Abstract
Background
Polycystic ovary syndrome (PCOS) is characterized by a hyperandrogenic state and frequently develops skeletal muscle insulin resistance. We determined whether testosterone adversely affects insulin action by increasing serine phosphorylation of IRS-1636/639 in differentiated rat skeletal muscle myotubes. The phosphorylation of Akt, mTOR, and S6K, downstream targets of the PI3-kinase-IRS-1 complex were also studied.
Methods
Primary differentiated rat skeletal muscle myotubes were subjected to insulin for 30 min after 16-hour pre-exposure to either low (20 ng/ml) or high (200 ng/ml) doses of testosterone. Protein phosphorylation of IRS-1 Ser636/639, Akt Ser473, mTOR-Ser2448, and S6K-Thr389 were measured by Western blot with signal intensity measured by immunofluorescence.
Results
Cells exposed to 100 nM of insulin had increased IRS-1 Ser636/639 and Akt Ser473 phosphorylation. Cells pre-exposed to low-dose testosterone had significantly increased insulin-induced mTOR-Ser2448 and S6K-Thr389 phosphorylation (p<0.05), and further increased insulin-induced IRS-1 Ser636/639 phosphorylation (p = 0.042) compared to control cells. High-dose testosterone pre-exposure attenuated the insulin-induced mTOR-Ser2448 and S6K-Thr389 phosphorylation.
Conclusions
The data demonstrated an interaction between testosterone and insulin on phosphorylation of intracellular signaling proteins, and suggests a link between a hyperandrogenic, hyperinsulinemic environment and the development of insulin resistance involving serine phosphorylation of IRS-1 Ser636/639. These results may guide further investigations of potential mechanisms of PCOS-related insulin resistance.
Introduction
Polycystic Ovary Syndrome (PCOS) is estimated to affect approximately 5–7% of premenopausal women in the U.S., and is associated with a significant risk of developing type 2 diabetes (T2D) independent of obesity [1]. PCOS is characterized by a hyperandrogenic state and exposure to exogenous testosterone (T) in vivo has been associated with insulin resistance in rats and human females [2]–[4]. However, the mechanism by which hyperandrogenism results in the development of insulin resistance remains incompletely defined. The risk of developing the metabolic syndrome in adolescent females with PCOS correlates strongly with increased bioavailable T concentrations, independent of obesity [5]. In addition, a recent systematic review demonstrated a significantly higher risk of developing T2D mellitus in women with elevated T concentrations [6].
Insulin receptor substrate-1 (IRS-1) is a signaling protein which couples the insulin receptor to the phosphoinositide-3-kinase (PI3K) signaling cascade [7]. Serine phosphorylation of IRS-1 disassociates coupling of IRS-1 signal transduction to PI3K and results in insulin resistance. Exposure to a number of metabolites, including free fatty acids, glucose, diacylglycerol, and fatty acyl-CoA's increase serine phosphorylation of IRS-1 and result in impaired insulin signaling [8]. Skeletal muscle and liver cells from obese rats have been shown to demonstrate elevated IRS-1 serine phosphorylation and impaired insulin signaling through the PI3K pathway [9]. In addition, IRS-1 associated PI3K activity has been shown to be decreased in vivo in skeletal muscle of PCOS patients, and cultured skeletal muscle from PCOS patients has been shown to have elevated levels of IRS-1 serine phosphorylation as compared to age and body mass index (BMI) matched controls [10], [11].
The purpose of the current study was to test the hypothesis that T exposure increases serine phosphorylation of IRS-1 in skeletal muscle, the dominant site of insulin mediated glucose uptake in the postprandial state, in a hyperinsulinemic environment. Previous studies have shown that increased phosphorylation of mTOR and S6K causes sub-cellular redistribution of IRS-1, and inactivates IRS-1 by increasing its serine phosphorylation leading to insulin resistance [9], [12]–[15].
In an effort to further investigate aberrations in insulin signaling under these experimental conditions, we also studied the phosphorylation of Akt, mTOR, and S6K, which lie downstream of the PI3-kinase-IRS-1 complex.
Results
Homogeneity of myoblast culture
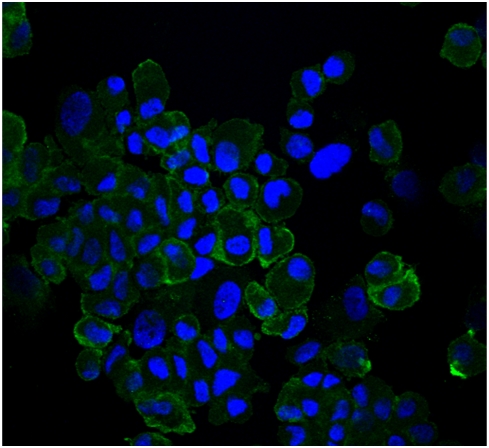
Immunohistochemistry staining of primary cultured rat myoblast cells showed positive MHC-1 in >95% of the cells, confirming the successful isolation and primary culture of skeletal muscle cells (Figure 1).
