Abstract
The specificities and sensitivities of five recombinant proteins of the surface protective antigen (SpaA) of Erysipelothrix rhusiopathiae were examined by indirect enzyme-linked immunosorbent assay (ELISA) with the aim of developing a reliable serological test for the detection of protective antibody against E. rhusiopathiae. Fully mature protein and the N-terminal 416 amino acids (SpaA416) showed sufficient antigenicities, and further examination was done with SpaA416 because of its higher yield. The antibody titers of pigs experimentally immunized with commercial live vaccine and two types of inactivated vaccines clearly increased after immunization, and all pigs were completely protected against challenge with virulent strains. On the other hand, the antibody titers of nonimmunized control pigs remained very low until they were challenged, and all showed severe symptoms or subsequently died. Interference with the production of antibody against live vaccine by maternal antibody or porcine respiratory and reproductive syndrome virus infection 1 week after vaccination was also clearly detected. Because the ELISA titer correlated well with the protection results, the specificity and sensitivity of the ELISA were further evaluated with sera collected from pigs reared on 1 farm on which animals had acute septicemia, 2 farms on which the animals were infected or free from infection, and 10 farms on which the animals were vaccinated with live vaccine, among others. The ELISA titers clearly revealed the conditions of the herds. These results indicate that the SpaA416 ELISA is an effective method not only for evaluating pigs for the presence of protective antibody levels resulting from vaccination or maternal antibody but also for detecting antibody produced by natural infection. This test has important potential for the effective control of swine erysipelas.
Swine erysipelas is a bacterial disease with a worldwide distribution that has a major economic impact on pork production: it causes acute septicemia, chronic arthritis, and endocarditis (26). Erysipelothrix rhusiopathiae, a slender gram-positive rod, is the causative agent. Although it is classified into 15 serotypes, serotypes 1, 2, 4 to 9, 11, 12, 15 to 17, 19, and 21 (23), only serotypes 1 (subdivided into 1a and 1b) and 2 (subdivided into 2a and 2b) are of importance in pigs (22, 26).
Ingestion of contaminated feed and water is believed to be the major mode of infection. The most important reservoir of E. rhusiopathiae is probably domestic pigs. They harbor the organism in their tonsils and other lymphoid tissues and can discharge the organism in their feces or oronasal secretions, creating an important source of infection (26). Theoretically, therefore, swine erysipelas could be controlled by the eradication of carrier pigs from the herd. However, it is difficult to detect carrier pigs effectively by serological testing or bacterial isolation. For this reason, vaccination is widely used as the most efficient and practical means of preventing the disease in animals. However, despite extensive vaccination, the impact of this disease has not decreased. In Japan, about 2,000 pigs are affected with acute or subacute swine erysipelas on farms each year, and each year meat inspection authorities condemn about 2,000 pigs because they have the subacute or chronic form of the disease.
Various serological methods for the diagnosis of chronic swine erysipelas or for assay of maternal antibody and acquired antibody before and after vaccination have been reported, e.g., growth agglutination tests (12, 20, 25), the latex agglutination test (19), and enzyme-linked immunosorbent assay (ELISA) (1, 2, 4, 9, 10, 11, 16, 17, 19). Unlike in other countries, the attenuated live vaccine is the most commonly used type of vaccine in Japan, and the growth agglutination test is used for the detection of maternal antibody and acquired antibody before and after vaccination. This double test is carried out since the production of antibody against the live vaccine is affected by the presence of maternal antibody (26). However, the growth agglutination test requires culture of live pathogenic bacteria, which can be hazardous to laboratory workers. For this reason, recently developed latex agglutination kits are increasingly being used. On the other hand, ELISA is the test of choice among existing serological procedures because it is simple, permits the testing of large numbers of samples in a short time, and gives precise, objective results.
The major protective antigen of E. rhusiopathiae is the so-called 64- to 66-kDa antigen (1, 6, 7, 13, 19). Makino et al. (14) cloned the gene encoding the 69.9-kDa protective antigen of strain Tama of serotype 2 and named it the protein surface protective antigen (SpaA). The spaA gene, which encodes a 69.0-kDa protective antigen, of the virulent Fujisawa strain of serotype 1a was also cloned, and it was shown for the first time that purified truncated recombinant SpaA (amino acids 61 to 408; SpaA348) of serotype 1a can elicit complete protection in pigs challenged with serotypes 1a and 2b (9). The antibody production of these immunized pigs was sensitively detected by an indirect ELISA with SpaA348 as the antigen and by a double-antibody sandwich ELISA with alkaline extracts of E. rhusiopathiae as the antigen (9). However, the sensitivity of the indirect SpaA348 ELISA was insufficient for the detection of antibody in pigs immunized with the live vaccine. In this study, we constructed five regions of SpaA and compared their sensitivities and specificities in an indirect ELISA. We also evaluated the applicability of the SpaA ELISA using sera collected from experimentally immunized pigs, nonimmunized control pigs, experimentally challenged pigs, and pigs reared on farms.
MATERIALS AND METHODS
Bacterial strains and viral strain.
The E. rhusiopathiae Fujisawa strain (a virulent strain of serotype 1a) and a Japanese official challenge strain for the assay of vaccine efficacy were used in most of the experiments for intradermal challenge of pigs. E. rhusiopathiae 82-875, a virulent strain of serotype 2b, was obtained from the National Veterinary Assay Laboratory and was used in the cross-protection tests with truncated recombinant SpaA348. E. rhusiopathiae strain C42 of serotype 1a was isolated from a pig that had died from a dual infection with porcine reproductive and respiratory syndrome virus (PRRSV) and E. rhusiopathiae in 1995. PRRSV strain E4 was isolated from a severely affected pig in 1993. E. rhusiopathiae strain C42 and PRRSV strain E4 were used for the challenge exposure of pigs to study whether PRRSV infection in pigs inhibits the effect of the attenuated E. rhusiopathiae vaccine (18). E. rhusiopathiae strain SE-9, an official strain used for the production of bacterin in the United States, was used to prepare alkaline extracts for the double-antibody sandwich ELISA.
