Abstract
Glycoprotein G-based herpes simplex virus type 2 (HSV-2) enzyme-linked immunosorbent assays from Focus and Kalon were performed with specimens from 118 patients with culture-documented genital herpes episodes, and their results were compared. Sensitivity was 52% by Kalon and 86% by Focus for first HSV-2 episodes and 100% (for each of the two tests) for recurrent HSV-2. Median times to seroconversion were 120 days by the Kalon assay, 21 days by the Focus assay, and 68 days by Western blotting assay. Values for specificity were 100% (Kalon) and 93% (Focus).
The Centers for Disease Control and Prevention's Sexually Transmitted Disease Treatment Guidelines 2002 (6) recommends that tests based on glycoprotein G-2 (gG-2) be used for diagnosis of genital herpes. Recently only three such products on the market are cleared by the Food and Drug Administration to diagnose HSV-2: HerpeSelect HSV-2 ELISA and HerpeSelect Immunoblot IgG (both from Focus Technologies, Cypress, Calif.) and POCkit-HSV-2 (Diagnology, Belfast, Northern Ireland [ceased production in September 2003]) (1). The Focus HSV-2 enzyme-linked immunosorbent assay (ELISA) is a standard, automatable immunoassay that is cost-effective for most laboratories. However, concerns have been raised over the specificity of Focus HSV-2 ELISA for sera from African countries (E. Van Dyck, A. Buvé, D. Brown, and M. Laga, 17th INSTI, abstr. T079 ISSTDR, 2001).
A recombinant gG-2-based test from Kalon Biological Ltd (Kalon, Aldershot, United Kingdom) is used in the United Kingdom and Europe, is commercially available in the United States, and reportedly has higher specificity than the Focus HSV-2 ELISA (Focus) when the two assays are compared to a third type-specific assay (Van Dyck et al., 17th INSTI). Kalon and Focus tests are nearly identical 96-well, indirect ELISAs, with the main differences being serum dilution (1:20 for Kalon and 1:101 for Focus) and enzyme substrate. This study was designed to compare the Kalon and Focus HSV-2 ELISAs by using clinical and virological evidence of infection as a “gold standard” rather than a comparator serology. Samples from HSV-2-infected persons were used to determine sensitivity, while samples from HSV-1-infected, HSV-2 seronegative persons were used to determine specificity.
Sera were obtained from patients with four distinct types of episodes (7): Group 1 comprised 16 patients with primary episodes. All had culture-positive HSV-2 lesions, none had detectable antibodies to either HSV-1 or HSV-2 by Western blotting (WB) at lesion onset, and all seroconverted to HSV-2 by WB, considered a gold standard for sensitivity (5). Fifty sequential sera (median, 3 per patient; range, 2 to 4) were tested. Group 2 comprised 14 patients with nonprimary first episodes who had culture-positive HSV-2 lesions and antibodies only to HSV-1 at lesion onset. Forty-nine sequential sera (median, 3 per patient; range, 3 to 5) were tested. All 14 were found to have seroconverted to HSV-2 by WB, although 4 became positive after this study was completed. Group 3 comprised one serum each from 30 HSV-2 seropositive persons with recurrent culture-positive genital episodes. Group 4 comprised one serum each from 58 patients with recurrent episodes of HSV-1; all had HSV-1 antibodies but were HSV-2 seronegative by WB. Seroconversion time in Groups 1 and 2 was defined as days from onset to first positive serum by a given test. Equivocal test results were considered negative for the percent seroconversion calculations and were omitted for sensitivity calculations.
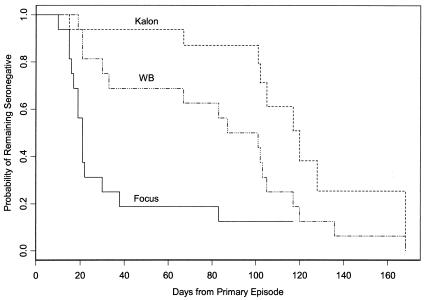
Median time to seroconversion in serum sets from primary episodes (Group 1) was significantly longer by paired Prentice-Wilcoxon test for the Kalon test (120 days) than for Focus (21 days; P < 0.001) or WB (87 days; P = 0.004) (Fig. 1). Seven (44%) of the 16 subjects did not become seropositive during follow-up by Kalon, whereas 2 (13%) did not become positive during follow-up by Focus. All 16 seroconverted by WB. Of 16 subjects, 9 seroconverted by all 3 tests; 4 seroconverted by only Focus and WB, and 1 by only WB. An additional patient seroconverted by Focus and WB but was equivocal by Kalon, while the final patient seroconverted by only WB with an equivocal result by Focus. Sensitivity for seroconversion was 9 of 15 (60%) for Kalon, 14 of 15 (93%) for Focus, and 100% for WB (Table 1).
FIG. 1.
Time to seroconversion following onset of genital infection in HSV-1-seronegative, HSV-2-seronegative patients (primary episode). Note that censored data (the day from onset that a subject remained seronegative when he/she left the study) affect the curves. Thus, seven subjects failed to seroconvert by Kalon at their latest serum postonset (days 66 to 136) and were dropped from further consideration. Of the remaining subjects, the final one seroconverted by Kalon at day 168. Only one subject who was still eligible failed to seroconvert by day 117 by Focus and was censored at the point where the curve is truncated.
TABLE 1.