Figure 1. There was no contamination of non-myoblast cells in our primary culture of rat skeletal muscle cells.
Cells were positively stained with MHCI-antibody (Green Florescence).
The impact of T exposure on the phosphorylation of insulin signaling proteins
IRS-1
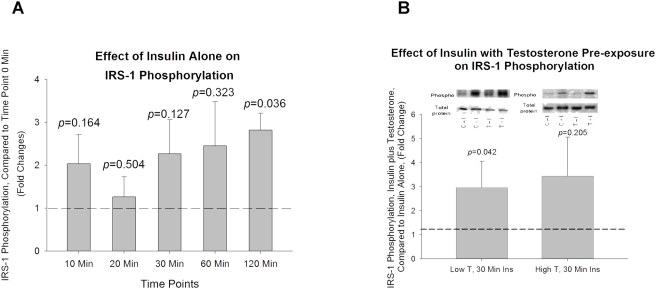
Exposure of differentiated rat skeletal muscle myotubes to 100 nM insulin significantly increased phosphorylation of IRS-1 Ser636/639 at 120 Min (p = 0.036,Figure 2A). Phosphorylation of IRS-1 Ser636/639 was also elevated at 30 and 60 Min (Figure 2A), however, these elevations in IRS-1 Ser636/639 phosphorylation did not reach the level of statistical significance. Cells pre-exposed for 16 hours to low T concentrations (20 ng/mL) had a significantly greater insulin-induced IRS-1 Ser636/639 phosphorylation compared to non-T exposed (control) cells (p = 0.042, figure 2B) following 30 minutes of exposure to 100 nM of insulin. Cells pre-exposed for 16 hours to high T concentrations (200 ng/ml) resulted in a non-significant increase in IRS-1 Ser636/639 compared to non-T exposed (control) cells (p = 0.205, Figure 2B) following 30 minutes of exposure to 100 nM of insulin. Exposure to neither low-dose T nor high-dose T alone affected IRS-1 Ser636/639 phosphorylation.
Figure 2. The effect of insulin in IRS-1 phosphorylation with or without pre-exposure of low and high dose testosterone.
(A). The effect of insulin alone on IRS-1 phosphorylation after insulin treatment for 10, 20, 30, 60, and 120 minutes; Insulin significantly increased IRS-1 Ser636/639 phosphorylation after 120 minutes; (B). Cells were pre-exposed to low-dose (20ng/ml) or high-dose (200ng/ml) of testosterone for 16 hours before insulin treatment. Compared to non-testosterone treated (control) cells, IRS-1 Ser636/639 phosphorylation was significantly increased with both low and high-dose testosterone exposure. Representative western blots are shown above the bar graphs. The four experimental groups are: control cells without insulin (C−I), control cells plus insulin (C+I), testosterone exposed cells without insulin (T−I), and testosterone exposed cells plus insulin (T+I). The phosphorylated signal of IRS-1 Ser636/639 is normalized to total IRS-1 in the sample.
Akt
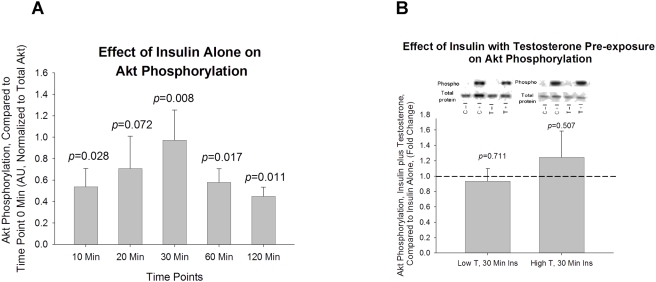
Exposure to 100 nM insulin resulted in increases in Akt phosphorylation at all time points: 10, 20, 30, 60, and 120 minutes (p = 0.028, p = 0.072, p = 0.007, p = 0.016, and p = 0.011, respectively, Figure 3A). Cells pre-exposed for 16 hours to both low (20 ng/mL) and high (200 ng/mL) T concentrations resulted in no significant changes in Akt phosphorylation compared to non-T exposed (control) cells (Figure 3B) following 30 minutes of exposure to 100 nM of insulin.
Figure 3. The effect of insulin in Akt Ser473 phosphorylation with or without pre-exposure of low and high dose testosterone.