Expression of SpaA in Escherichia coli as a fusion protein.
SpaA348, which corresponds to amino acid residues 61 to 408 of the mature protein (598 amino acids), was expressed by E. coli XL1-Blue transformed with pA1.0, a recombinant plasmid of pQE32 (Qiagen) constructed from an Sau3AI clone encoding the spaA gene (9). The nucleotide sequence of spaA of the Fujisawa strain is available from the DDBJ/EMBL/GenBank nucleotide sequence databases under accession no. AB019124. Four other regions of the SpaA protein, SpaA89, SpaA416, SpaA594, and SpaA113, which correspond to amino acid residues 1 to 89, 1 to 416, 1 to 594, and 272 to 384 of the mature protein, respectively, were generated by using the histidine hexamer fusion system (Qiagen). Relevant DNA fragments were produced from the template DNA of E. rhusiopathiae strain Fujisawa by PCR with the six oligonucleotide primers listed in Table 1. PCR was performed with 100-μl volumes containing 400 ng of E. rhusiopathiae genomic DNA; 1.5 mM MgCl2; 50 pmol each primer; 2.5 U of AmpliTaq Gold DNA polymerase (Applied Biosystems); and 200 μM each dCTP, dGTP, dATP, and dTTP in 10 mM Tris-HCl (pH 8.3)-50 mM KCl under 2 drops of mineral oil. The cycling program was 1 cycle of 95°C for 9 min; 35 cycles of 94°C for 30 s, 54°C for 30 s, and 72°C for 1 min; and then 72°C for 7 min. The PCR products were digested with BamHI and SalI and inserted into pQE30, a type IV histidine hexamer fusion protein expression plasmid (Qiagen), in frame by the use of engineered restriction sites located in forward and reverse primers. Each plasmid was used to transform E. coli XL1-Blue (Stratagene), and the resultant colonies were examined for expression of SpaA regions by Western blotting with sera from pigs immunized with SpaA348.
TABLE 1.
Sequences and orientations of the PCR primers used for the cloning of four spaA gene fragments
| Amino acid residuesa | Primer name | Orientationb | Sequence (5′-3′) |
|---|---|---|---|
| 1- | F1 | Forward | 5′-TTTTGGATCCGATTCGACAGATATTTCTGTG-3′ |
| -89 | R89 | Reverse | 5′-TTAAGTCGACGGTGAATGTTTTGAAGACTC-3′ |
| -416 | R416 | Reverse | 5′-TTTTGTCGCTGAATTCGATTCGGGTTTTG-3′ |
| -594 | R594 | Reverse | 5′-CTATGTCGACCTTCCATCGTTCTTAAATGC-3′ |
| 272- | F272 | Forward | 5′-GATAGGATCCAAAGAGGGAAGAAAATACAG-3′ |
| -384 | R384 | Reverse | 5′-TTAAGTCGACAGGCTCTACTTTAGAGTCAC-3′ |
The target site is indicated by the positions of the residues corresponding to the amino acid sequence of the mature SpaA of strain Fujisawa.
Forward and reverse refer to the orientations of the coding and the complementary strands, respectively.
Expression of the recombinant protein in E. coli was induced with 2 mM isopropyl-β-d-thiogalactopyranoside at 37°C for 5 h. The resultant fusion protein was purified as instructed by the manufacturer (Qiagen) under denaturing conditions. Briefly, induced cells were resuspended in 6 M guanidine buffer at pH 8.0 and stirred for 1 h at room temperature. After centrifugation, the supernatant was filter sterilized, and the fusion protein was purified by affinity chromatography on a nickel nitrilotriacetic acid Sepharose column. The purified proteins were eluted at pH 4.5, dialyzed against 10 mM phosphate-buffered saline at pH 7.2, and kept at 4°C with 0.1% sodium azide. Fusion proteins were further examined for their ability to induce protection in immunized mice. Groups of four 4-week-old female mice (ddY outbred strain; Japan SLC, Hamamatsu, Japan) were immunized intramuscularly with two doses of 25 μg of purified protein mixed with Freund's complete adjuvant 2 weeks apart, and 2 weeks later they were challenged intradermally with 100 50% lethal doses of the Fujisawa strain. Mice immunized with fusion proteins other than SpaA113 showed no symptoms in response to challenge, and no E. rhusiopathiae organisms were isolated from their organs 1 week after the challenge. However, mice immunized with SpaA113 showed severe depression, and large numbers of E. rhusiopathiae organisms were isolated from the heart blood 1 week after the challenge.
Vaccines.
Three kinds of commercially available vaccines, lyophilized live vaccine (Nisseiken, Ohme, Japan), lysate vaccine (Intervet, Tokyo, Japan), and aluminum-adsorbed vaccine (Nisseiken), were used to immunize the pigs. Reconstituted live vaccine contained approximately 108.0 CFU of the Koganei strain 65-0.15 of E. rhusiopathiae per ml. Strain 65-0.15 is an attenuated acriflavine-resistant strain of serotype 1a (21). One dose of each vaccine was 1 ml. The pigs were immunized subcutaneously with one dose of live vaccine and were immunized intramuscularly with two doses of lysate vaccine, adsorbed vaccine, or SpaA348 3 to 4 weeks apart.
Isolation of bacteria from experimentally immunized and challenged pigs.
