in myeloproliferative disorders. Blood. 2006;107:3676–82. doi: 10.1182/blood-2005-09-3826. [DOI] [PubMed] [Google Scholar]
- 12.Moliterno AR, Williams DM, Rogers O, Spivak JL. Molecular mimicry in the chronic myeloproliferative disorders: reciprocity between quantitative JAK2 V617F and Mpl expression. Blood. 2006;108:3913–5. doi: 10.1182/blood-2006-03-008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antonioli E, Guglielmelli P, Poli G, Bogani C, Pancrazzi A, Longo G, et al. Influence of JAK2V617F allele burden on phenotype in essential thrombocythemia. Haematologica. 2008;93:41–8. doi: 10.3324/haematol.11653. [DOI] [PubMed] [Google Scholar]
- 14.Vannucchi AM, Antonioli E, Guglielmelli P, Longo G, Pancrazzi A, Ponziani V, et al. Prospective identification of high-risk polycythemia vera patients based on JAK2(V617F) allele burden. Leukemia. 2007;21:1952–9. doi: 10.1038/sj.leu.2404854. [DOI] [PubMed] [Google Scholar]
- 15.Tefferi A, Strand JJ, Lasho TL, Knudson RA, Finke CM, Gangat N, et al. Bone marrow JAK2V617F allele burden and clinical correlates in polycythemia vera. Leukemia. 2007;21:2074–5. doi: 10.1038/sj.leu.2404724. [DOI] [PubMed] [Google Scholar]
- 16.Passamonti F, Rumi E, Caramella M, Elena C, Arcaini L, Boveri E, et al. A dynamic prognostic model to predict survival in post-polycythemia vera myelofibrosis. Blood. 2008;111:3383–7. doi: 10.1182/blood-2007-11-121434. [DOI] [PubMed] [Google Scholar]
- 17.Kittur J, Knudson RA, Lasho TL, Finke CM, Gangat N, Wolanskyj AP, et al. Clinical correlates of JAK2V617F allele burden in essential thrombocythemia. Cancer. 2007;109:2279–84. doi: 10.1002/cncr.22663. [DOI] [PubMed] [Google Scholar]
- 18.Tefferi A, Lasho TL, Huang J, Finke C, Mesa RA, Li CY, et al. Low JAK2V617F allele burden in primary myelofibrosis, compared to either higher allele burden or unmutated status, is associated with inferior overall and leukemia-free survival. Leukemia. 2008;22:756–61. doi: 10.1038/sj.leu.2405097. [DOI] [PubMed] [Google Scholar]
- 19.Barosi G, Bergamaschi G, Marchetti M, Vannucchi AM, Guglielmelli P, Antonioli E, et al. JAK2 V617F mutational status predicts progression to large splenomegaly and leukemic transformation in primary myelofibrosis. Blood. 2007;110:4030–6. doi: 10.1182/blood-2007-07-099184. [DOI] [PubMed] [Google Scholar]
- 20.Falanga A, Marchetti M, Vignoli A, Balducci D, Russo L, Guerini V, et al. V617F JAK-2 mutation in patients with essential thrombocythemia: relation to platelet, granulocyte, and plasma hemostatic and inflammatory molecules. Exp Hematol. 2007;35:702–11. doi: 10.1016/j.exphem.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 21.Ziakas PD. Effect of JAK2 V617F on thrombotic risk in patients with essential thrombocythemia: measuring the uncertain. Haematologica. 2008;93:1412–4. doi: 10.3324/haematol.12970. [DOI] [PubMed] [Google Scholar]
- 22.Vannucchi AM, Antonioli E, Guglielmelli P, Rambaldi A, Barosi G, Marchioli R, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. 2007;110:840–6. doi: 10.1182/blood-2006-12-064287. [DOI] [PubMed] [Google Scholar]
- 23.Tefferi A, Lasho TL, Schwager SM, Strand JS, Elliott M, Mesa R, et al. The clinical phenotype of wild-type, heterozygous, and homozygous JAK2V617F in polycythemia vera. Cancer. 2006;106:631–5. doi: 10.1002/cncr.21645. [DOI] [PubMed] [Google Scholar]