(A). The effect of insulin alone on Akt phosphorylation after insulin treatment for 10, 20, 30, 60, and 120 minutes; Insulin significantly increased Akt Ser473 phosphorylation at all time points except 20 minutes; (B). Cells were pre-exposed to low-dose (20ng/ml) or high-dose (200ng/ml) of testosterone for 16 hours before insulin treatment. No change in Akt Ser473 phosphorylation was observed between T treated and non-T treated cells. Representative western blots are shown above the bar graphs. The four experimental groups are: control cells without insulin (C−I), control cells plus insulin (C+I), testosterone exposed cells without insulin (T−I), and testosterone exposed cells plus insulin (T+I). The phosphorylated signal of Akt Ser473 is normalized to total Akt in the sample.
mTOR
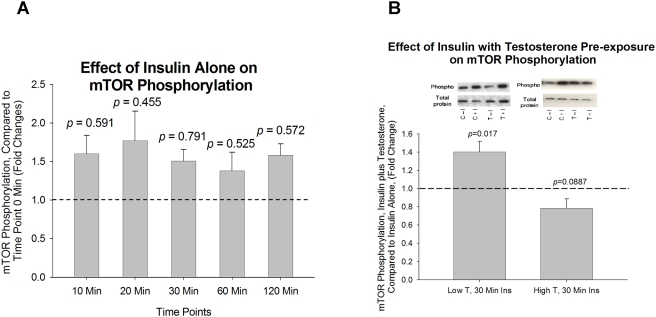
Exposure to 100 nM insulin at 10, 20, 30, 60, and 120 minutes did not change mTOR phosphorylation at any time point (figure 4A). Cells pre-exposed for 16 hours to low T concentrations (20 ng/mL) had a 40% increase in insulin-induced mTOR phosphorylation compared to non-T exposed (control) cells (p = 0.017, Figure 4B) following 30 minutes of exposure to 100 nM of insulin. Cells pre-exposed for 16 hours to high T concentrations (200 ng/mL) resulted in a borderline-significant reduction in mTOR phosphorylation compared to non-T exposed (control) cells (p = 0.088, Figure 4B) following 30 minutes of exposure to 100 nM of insulin.
Figure 4. The effect of insulin in mTOR Ser2448 phosphorylation with or without pre-exposure of low and high dose testosterone.
(A). The effect of insulin alone on mTOR Ser2448 phosphorylation after insulin treatment for 10, 20, 30, 60, and 120 minutes; Insulin had no effect on mTOR Ser2448 phosphorylation at all time points; (B). Cells were pre-exposed to low-dose (20ng/ml) or high-dose (200ng/ml) of testosterone for 16 hours before insulin treatment. Compared to non-testosterone treated cells, mTOR Ser2448 phosphorylation was significantly increased with low-dose testosterone treatment. Representative western blots are shown above the bar graphs. The four experimental groups are: control cells without insulin (C−I), control cells plus insulin (C+I), testosterone exposed cells without insulin (T−I), and testosterone exposed cells plus insulin (T+I). The phosphorylated signal of mTOR Ser2448 is normalized to total mTOR in the sample.
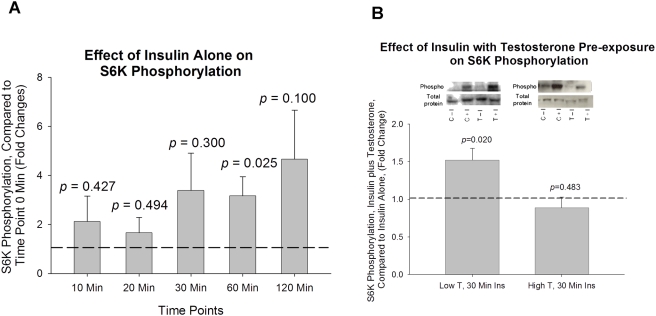
S6K
Exposure to 100 nM insulin resulted in a significant increase in S6K phosphorylation at time point 60 minute compared to that of baseline (p = 0.025, Figure 5A). The cells pre-exposed for 16 hours to low T concentrations (20 ng/mL) had a 52% increase in insulin stimulated S6K phosphorylation compared to non-T exposed (control) cells following 30 minutes of exposure to 100 nM of insulin (p = 0.020, Figure 5B). When cells were pre-exposed to high T concentrations (200 ng/mL), there were no significant changes in S6K phosphorylation compared to that of non-T exposed (control) cells following 30 minutes of exposure to 100 nM of insulin (Figure 5B).
Figure 5. The effect of insulin in S6K Thr389 phosphorylation with or without pre-exposure of low and high dose testosterone.
(A). The effect of insulin alone on S6K Thr389 phosphorylation after insulin treatment for 10, 20, 30, 60, and 120 minutes; Insulin significantly increased S6K Thr389 phosphorylation after 60 minutes; (B). Cells were pre-exposed to low-dose (20ng/ml) or high-dose (200ng/ml) of testosterone for 16 hours before insulin treatment. Compared to non-testosterone treated cells, S6K Thr389 phosphorylation was significantly increased with low-dose testosterone treatment. Representative western blots are shown above the bar graphs. The four experimental groups are: control cells without insulin (C−I), control cells plus insulin (C+I), testosterone exposed cells without insulin (T−I), and testosterone exposed cells plus insulin (T+I). The phosphorylated signal of S6K Thr389 is normalized to total S6K in the sample.