All experiments with animals described in this work complied with the relevant policies of our institutes. All surviving pigs except for the pigs used in experiment 1 were killed and examined by bacterial isolation 1 week or 12 days after challenge. The pigs used in Experiment 1 were examined 11 weeks after challenge. For bacterial isolation, brain heart infusion broth and agar (Difco) supplemented with 0.1% Tween 80, 0.3% Tris, 500 μg of kanamycin per ml, and 25 μg of gentamicin per ml (pH 7.8) (24) were used. Each 1 g of organ (heart, lung, liver, spleen, kidney, lymph nodes near the challenge site, and tonsils) was stamped on selective agar medium. Each organ was then cut into small pieces and cultivated in 10 ml of selective broth medium at 37°C for 48 h. After 24 and 48 h of incubation, the broth culture was streaked on selective agar medium and incubated at 37°C for 48 h. Suspected colonies of E. rhusiopathiae were identified by PCR (15) and serotyped by the agar gel precipitation test (22).
Serum samples collected from experimentally immunized and challenge-exposed pigs.
Pigs were immunized and challenge exposed in seven independent experiments, characterized in Table 2. Pigs with no maternal antibody were used in all experiments except experiment 7. In experiments 3 to 5, the pigs were immunized intramuscularly with two doses of 100 μg of SpaA348 mixed with Freund's complete adjuvant; however, two pigs in experiment 4 were immunized with two doses of 500 μg of SpaA348 mixed with Freund's complete adjuvant. Experiment 5 was carried out to test the ability of immunization with SpaA348 against serotype 1a to cross-protect pigs against challenge with a virulent strain of serotype 2b (9). Experiment 6 was conducted to determine the effect of PRRSV infection on immunization with live vaccine (18). Experiment 7 retrospectively revealed that all pigs had had some maternal antibody at the time of vaccination and challenge.
TABLE 2.
Characterization of experimental immunization and challenge exposure of pigs
| Expt no. (y) | Pig typea | Pig group (no.) | Age (wk) at immunization
|
Challenge
|
Systemic symptomb
|
Local skin lesionc | Isolation of E. rhusiopathiaed
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First | Second | Age (wk) | Strain | Dose (CFU/ml) | Depression | Skin lesion | Tonsil | Other organse | ||||
| 1 (1992) | SPF | Control (2) | —f | — | 10 | Fujisawa | 106.0 | +/++ | +/++ | +/+ | − | − |
| Live Vaccine (2) | 7 | — | 10 | Fujisawa | 108.0 | −/− | −/− | −/− | − | − | ||
| 2 (1997) | Conv. | Control (2) | — | — | 10 | Fujisawa | 107.3 | D/++ | ++/+ | +/+ | ++/(+) | ++/(+) |
| Lysate vaccine (1) | 4 | 8 | 10 | Fujisawa | 107.3 | − | − | − | − | − | ||
| Adsorbed vaccine (2) | 4 | 8 | 10 | Fujisawa | 107.3 | −/− | −/− | −/− | −/− | −/− | ||
| 3 (1999) | SPF | Control (2) | — | — | 12 | Fujisawa | 107.5 | D/++ | −/++ | −/− | ++/(+) | ++/(+) |
| Live Vaccine (2) | 4 | — | 12 | Fujisawa | 107.5 | −/− | −/− | −/− | −/− | −/− | ||
| SpaA348 (1) | 4 | 8 | 12 | Fujisawa | 107.5 | − | − | − | − | − | ||
| Lysate vaccine (2) | 4 | 8 | 12 | Fujisawa | 107.5 | −/− | −/− | −/− | −/− | −/− | ||
| Adsorbed vaccine (2) | 4 | 8 | 12 | Fujisawa | 107.5 | −/− | −/− | −/− | −/− | −/− | ||
| 4 (1996) | Conv. | Control (2) | — | — | 9 | Fujisawa | 107.6 | DD | +/++ | +/+ | ++/++ | ++/++ |
| SpaA348 (4) | 4 | 7 | 9 | Fujisawa | 107.6 | −/−/−/−/− | −/−/−/−/− | −/−/−/−/− | −/−/−/−/− | −/−/−/−/− | ||
| 5 (1998) | Conv. | Control (2) | — | — | 10 | 82-875 | 107.9 | +/++ | ++/+ | +/+ | ++/++ | (+)/+ |
| SpaA348 (2) | 4 | 8 | 10 | 82-875 | 107.9 | −/− | −/− | −/− | −/− | −/− | ||
| Live vaccine (4) | 4 | — | 10 | Fujisawa | 107.0 | −/−/−/− | −/−/−/− | +/−/+/+ | −/−/−/− | −/−−/− | ||
| Lysate vaccine (1) | 4 | 8 | 10 | Fujisawa | 107.0 | − | − | − | − | − | ||
| 6 (1995) | SPF | Control (5) | — | — | 10 | C42 | 105.5 | −/++/+/−/++ | −/++/−/−/++ | NT | −/+/+/+/− | −/+/−/−/++ |
| Live vaccine (5) | 7 | — | 10 | C42 | 105.5 | −/−/−/−/− | −/−/−/−/− | NT | −/−/−/−/− | −/−/−/−/− | ||
| Live vaccine (5) | 7 | — | 10 | PRRSV + C42 | 105.5 | −/−/−/−/− | −/−/−/−/− | NT | −/−/−/+/+ | −/−/−/−/− | ||
| Live vaccine (5) | 7 | — | 10 | C42 + PRRSV | 105.5 | −/−/+/−/−/− | −/++/+/−/− | NT | −/−/−/−/− | −/−/+/+/− | ||
| 7 (1998) | SPF | Control (6) | — | — | 7 | Fujisawa | 108.6 | −/−/−/−/− | −/−/−/−/− | +/+/+/+/+ | NT | −/−/−/−/− |
| Live vaccine (6) | 4 | — | 7 | Fujisawa | 108.6 | −/−/−/−/− | −/−/−/−/− | −/−/−/−/− | NT | −/−/−/−/− | ||
Secondary specific-pathogen-free (SPF) pigs or conventional (Conv.) pigs were immunized and challenged at the indicated ages.