Discussion
The current study reports a link between a hyperinsulinemic/hyperandrogenic environment and an elevation in serine phosphorylation of IRS-1, which has been associated with insulin resistance. More specifically, these results suggest there is a synergistic interaction between T and hyperinsulinemia in its effect beyond that of hyperinsulinemia alone. At both the low and high doses of T, IRS-1 demonstrated an ∼300% increase in insulin stimulated Ser636/639 phosphorylation. Regarding the Akt-mTOR-S6K pathway, low-dose testosterone exposure significantly increased both mTOR and S6K phosphorylation by insulin compared to those from control cells that were not pre-exposed to testosterone. There are other factors causing insulin resistance, known to be involved in elevated IRS-1 serine phosphorylation including tumor necrosis factor α (TNF-α), free fatty acids, cellular stress, amino acids, and insulin [16]. These molecules are thought to act on IRS-1 through a variety of serine/threonine kinases including mTOR and S6K [9], [12], [16], [17]. Therefore, low-dose testosterone seems to elevate insulin-induced IRS-1 serine phosphorylation similar to TNF-α, free fatty acids, amino acids, and insulin.
Of note, we did not observe any significant changes in insulin-associated mTOR/S6K phosphorylation when cells were pre-exposed to a higher dose of testosterone, which may be due to the desensitization of mTOR/S6K to insulin under current in vitro conditions. Other studies have suggested that mTOR's effect on IRS-1 Ser636/639 phosphorylation is mediated through TNF-α [17]. Our data demonstrating increased phosphorylation of mTOR and S6K paralleling that of IRS-1 Ser636/639 phosphorylation in response to a hyperandrogenic, hyperinsulinemic stimulus are consistent with these prior studies. Additionally, the observed increase in mTOR and S6K insulin stimulated phosphorylation following T exposure was not observed for Akt, which is consistent with the findings of Khamzina et al [9], which demonstrated elevated IRS-1 Ser636/639 resulted in suppression of Akt phosphorylation.
Increased IRS-1 Ser636/639 phosphorylation has previously been reported in liver and skeletal muscles of obese, insulin-resistant rats under insulin stimulation [9], which is similar to our observations where insulin alone significantly stimulated IRS-1 Ser636/639 phosphorylation at time point 120 minute (Figure 2A). The same study also suggested that suppressed Akt activity in liver and skeletal muscle of the obese animals is potentially due to the IRS-1 Ser636/639 phosphorylation secondary to insulin resistance, which uncouples PI3-kinase from the insulin receptor and results in reduced insulin signaling through Akt [9].
The results from the current study offer an opportunity to better understand the underlying mechanism of insulin resistance in PCOS patients. The association between hyperandrogenism and insulin resistance is well established in both animal models and humans. Multiple mechanisms have been suggested based on animal studies including reduced skeletal muscle capillary formation, muscle fiber isoform switching, reduced glycogen synthase activity, and impaired insulin-induced glucose transporter 4 (GLUT4) plasma membrane translocation [18]–[21]. However, much of the data regarding hyperandrogenic insulin resistance in humans has been observational [5], [6], [22], [23].
Studies of the mechanism of insulin resistance of PCOS have found evidence of altered serine phosphorylation in the insulin receptors of cultured skin fibroblasts from PCOS patients, which inhibited IR activation by tyrosine autophosphorylation in response to insulin stimulation [24], [25]. Of note, PCOS patients had significant elevations in both total and bioavailable T compared to the control subjects. A recent study using cultured skeletal muscle from 10 PCOS patients and matched control subjects reported a 35% significant increase in IRS-1 protein abundance in cultured skeletal muscle of PCOS patients, and the insulin stimulated IRS-1 associated PI3K activity was reduced in the PCOS patients [11]. In addition, insulin stimulated IRS-1 Ser312 was 55% higher in the PCOS patients as compared to controls, even after controlling for the elevation in IRS-1 protein abundance in these subjects [11]. The present study took the next logical step by examining the effects of both low-dose and high-dose testosterone exposure on insulin-induced phosphorylation of serine (inhibitory) residues on IRS-1 as well as some of its downstream targets. This finding from the current study is consistent with that of Courbold et al and demonstrates that increased insulin stimulated IRS-1 Ser phosphorylation occurs in cultured rat skeletal muscle after pre-exposure to T. Although there are at least 50 known potential serine/threonine phosphorylation sites on IRS-1, most studies relating IRS-1 serine phosphorylation to insulin resistance study one, or at most several, sites in response to a given stimulus [9], [11], [14], [16], [17], [26], [27]. Elevations in phosphorylation at both IRS-1 sites, Ser312 and Ser636/639, have been reported in other studies in association with insulin resistance [9], [17], [27].
The fact that we observed a similar trend to increase insulin-induced IRS-1 serine phosphorylation with both doses of T indicates that both the low- and high-dose T exposure enhanced insulin-induced IRS-1 serine phosphorylation. It should be noted that the low-dose T concentrations is at the upper limit of the physiological range, which demonstrated a significant IRS-1 serine phosphorylation and although the larger dose of T showed similar trend it did not reach the level of statistical significance. There may be multiple pathways and signaling proteins other than IRS-1 involved in this interaction between T and insulin. Previous studies suggested other pathways such as the mitogen activated protein kinase (MAPK) pathway and the stress activated protein kinase JNK, which are implicated in insulin induced IRS-1 serine phosphorylation and insulin resistance [28], [29].
Another intriguing finding of the present study is the observation that insulin stimulated IRS-1 serine phosphorylation in the absence of T, while concomitantly inhibiting the phosphorylation AKT beyond 30 minutes of insulin exposure (Figures 2A and 3A). This additional finding indicates that insulin can modulate its own signaling pathway. This finding is consistent with a recent report that indicated that continually high insulin levels impaired Akt phosphorylation and glucose transport in human myoblasts [30].
We chose an in vitro model to test our hypothesis. The in vitro model of the experimental design has limitations in that the model may not always accurately represent to in vivo biology. However, in vitro studies allow for the isolation of the effects of specific stimuli (in this case testosterone) on aberrant protein signaling (in this case insulin-stimulated IRS-1 Ser636/639 phosphorylation) from complexities of in vivo model. In addition, we chose to initially test our hypothesis in this primary rat skeletal muscle myotubes, but future investigations are warranted to determine whether T exposure has similar effects on insulin-stimulated IRS-1 Ser636/639 phosphorylation in primary human skeletal muscle myotubes as well as in vivo in humans.
In conclusion, we report a specific link between a hyperinsulinemic, hyperandrogenic environment and an elevation of IRS-1 serine phosphorylation of IRS-1, which has been associated with the development of insulin resistance. Specifically, our results suggest that there is a synergistic interaction between T and insulin in this effect beyond that of insulin alone. Additionally, we suggest future studies utilizing primary skeletal muscle culture exposed to T in the presence of a hyperinsulinemic environment may serve as models for PCOS-related insulin resistance. These results agree with the recent findings of elevated serine phosphorylation of IRS-1 in cultured skeletal muscle of PCOS patients [11].
Materials and Methods
Skeletal Muscle Culture
The study protocol was approved by the Institutional Animal Care and Use Committee of Mayo Clinic and Foundation. Skeletal muscle was obtained from the soleus and EDL muscle of an adult female Sprague-Dawley rat from which myoblasts were isolated and cultured according to established protocols [31]–[33]. Specifically, muscle biopsy samples were collected in ice-cold phosphate-buffered saline (PBS) supplemented with 1% PeSt (100 units/ml penicillin/100 μg/ml streptomycin). Fat and connective tissue were dissected from the specimen in a sterile tissue culture hood. The muscle was placed into a filter-sterilized collagenase solution (0.2% type I collagenase (Sigma) in Dulbecco's modified Eagles medium (DMEM, Sigma) and incubated for 1.5 h at 37°C in an oscillating water bath. Following digestion, the muscle cells were transferred to a 100mm petri dish (Falcon) pre-rinsed with DMEM containing horse serum (HS). A wide-bore pipet (Falcon) was then used to gently separate muscle fibers while the mass of muscle cells were then transferred to a fresh identically-prepared petri dish and the separation process repeated. The supernatant from each separation process was transferred to a non-coated 100mm petri dish and incubated for 1 h in a tissue culture incubator at 37°C and 5% CO2 to promote adherence of non-myogenic cells. The non-adherent cells from each non-coated dish were then transferred to a 24-well tissue culture plate (Falcon) precoated with 10% Matrigel and allowed to settle and attach for 3 min prior to the placement of 0.5 ml plating media (DMEM, 10% HS, 0.5% chick embryo extract (CEE), 1% PeSt) per well. After 3 days, the media was changed to proliferation media containing 20% fetal bovine serum (FBS), 10% HS, 0.5% CEE, and 1% PeSt. The cells, when reached ∼80% confluence, were then subcultured according to standard techniques and transferred to non-coated 75-cm2 flasks (Falcon) for continued culture.
Myoblasts were differentiated into multi-nucleated myotubes after 4 days in low-serum media containing DMEM, 2% FBS, and 1% PeSt. All protein experiments were performed using myotubes.