The level of depression or generalized urticarial skin lesion is indicated for each pig, as follows: D, death; ++, severe; +, moderate; −; no symptom.
The presence of a local urticarial skin lesion at the site of injection is indicated for each pig, as follows: +, positive; −, negative; NT, not tested.
The isolation result for each pig is indicated as follows: ++, enormous numbers of colonies by direct culture; +, positive by direct culture; (+), positive by enrichment culture; −, negative by both methods; NT, not tested.
Heart, liver, lung, spleen, and kidney.
—, no immunization.
Serum samples from pigs reared on farms and nondomesticated wild boars.
Group 1 consisted of sera from 18 sows reared on two farms where pigs had not received swine erysipelas vaccine for a long period, since they supply piglets to several laboratories for experimental use. The animals on one farm were proved to be infected with E. rhusiopathiae because the piglets had medium to high antibody titers and five piglets harbored E. rhusiopathiae of serotype N in their tonsils. The animals on another farm were thought to be free from infection because none of the 23 piglets had maternal antibody and E. rhusiopathiae was not isolated. Group 2 consisted of serum samples collected from 6- to 7-month-old fattening pigs for 3 years, 10 pigs per year, just before the farm had been affected by acute swine erysipelas. The pigs on this farm had not received swine erysipelas vaccine for many years because it had had no problems with the disease. Group 3 consisted of serum samples from sows and fattening pigs reared on two farms that had no problems with swine erysipelas. On one farm, only sows were immunized with live vaccine once a year; fattening pigs were not vaccinated. On another farm, both sows and fattening pigs were immunized with live vaccine at about 70 days of age and sows were further immunized once a year. Group 4 consisted of serum samples from 284 sows and 144 fattening pigs ages 6 to 7 months reared on 10 farms. All sows and fattening pigs were immunized with one dose of live vaccine at about 2 months of age. Group 5 consisted of 104 serum samples from wild boars hunted in Saitama and Yamaguchi Prefectures, which are 800 km apart, from 1999 to 2000.
Indirect ELISA.
The reactivities of five recombinant SpaA fragments were compared by indirect ELISA, as follows. All incubation steps were done at room temperature. First, each well of a medium-binding ELISA plate (Immulon 200; Greiner) was coated with 100 μl of a 2.5-μg/ml dilution of the respective SpaA proteins in 0.05 M bicarbonate buffer at pH 9.6. After incubation for 1 h the plates were washed three times with 0.85% saline containing 0.05% Tween 20. Each well of the plates was blocked with 150 μl of 3% skim milk in 0.15 M phosphate-buffered saline (PBS) at pH 7.2 containing 0.05% Tween 20 (PBST) for 30 min. The plates were then washed, and 100 μl of a serum sample diluted 1:100 in 1% skim milk in PBST was applied to each well. The plates were incubated for 1 h and washed, and then 100 μl of horseradish peroxidase-conjugated goat anti-pig immunoglobulin G (heavy and light chains; Rockland) at a dilution of 1:28,000 was added to each well. The plates were incubated for 1 h and washed, and then 100 μl of 0.1 M disodium phosphate-0.05 M citric acid buffer at pH 4.5 containing 0.2 mg of tetramethylbenzidine per ml and 0.01% hydrogen peroxide was added to each well. The reaction was terminated after 30 min by adding 100 μl of 2 N sulfuric acid to each well. The absorbance at 450 nm was then monitored.
SpaA416 ELISA.
The SpaA416 ELISA was performed as described above. In preliminary experiments, the optimal SpaA416 concentration was determined to be 0.5 μg/ml for serum samples from pigs immunized with lysate vaccine and SpaA348 and 2.5 μg/ml for all other serum samples. The SpaA416 ELISA titer was shown by the absorbance of sample serum to absorbance of positive reference serum (S/P) ratio, which was calculated as follows: (sample absorbance − negative reference absorbance)/(positive reference absorbance − negative reference absorbance). Serum samples from nonimmunized control pigs were used as common negative reference samples, and serum samples from pigs experimentally immunized with live vaccine or lysate vaccine on the day of challenge exposure were used as positive reference samples for the 2.5- and 0.5-μg/ml antigen systems, respectively. The absorbances obtained with the negative reference serum sample were 0.01 to 0.07, and the absorbances obtained with these two positive reference serum samples were near 2.0 under the respective conditions for the positive reference samples.
Double-antibody sandwich ELISA.
The double-antibody sandwich ELISA was carried out as reported previously (9) with antigen extracted with 10 mM NaOH from cells of E. rhusiopathiae strain SE-9 cultivated in modified Feist broth (8). Briefly, each well of high-adsorption ELISA plates (Immulon 600; Greiner) was coated with 100 μl of rabbit anti-SpaA348 serum diluted 1:1,000 in 0.05 M bicarbonate buffer at pH 9.6. After incubation for 1 h, the plates were washed three times and incubated with 100 μl (per well) of alkaline-extracted antigen diluted 1:400 for 1 h. The plates were then incubated with pig sera diluted 1:100, anti-pig immunoglobulin G conjugated with horseradish peroxidase diluted 1:12,000, and substrate solution as in the indirect ELISA.
Latex agglutination test.
To compare the sensitivities and specificities of the different assays, sera were also assayed by the latex agglutination test with a commercially available kit (Nisseiken). In this kit, latex beads were sensitized with a crude alkaline extract of E. rhusiopathiae strain Tama of serotype 2. The test was performed according to the instructions provided by the manufacturer in 96-well V-bottom microplates. Briefly, 25 μl of a latex bead suspension was mixed with 25 μl of serially diluted serum. The plate was then sealed and incubated at 37°C overnight. The antibody titer was the maximum serum dilution that gave positive agglutination. The instruction manual claims that the latex agglutination titers correlate well with growth agglutination titers. Sawada et al. (20) reported that pigs with a growth agglutination titer of more than 1:8 in the early stage (on the 10th or 15th day) after immunization with live vaccine were protected against challenge.