Immunohistochemical (IHC) Staining
Myoblasts, upon reaching 80% confluence, were trypsinized and mounted on microscope slides for homogeneity estimation with IHC staining using the myoblast-specific myosin heavy chain-1 (MHC-1) antibody (Sigma, M8421) Briefly, following trypsinization the cells were suspended in 1% bovine serum albumin (BSA)/PBS. The cell concentration was adjusted to 1×105 cells/ml by serial dilution. Two-hundred microliters of the cell suspension was loaded into the cytofunnel to provide a final concentration of 2×104 cells per slide. The cells were spun at 600 rpm for 5 min to mount them on the slide. The slides were dried for 2 hours prior to antibody staining. Following drying, each slide was washed with PBS times for 1–2 min, followed by acetone fixation for 1 min. The slides were dried for 10 min, followed by an additional 1 min PBS wash. The slides were then incubated for 10 min at room temperature (RT) in 100 μl of Dako Cytomation Protein Block (DPBB) (DakoUSA x0909) with 1% saponin (Sap) (Sigma S-7900 100X stock). Slides were exposed to MHC-1 antibody (Ab) diluted 1:500 in DPBB-Sap for 1 hr at RT. Negative control slides were incubated in DPBB-Sap for 1 hr at RT. Slides were then washed 3 times in PBS for 5 min. Secondary Ab (Molecular Probes Alexa Flour 488) was applied for 30 min. Following PBS wash, slides were counterstained with a Hoescht counterstain (Sigma B2883). Slides were then mounted with an anti-fade agent (Molecular Probes P7481) and inspected using a Zeiss LSM 510 confocal laser scanning microscope (Carl Zeiss, Inc., Germany) set for excitation at 488 nm utilizing an argon/krypton laser. Negative control slides were used to calibrate the microscope prior to image analysis of the MHC-1 slides.
Western Blot Analysis
Sixteen hours prior to insulin exposure, differentiated myotube cells were adapted to serum-free media containing 1% BSA without testosterone (control, C) or with testosterone (T) at two different concentrations, 20 ng/mL and 200 ng/mL. Then C and T pre-exposed cells were treated with or without 100 nM insulin (I) resulting in 6 experimental groups: C−I, C+I low T−I, low T+I, high T−I and high T+I. The T concentrations were empirically chosen. In brief, we used the upper physiologic limit for T concentration for the low-dose T and multiplied it by a factor of 10 base on our understanding that metabolic processes occur at much faster rates in rats. We then performed preliminary Western blots to determine if we could see a difference in protein phosphorylation. Based on our preliminary results we designed the experiment to compare the 2 concentrations. The 100 nM insulin concentration was chosen, because it represents a standard in vitro insulin concentration for stimulating glucose uptake [34]. Cells and total proteins from the cells were collected using cell lysis buffer supplemented with protease inhibitor cocktail (Cell Signaling #9803, Roche complete mini #1836153) after 30 minutes, 1 hour, and 2 hours of insulin stimulation for the low-dose T pre-exposed cells, and after 10, 20 and 30 min of insulin stimulation for the high-dose T treated cells . For both the low-dose and high-dose T exposed cells, the 30 min data is presented based on preliminary experiments which suggested that at this time point the phosphorylation status of IRS1 Ser636/639 and S6k plateaued, Akt reached its peak phosphorylation, and mTOR phosphorylation remained unchanged. Total proteins were separated by gel electrophoresis using the Invitrogen Mini Cell© system and NuPage 4–12% Tris-Bis gels. Proteins were then transferred to PVDF membranes, and western blot analysis was performed using antibodies against total and phosphorylated Akt Ser473 (Cell Signaling #9272, #9271), mTOR Ser2448 (Cell Signaling #2972, #2971), S6 kinase Thr389 (Cell Signaling #9202, #9205), and IRS-1Ser636/639 (Cell Signaling #2382, #2388). Signal intensities of phosphorylated and total proteins were quantified and analyzed using Kodak image station 1000 and the accompanying software packages. Phosphorylated signals were normalized to total protein for each measurement. The net effect of insulin on protein phosphorylation was determined using the ratio of the insulin-treated phosphorylation signal to the non-insulin treated phosphorylation signal for both control cells and T exposed cells. The experiment was replicated six times for each condition.
Statistics
Data are presented as the mean±SE. Statistical significances were calculated using unpaired t-tests.
Acknowledgments
We appreciate the secretarial assistance of Melissa Aakre.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: NIH RO1 DK 41973 and David Murdock Dole Professorship (KSN), KL2 RR024151 (BAI). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165–169. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 2.Rincon J, Holmang A, Wahlstrom EO, Lonnroth P, Bjorntorp P, et al. Mechanisms behind insulin resistance in rat skeletal muscle after oophorectomy and additional testosterone treatment. Diabetes. 1996;45:615–621. doi: 10.2337/diab.45.5.615. [DOI] [PubMed] [Google Scholar]
- 3.Diamond MP, Grainger D, Diamond MC, Sherwin RS, Defronzo RA. Effects of methyltestosterone on insulin secretion and sensitivity in women. J Clin Endocrinol Metab. 1998;83:4420–4425. doi: 10.1210/jcem.83.12.5333. [DOI] [PubMed] [Google Scholar]
- 4.Holmang A, Niklasson M, Rippe B, Lonnroth P. Insulin insensitivity and delayed transcapillary delivery of insulin in oophorectomized rats treated with testosterone. Acta Physiol Scand. 2001;171:427–438. doi: 10.1046/j.1365-201X.2001.00801.x. [DOI] [PubMed] [Google Scholar]
- 5.Coviello AD, Legro RS, Dunaif A. Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2006;91:492–497. doi: 10.1210/jc.2005-1666. [DOI] [PubMed] [Google Scholar]
- 6.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. Jama. 2006;295:1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 7.White MF. IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab. 2002;283:E413–422. doi: 10.1152/ajpendo.00514.2001. [DOI] [PubMed] [Google Scholar]
- 8.Shulman G. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473–1481. doi: 10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- 10.Dunaif A, Wu X, Lee A, Diamanti-Kandarakis E. Defects in insulin receptor signaling in vivo in the polycystic ovary syndrome (PCOS). Am J Physiol Endocrinol Metab. 2001;281:E392–399. doi: 10.1152/ajpendo.2001.281.2.E392. [DOI] [PubMed] [Google Scholar]
- 11.Corbould A, Kim YB, Youngren JF, Pender C, Kahn BB, et al. Insulin resistance in the skeletal muscle of women with PCOS involves intrinsic and acquired defects in insulin signaling. Am J Physiol Endocrinol Metab. 2005;288:E1047–1054. doi: 10.1152/ajpendo.00361.2004. [DOI] [PubMed] [Google Scholar]
- 12.Tremblay F, Marette A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J Biol Chem. 2001;276:38052–38060. doi: 10.1074/jbc.M106703200. [DOI] [PubMed] [Google Scholar]
- 13.Takano A, Usui I, Haruta T, Kawahara J, Uno T, et al. Mammalian target of rapamycin pathway regulates insulin signaling via subcellular redistribution of insulin receptor substrate 1 and integrates nutritional signals and metabolic signals of insulin. Mol Cell Biol. 2001;21:5050–5062. doi: 10.1128/MCB.21.15.5050-5062.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouzakri K, Roques M, Gual P, Espinosa S, Guebre-Egziabher F, et al. Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes. 2003;52:1319–1325. doi: 10.2337/diabetes.52.6.1319. [DOI] [PubMed] [Google Scholar]
- 15.O'Connor JC, Freund GG. Vanadate and rapamycin synergistically enhance insulin-stimulated glucose uptake. Metabolism. 2003;52:666–674. doi: 10.1016/s0026-0495(03)00026-x. [DOI] [PubMed] [Google Scholar]
- 16.Gual P, Marchand-Brustel Y, Tanti JF. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie. 2005;87:99–109. doi: 10.1016/j.biochi.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Ozes ON, Akca H, Mayo LD, Gustin JA, Maehama T, et al. A phosphatidylinositol 3-kinase/Akt/mTOR pathway mediates and PTEN antagonizes tumor necrosis factor inhibition of insulin signaling through insulin receptor substrate-1. Proc Natl Acad Sci USA. 2001;98:4640–4645. doi: 10.1073/pnas.051042298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmang A, Niklasson M, Rippe B, Lonnroth P. Insulin insensitivity and delayed transcapillary delivery of insulin in oophorectomized rats treated with testosterone. Acta Physiol Scand. 2001;171:427–438. doi: 10.1046/j.1365-201X.2001.00801.x. [DOI] [PubMed] [Google Scholar]
- 19.Holmang A, Svedberg J, Jennische E, Bjorntorp P. Effects of testosterone on muscle insulin sensitivity and morphology in female rats. Am J Physiol Endocrinol Metab. 1990;259:E555–560. doi: 10.1152/ajpendo.1990.259.4.E555. [DOI] [PubMed] [Google Scholar]