RESULTS
Reactivities of the five recombinant SpaA fragments.
Of the five recombinant SpaA fragments, only SpaA416 and SpaA594 showed sufficient reactivities in the indirect ELISA even for pigs immunized with the live vaccine, and the reaction pattern was similar to that obtained by the double-antibody sandwich ELISA with intact antigen. SpaA89 and SpaA113 showed little reactivy. Because of the higher yield, SpaA416 was selected as the ELISA antigen for the following examination. The cutoff values of the SpaA416 ELISA titer in pigs under age 3 months were defined as 0.040 in the 0.5-μg/ml antigen system and 0.090 in the 2.5-μg/ml antigen system on the basis of the mean plus 3 standard deviations (SDs) for a total of 68 serum samples collected from 53 pigs on the day of immunization and 15 control pigs on the day of challenge in experiments 1 to 6.
Reactions of pigs immunized and challenged in experiments 1 to 5.
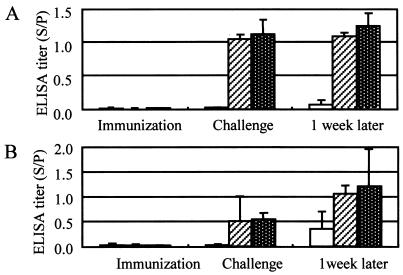
After challenge exposure, no immunized pigs showed any symptoms and no E. rhusiopathiae organisms were isolated from their organs. In contrast, all control pigs showed severe to moderate systemic symptoms, and E. rhusiopathiae was isolated from their organs. In experiment 1, even control pigs were negative for the isolation of E. rhusiopathiae, since they were treated and autopsied 11 weeks after challenge. The ELISA results agreed closely with the immunization and protection results (Fig. 1).
FIG. 1.
Results of the SpaA416 ELISA with the 0.5-μg/ml antigen (A) and the 2.5-μg/ml antigen (B) with sera from pigs immunized and challenged in experiments 1 to 5. The clear columns, hatched columns, and white-dotted columns represent the antibody titers (means and SDs) of nonimmunized control pigs (n = 10) and pigs immunized with the lysate vaccine (n = 3) and SpaA348 (n = 7), respectively (A), and the antibody titers of control pigs (n = 10) and pigs immunized with the live vaccine (n = 8) and the adsorbed vaccine (n = 5), respectively (B). Sera were collected on the day of the first immunization, the day of challenge exposure, and 1 week after challenge.
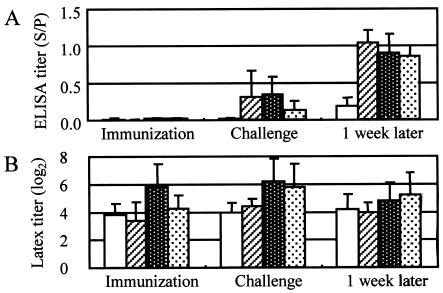
Effect of PRRSV infection on immunization with live vaccine.
PRRSV infection 1 week after vaccination significantly interfered (P = 0.097) with the antibody responses of the pigs in experiment 6 (Fig. 2). Although their titers were higher (P = 0.102) than those of the control group, they manifested milder symptoms than those of the control group because of the synergism of PRRSV proliferation and challenge exposure. The ELISA results correlated well with the results of immunization and protection. However, by the latex agglutination test, all control pigs had sufficiently high antibody titers to provide protection on the day of challenge exposure.
FIG. 2.
Results of the SpaA416 ELISA with the 2.5-μg/ml antigen (A) and the latex agglutination test (B) with sera from pigs immunized with the live vaccine with or without PRRSV infection and challenged in experiment 6. The clear columns, hatched columns, white-dotted columns, and black-dotted columns represent the antibody titers (means and SDs) of nonimmunized control pigs (n = 5), pigs immunized and not infected with PRRSV (n = 5), pigs immunized and infected with PRRSV 1 week before vaccination (n = 5), and pigs immunized and infected with PRRSV 1 week after vaccination (n = 5), respectively. Sera were collected on the day of immunization, on the day of challenge exposure, and 1 week after challenge.
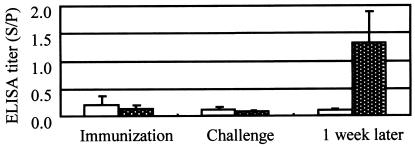
Effect of maternal antibody on immunization with live vaccine.
The ELISA titers decreased even after vaccination in both groups in experiment 7 and increased rapidly after challenge exposure only in the immunized group 1 week after challenge (Fig. 3). In this experiment, the systemic symptoms of the control pigs after challenge exposure were much milder than those observed in the pigs in experiments 1 to 6 because they had still small amounts of maternal antibody.
FIG. 3.
Results of the SpaA416 ELISA with the 2.5-μg/ml antigen with sera from pigs immunized with the live vaccine and challenge exposed in the presence of maternal antibody in experiment 7. The clear columns and white-dotted columns represent the antibody titers (means and SDs) of nonimmunized control pigs (n = 6) and pigs immunized with the live vaccine (n = 6), respectively. Sera were collected on the day of immunization, on the day of challenge, and 1 week after challenge.
SpaA416 ELISA results for pigs reared on farms and nondomesticated wild boars.