- 20.Holmang A, Larsson BM, Brzezinska Z, Bjorntorp P. Effects of short-term testosterone exposure on insulin sensitivity of muscles in female rats. Am J Physiol Endocrinol Metab. 1992;262:E851–855. doi: 10.1152/ajpendo.1992.262.6.E851. [DOI] [PubMed] [Google Scholar]
- 21.Rincon J, Holmang A, Wahlstrom EO, Lonnroth P, Bjorntorp P, et al. Mechanisms behind insulin resistance in rat skeletal muscle after oophorectomy and additional testosterone treatment. Diabetes. 1996;45:615–621. doi: 10.2337/diab.45.5.615. [DOI] [PubMed] [Google Scholar]
- 22.Diamond MP, Grainger D, Diamond MC, Sherwin RS, DeFronzo RA. Effects of methyltestosterone on insulin secretion and sensitivity in women. J Clin Endocrinol Metab. 1998;83:4420–4425. doi: 10.1210/jcem.83.12.5333. [DOI] [PubMed] [Google Scholar]
- 23.Moghetti P, Tosi F, Castello R, Magnani CM, Negri C, et al. The insulin resistance in women with hyperandrogenism is partially reversed by antiandrogen treatment: evidence that androgens impair insulin action in women. J Clin Endocrinol Metab. 1996;81:952–960. doi: 10.1210/jcem.81.3.8772557. [DOI] [PubMed] [Google Scholar]
- 24.Dunaif A, Xia J, Book CB, Schenker E, Tang Z. Excessive insulin receptor serine phosphorylation in cultured fibroblasts and in skeletal muscle. A potential mechanism for insulin resistance in the polycystic ovary syndrome. J Clin Invest. 1995;96:801–810. doi: 10.1172/JCI118126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M, Youngren JF, Dunaif A, Goldfine ID, Maddux BA, et al. Decreased insulin receptor (IR) autophosphorylation in fibroblasts from patients with PCOS: effects of serine kinase inhibitors and IR activators. J Clin Endocrinol Metab. 2002;87:4088–4093. doi: 10.1210/jc.2002-020363. [DOI] [PubMed] [Google Scholar]
- 26.Gual P, Gremeaux T, Gonzalez T, Marchand-Brustel Y, Tanti JF. MAP kinases and mTOR mediate insulin-induced phosphorylation of insulin receptor substrate-1 on serine residues 307, 612 and 632. Diabetologia. 2003;46:1532–1542. doi: 10.1007/s00125-003-1223-4. [DOI] [PubMed] [Google Scholar]
- 27.Bouzakri K, Karlsson HK, Vestergaard H, Madsbad S, Christiansen E, et al. IRS-1 serine phosphorylation and insulin resistance in skeletal muscle from pancreas transplant recipients. Diabetes. 2006;55:785–791. doi: 10.2337/diabetes.55.03.06.db05-0796. [DOI] [PubMed] [Google Scholar]
- 28.Mussig K, Fiedler H, Staiger H, Weigert C, Lehmann R, et al. Insulin-induced stimulation of JNK and the PI 3-kinase/mTOR pathway leads to phosphorylation of serine 318 of IRS-1 in C2C12 myotubes. Biochem Biophys Res Commun. 2005;335:819–825. doi: 10.1016/j.bbrc.2005.07.154. [DOI] [PubMed] [Google Scholar]
- 29.Hiratani K, Haruta T, Tani A, Kawahara J, Usui I, et al. Roles of mTOR and JNK in serine phosphorylation, translocation, and degradation of IRS-1. Biochem Biophys Res Commun. 2005;335:836–842. doi: 10.1016/j.bbrc.2005.07.152. [DOI] [PubMed] [Google Scholar]
- 30.Bertacca A, Ciccarone A, Cecchetti P, Vianello B, Laurenza I, et al. Continually high insulin levels impair Akt phosphorylation and glucose transport in human myoblasts. Metabolism. 2005;54:1687–1693. doi: 10.1016/j.metabol.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 31.Rosenblatt JD, Lunt AI, Parry DJ, Partridge TA. Culturing satellite cells from living single muscle fiber explants. In Vitro Cellular&Developmental Biology Animal. 1995;31:773–779. doi: 10.1007/BF02634119. [DOI] [PubMed] [Google Scholar]
- 32.Partridge TA. Tissue culture of skeletal muscle. Methods in Molecular Biology. 1997;75:131–144. doi: 10.1385/0-89603-441-0:131. [DOI] [PubMed] [Google Scholar]
- 33.Al Khalili L, Chibalin AV, Kannisto K, Zhang BB, Permert J, et al. Insulin action in cultured human skeletal muscle cells during differentiation: assessment of cell surface GLUT4 and GLUT1 content. Cellular & Molecular Life Sciences. 2003;60:991–998. doi: 10.1007/s00018-003-3001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sweeney G, Somwar R, Ramlal T, Volchuk A, Ueyama A, et al. An inhibitor of p38 mitogen-activated protein kinase prevents insulin-stimulated glucose transport but not glucose transporter translocation in 3T3-L1 adipocytes and L6 myotubes. J Biol Chem. 1999;274:10071–10078. doi: 10.1074/jbc.274.15.10071. [DOI] [PubMed] [Google Scholar]