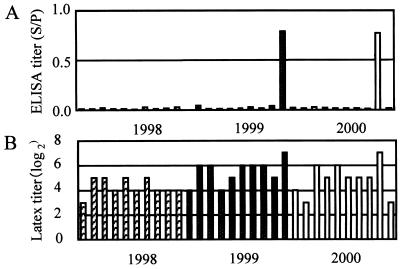
For group 1, the ELISA titers (means ± SDs) of sows at the infected farm (1.086 ± 0.637) were significantly (P = 0.0004) higher than the titers of sows at the infection-free farm (0.082 ± 0.030), although the pigs on both farms had not received the swine erysipelas vaccine for a long time. The ELISA titers of the sows closely reflected the infection status of the herds. For group 2, although the pigs had not been vaccinated for a long time, 2 of 30 serum samples had significantly (P = 0.030) high ELISA titers, and the titers of all other serum samples were negative (Fig. 4). This result suggests that these two pigs were carriers and that the others were highly sensitive individuals, because a mixture of a few carrier pigs and many highly sensitive pigs is essential for an outbreak of the acute septicemia type of swine erysipelas. However, the latex agglutination test results did not accurately describe the situation in the herd. For the first farm in group 3, where only sows were immunized with live vaccine every year and fattening pigs were not vaccinated, the sows had high ELISA titers and the fattening pigs had only maternal antibody (Fig. 5). For the second farm in group 3, where sows were immunized with live vaccine every year and fattening pigs were immunized with live vaccine at about 70 days of age, sows had high ELISA titers and fattening pigs had both maternal antibody and vaccine-derived antibody. The ELISA results closely reflected the vaccination programs on the respective farms. For group 4, although the mean ELISA titer for the sows and fattening pigs reared on 10 farms varied remarkably, they correlated well on the same farm and were closely similar (r = 0.9674; P = 0.690) (Fig. 6). The ELISA results clearly suggest that E. rhusiopathiae had been constantly transmitted from carrier sows to their progeny on the same farm and that the maternal antibody could not prevent the oral transmission of E. rhusiopathiae. For group 5, almost all sera from wild boars hunted in two prefectures had very high ELISA titers, and the means ± SDs for the respective groups were 0.979 ± 0.320 (n = 64) and 1.115 ± 0.457 (n = 40). This result suggests that in Japan, most nondomesticated wild boar are naturally infected with E. rhusiopathiae without manifesting any symptoms and are resistant to the acute type of swine erysipelas.
FIG. 4.
Results of the SpaA416 ELISA with the 2.5-μg/ml antigen (A) and the latex agglutination test (B) with sera from 6- to 7-month-old fattening pigs in 1998, 1999, and 2000, just before the farm experienced an epidemic of acute swine erysipelas in 2000. The hatched columns, white-dotted columns, and clear columns represent the antibody titers of sera collected in each of the three years, respectively (n = 10 per year).
FIG. 5.
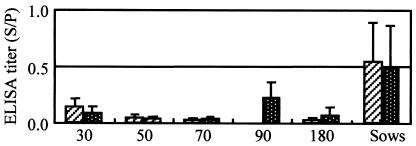
Results of the SpaA416 ELISA with the 2.5-μg/ml antigen with sera collected from fattening pigs of different ages and sows on two farms. On the first farm, fattening pigs were immunized with live vaccine at age 70 days and sows were immunized every year. On the second farm, fattening pigs were not immunized and only sows were immunized with the live vaccine every year. The hatched columns represent the antibody titers (means and SDs) for sows (n = 15) and fattening pigs at about 30, 50, 70, and 180 days of age (n = 8 at each age) on the first farm; and the white-dotted columns represent the antibody titers (means and SDs) for sows (n = 12) and fattening pigs at about 30, 50, 70, 90, and 180 days of age (n = 8 at each age) on the second farm.
FIG. 6.
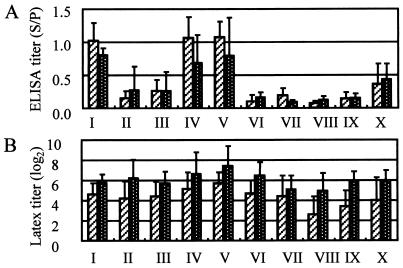
Results of the SpaA416 ELISA with the 2.5-μg/ml antigen (A) and the latex agglutination test (B) with sera from sows and 6- to 7-month-old fattening pigs reared on 10 farms where all sows and fattening pigs were immunized with live vaccine at about 2 months of age. The hatched columns represent the antibody titers (means and SDs) in sera from sows (n = 58, 59, 27, 31, 35, 15, 26, 12, 26, and 10 on farms I to X, respectively), and the white-dotted columns represent the antibody titers (means and SDs) in sera from fattening pigs (n = 15, 20, 15, 15, 15, 11, 15, 15, 16, and 15 on farms I to X, respectively).
DISCUSSION
Many reports have found that the 64- to 66-kDa protein of E. rhusiopathiae is the major protective antigen (1, 6, 7, 8, 9, 11, 12, 13, 14, 19). Galan and Timoney (6) are thought to have been the first to succeed in the cloning and expression of this gene from a highly virulent strain of serotype 1a; however, they did not report the gene sequence. Makino et al. (14) cloned the gene from a serotype 2 strain and named the protein SpaA (surface protective antigen A); however, they reported that a 20-amino-acid repeat region at the C terminus was essential for protection in mice. Imada et al. (9) also cloned a gene encoding the 69-kDa SpaA protein from a highly virulent strain of serotype 1a and were the first to show that 348 amino acids (amino acid residues 61 to 408 of mature SpaA) at the N terminus could elicit complete protection in pigs against challenge with virulent strains of serotypes 1a and 2b. Serotypes 1 and 2 are the most important in swine erysipelas (22, 26), and the cross-protection seen between these serotypes could be explained by the highly conserved amino acid sequences seen in the protective region of SpaA in all five strains of serotypes 1 and 2 (9).
The antibody responses of pigs immunized with truncated recombinant SpaA were sensitively detected by the indirect SpaA348 ELISA and also by the double-antibody sandwich ELISA with rabbit anti-SpaA348 as the capture antibody and an alkaline extract of E. rhusiopathiae cells as the antigen (9). Although rabbit antiserum against SpaA348 enhanced in vitro phagocytosis and the killing of a virulent strain of E. rhusiopathiae by swine neutrophils (9), the sensitivity of the SpaA348 ELISA was insufficient for the detection of protective antibody in pigs immunized with the live vaccine.
To overcome the problem, in this study, we constructed and compared five regions of the SpaA protein and found that truncated SpaA416 and an almost full-size SpaA protein, SpaA594, had sufficient reactivities in the indirect ELISA for the detection of protective antibody in pig sera. Because of its higher yield, SpaA416 was selected as the ELISA antigen. In pigs experimentally immunized with vaccines and subsequently exposed to challenge, the SpaA416 ELISA titers exactly mirrored the immunization patterns, and the titers at the time of challenge correlated well with the protection results. The sensitivity and specificity of the SpaA416 ELISA were also confirmed with many kinds of sera in terms of immunization status from pigs on conventional farms.
Latex agglutination kits have recently became commercially available and have been widely used in Japan as an alternative to the growth agglutination test. However, many nonimmunized control pigs in experiments 1 to 6 that showed severe symptoms after experimental challenge exposure had sufficient titers, according to the latex agglutination test, for protection on the day of challenge. In addition, it could not warn of the dangerous status of a herd shortly before it suffered an epidemic of the acute septicemia type of swine erysipelas. When these results are taken into account, it must be concluded that the specificity of the latex agglutination kit was not sufficiently high.
Many ELISA methods for the detection of antibody against E. rhusiopathiae have been reported, and most of them use crude antigens such as sonicated bacterial cell suspensions (2, 16), sodium dodecyl sulfate extract (10), and alkaline extract (9, 17). In contrast, some use semipurified antigens such as gel filtration fractions of autoclaved extract (4) and the 64- to 67-kDa protein separated from the alkaline extract by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (19) or Western blotting (1). Although the purified 64- to 67-kDa protein appears to work specifically, it is difficult to prepare enough purified protein by these methods. In general, recombinant antigens have great advantages over intact antigens: it is easy to prepare large amounts of purified antigen of consistent quality; one can use a desired single antigen or multiple antigens for serological testing and detect specific antibodies against them (3, 5, 25); and, moreover, purified antigen is usually more specific than crude antigen.
We developed a SpaA416 ELISA that used the truncated recombinant surface protective antigen of E. rhusiopathiae for the assay of protective antibody in pig sera. The specificity and sensitivity of the SpaA416 ELISA were confirmed by the use of numerous kinds of sera collected from experimentally immunized and challenge-exposed pigs and sera collected from pigs reared on different types of farms. The results of the SpaA416 ELISA closely mirrored the immunization and protection results and the infection status of the herds. These results indicate that the SpaA416 ELISA has the potential for use as an effective tool not only for herd management on farms but also for the quality control of live and inactivated vaccines in laboratories.
Acknowledgments
We thank Yohko Nagayama, Shohko Suzuki, Kiyohito Nishimoto, and Tohru Yoshida for providing sera from pigs reared on farms and from wild boars. We also thank Akira Taneno for providing the lysate vaccine before it became commercially available.
REFERENCES
- 1.Chin, J. C., B. Turner, and G. J. Eamens. 1992. Serological assay for swine erysipelas using nitrocellulose particles impregnated with an immunodominant 65-kDa antigen from Erysipelothrix rhusiopathiae. Vet. Microbiol. 31:169-180. [DOI] [PubMed] [Google Scholar]
- 2.Dahms, H., W. F. Schilow, and G. Hagemann. 1989. Detection of Erysipelothrix rhusiopathiae specific antibodies in the serum of experimentally infected swine by ELISA and immunoblotting. Arch. Exp. Veterinarmed. 43:907-916. (In German.) [PubMed] [Google Scholar]
- 3.Davis, B. S., G. J. Chang, B. Cropp, J. T. Roehrig, D. A. Martin, C. J. Mitchell, R. Bowen, and M. L. Bunning. 2001. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J. Virol. 75:4040-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eamens, G. J., J. C. Chin, and P. J. Nicholls. 1989. Comparison of inoculation regimes for the experimental production of swine erysipelas arthritis. II. Serological findings in a gel diffusion precipitin test and enzyme-linked immunosorbent assay. Aust. Vet. J. 66:216-220. [DOI] [PubMed] [Google Scholar]
- 5.Ferreira, A. W., Z. R. Belem, E. A. Lemos, S. G. Reed, and A. Campos-Neto. 2001. Enzyme-linked immunosorbent assay for serological diagnosis of Chagas' disease employing a Trypanosoma cruzi recombinant antigen that consists of four different peptides. J. Clin. Microbiol. 39:4390-4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galan, J. E., and J. F. Timoney. 1990. Cloning and expression in Escherichia coli of a protective antigen of Erysipelothrix rhusiopathiae. Infect. Immun. 58:3116-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groschup, M. H., K. Cussler, R. Weiss, and J. F. Timoney. 1991. Characterization of a protective protein antigen of Erysipelothrix rhusiopathiae. Epidemiol. Infect. 107:637-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groschup, M. H., and J. F. Timoney. 1990. Modified Feist broth as a serum-free alternative for enhanced production of protective antigen of Erysipelothrix rhusiopathiae. J. Clin. Microbiol. 28:2573-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imada, Y., N. Goji, H. Ishikawa, M. Kishima, and T. Sekizaki. 1999. Truncated surface protective antigen (SpaA) of Erysipelothrix rhusiopathiae serotype 1a elicits protection against challenge with serotypes 1a and 2b in pigs. Infect. Immun. 67:4376-4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirchhoff, H., H. Dubenkropp, G. Kerlen, H. W. Steffens, W. Hermanns, G. Trautwein, and K. H. Böhm. 1985. Application of the indirect enzyme immunoassay for the detection of antibodies against Erysipelothrix rhusiopathiae. Vet. Microbiol. 10:549-559. [DOI] [PubMed] [Google Scholar]
- 11.Kitajima, T., E. Oishi, K. Amimoto, S. Ui, H. Nakamura, K. Oda, S. Katayama, A. Izumida, and Y. Shimizu. 2000. Quantitative diversity of 67 kDa protective antigen among serovar 2 strains of Erysipelothrix rhusiopathiae and its implication in protective immune response. J. Vet. Med. Sci. 62:1073-1077. [DOI] [PubMed] [Google Scholar]
- 12.Kitajima, T., E. Oishi, K. Amimoto, S. Ui, H. Nakamura, N. Okada, O. Sasaki, and H. Yasuhara. 1998. Protective effect of NaOH-extracted Erysipelothrix rhusiopathiae vaccine in pigs. J. Vet. Med. Sci. 60:9-14. [DOI] [PubMed] [Google Scholar]
- 13.Lachmann, P. G., and H. Deicher. 1986. Solubilization and characterization of surface antigenic components of Erysipelothrix rhusiopathiae T28. Infect. Immun. 52:818-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makino, S., K. Yamamoto, S. Murakami, T. Shirahata, K. Uemura, T. Sawada, H. Wakamoto, and Y. Morita. 1998. Properties of repeat domain found in a novel protective antigen, SpaA, of Erysipelothrix rhusiopathiae. Microb. Pathog. 25:101-109. [DOI] [PubMed] [Google Scholar]
- 15.Makino, S., Y. Okada, T. Maruyama, K. Ishikawa, T. Takahashi, M. Nakamura, T. Ezaki, and H. Morita. 1994. Direct and rapid detection of Erysipelothrix rhusiopathiae DNA in animals by PCR. J. Clin. Microbiol. 32:1526-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molin, G., O. Söderlind, J. Ursing, V. Nørrung, A. Ternström, and C. Löwenhielm. 1989. Occurrence of Erysipelothrix rhusiopathiae on pork and in pig slurry, and the distribution of specific antibodies in abattoir workers. J. Appl. Bacteriol. 67:347-352. [DOI] [PubMed] [Google Scholar]
- 17.Rosskopf-Streicher, U., S. Johannes, M. Wilhelm, and K. Cussler. 2001. Quality control of inactivated erysipelas vaccines: results of an international collaborative study to establish a new regulatory test. Vaccine 19:1477-1483. [DOI] [PubMed] [Google Scholar]
- 18.Sakano, T., I. Shibata, T. Namimatsu, M. Mori, M. Ono, K. Uruno, and T. Osumi. 1997. Effect of attenuated Erysipelothrix rhusiopathiae vaccine in pigs infected with porcine reproductive respiratory syndrome virus. J. Vet. Med. Sci. 59:977-981. [DOI] [PubMed] [Google Scholar]
- 19.Sato, H., Y. Yamazaki, K. Tsuchiya, T. Aoyama, N. Akaba, T. Suzuki, A. Yokoyama, H. Saito, and N. Maehara. 1998. Use of the protective antigen of Erysipelothrix rhusiopathiae in the enzyme-linked immunosorbent assay and latex agglutination. Zentbl. Veterinarmed. B 45:407-420. [DOI] [PubMed] [Google Scholar]
- 20.Sawada, T., M. Muramatsu, and K. Seto. 1979. Response of growth agglutinating antibody and protection of pigs inoculated with swine erysipelas live vaccine. Jpn. J. Vet. Sci. 41:593-600. [DOI] [PubMed] [Google Scholar]
- 21.Seto, K., Y. Nishimura, M. Fujiki, H. Azechi, and K. Suzuki. 1971. Studies on acriflavin-fast attenuated Erysipelothrix insidiosa. Comparison on pathogenicity and immunogenicity between mice and pigs. Jpn. J. Vet. Sci. 33:161-171. (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 22.Takahashi, T., N. Nagamine, M. Kijima, S. Suzuki, M. Takagi, Y. Tamura, M. Nakamura, M. Muramatsu, and T. Sawada. 1996. Serovars of Erysipelothrix strains isolated from pigs affected with erysipelas in Japan. J. Vet. Med Sci. 58:587-589. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi, T., T. Fujisawa, Y. Tamura, S. Suzuki, M. Muramatsu, T. Sawada, Y. Benno, and T. Mitsuoka. 1992. DNA relatedness among Erysipelothrix rhusiopathiae strains representing all twenty-three serovars and Erysipelothrix tonsillarum. Int. J. Syst. Bacteriol. 42:469-473. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi, T., K. Zarkasie, S. Mariana, Sumadi, and M. Ogata. 1989. Serological and pathogenic characterization of Erysipelothrix rhusiopathiae isolates from tonsils of slaughter pigs in Indonesia. Vet. Microbiol. 21:165-175. [DOI] [PubMed] [Google Scholar]
- 25.Wellman, G. 1955. Die subklinische Rotlaufinfektion und ihre Bedeutung fur die Epidemiologie des Schweinerotlaufs. Zentbl. Bakteriol. Parasitenkd. Infektkrankh. Hyg. Abt. 1 Orig. Reihe A 162:265-274. (In German.) [PubMed] [Google Scholar]
- 26.Wood, R. L. 1992. Erysipelas, p. 475-486. In A. D. Leman et al. (ed.), Diseases of swine. Iowa State University Press, Ames.
- 27.Yamasaki, H., K. Araki, P. K. C. Lim, N. Zasmy, J. W. Mak, R. Taib, and T. Aoki. 2000. Development of a highly specific recombinant Toxocara canis second-stage larva excretory-secretory antigen for immunodiagnosis of human toxocariasis. J. Clin. Microbiol. 38:1409-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]