Sensitivity and specificity of Kalon and Focus ELISAs for sera from culture-documented and WB-confirmed genital herpes episodes
| Group no. | Patient status at onseta | No. of patients | Median no. of days postonset (range)b | Kalon
|
Focus
|
WB sensitivity (%)e | ||
|---|---|---|---|---|---|---|---|---|
| Sensitivity (%)c | Specificity (%)d | Sensitivity (%) | Specificity (%) | |||||
| 1 | First HSV-2 episode; HSV-1 and HSV-2 seronegative | 16 | 104 (66-168) | 9/15 (60%) | NAf | 14/15 (93%) | NA | 16/16 (100%) |
| 2 | First HSV-2 episode; HSV-1 seropositive, HSV-2 seronegative | 14 | 81 (47-193) | 6/14 (43%) | NA | 10/13 (77%) | NA | 10/14 (71%) |
| 3 | Recurrent HSV-2 episode; HSV-1 seronegative, HSV-2 seropositive | 30 | 68 (11-150) | 30/30 (100%) | NA | 30/30 (100%) | NA | NA |
| 4 | Recurrent HSV-1 episode; HSV-1 seropositive, HSV-2 seronegative | 58 | 67 (0-541) | NA | 58/58 (100%) | NA | 54/58 (93%) | NA |
Genital herpes status was determined by monoclonal antibody typing of the HSV isolate cultured from the presenting lesion. Seropositivity for HSV-1 or HSV-2 was determined at the onset of the lesion by WB. All Group 1 and 2 subjects eventually seroconverted to HSV-2 by WB.
Days from presenting episode to seroconversion for patients who became positive or last available sample for those who remained seronegative.
Sensitivity percent was 100 times the number of positive results for infected patients divided by the sum of the number of positive results for infected patients plus the number of negative results for infected patients. Culture status was used as the gold standard for infected persons. All infected persons had HSV-2 cultured from lesions. Sera with equivocal test results were excluded.
Specificity percent was 100 times the number of negative results for uninfected patients divided by the sum of the number of negative results for uninfected patients plus the number of positive results for uninfected patients. HSV-2-uninfected status was determined by culture (HSV-1 only was isolated from the lesion) and WB (HSV-1 positive but HSV-2 negative).
WB sensitivity was determined only for Groups 1 and 2 in sera drawn after the first episode. Groups 3 and 4 were preselected, in part, by WB results.
NA, not available.
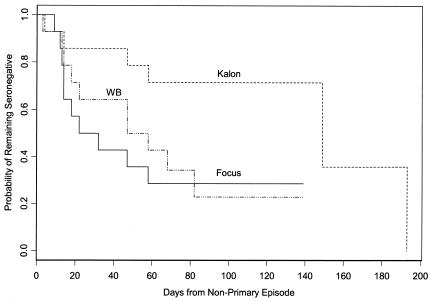
Median time to seroconversion in serum sets from nonprimary first episodes of HSV-2 (Group 2) was significantly longer by Kalon (149 days) than by Focus (22 days) or WB (47 days; P = 0.03 for both comparisons) (Fig. 2). Eight (57%) of the 14 subjects failed to show seroconversion by Kalon versus 4 each by Focus and WB (29%). Of 14 subjects, 6 seroconverted by all three tests, 3 seroconverted by only Focus and WB, 1 seroconverted by WB only, 1 seroconverted by Focus only, and 2 failed to seroconvert in any of the three tests. The final subject was negative by Kalon and WB but equivocal by Focus in the last serum.
FIG. 2.
Time to seroconversion following onset of genital HSV-2 infection in HSV-1-seropositive, HSV-2-seronegative patients (nonprimary episode). Note that these survival curves include censored data (when the latest serum available remained seronegative). For example, samples from eight subjects drawn 58 to 139 days postonset were negative by Kalon and were, therefore, censored from further analyses. At day 193 the sixth eligible subject seroconverted by Kalon. Day 139 was the last day for which subjects were neither eligible nor seropositive by Focus or WB.
Overall sensitivity for seroconversion (Groups 1 and 2) was 15 of 29 (52%) for Kalon, 24 of 28 (86%) for Focus, and 26 of 30 (87%) for WB. Median follow-up time for Kalon nonseroconverters was 86 days (range, 58 to 139), while median follow-up time for Kalon seroconverters was over 3 months (median, 105 days; range, 47 to 193). Sensitivity for all three tests was lower in nonprimary than in primary patients (Table 1) in contrast to findings with other gG-2 assays (3, 8), possibly due to the fact that follow-up time was shorter for nonprimary subjects (median, 81 days) than for primary patients (median, 104 days).
Thirty subjects from Group 3 had their sera drawn within 6 weeks of a culture-documented recurrence of HSV-2 and were HSV-2 seropositive by WB. Twenty-four had a history of when their first episode occurred; their length of time with HSV-2 infection was a median of 7.5 years (range, 1 to 31 years). All 30 (100%) were positive by the Kalon and Focus tests, suggesting that both are sensitive for antibodies in patients with established infection.
Of 58 sera from Group 4 patients with culture-documented recurrent HSV-1 episodes and only HSV-1 antibodies by WB, all were negative for HSV-2 antibodies by Kalon, for a specificity of 100% (Table 1). Fifty-four were negative for HSV-2 by the Focus test, giving a specificity (93%) slightly lower than that previously published in a study using WB as a comparator test (2). The four Focus positive sera from Group 4 had index values barely above the cutoff of 1.10 in the Focus test (median, 1.28; range, 1.21 to 1.81). These four sera could represent false-positive Focus test results or inaccurate culture typing. Alternatively, the patients could have been seroconverting to HSV-2. Previous studies have shown that patients can become positive by Focus before they seroconvert by WB (4). Further testing by more-refined methods might help resolve this discordance (9).
In summary, while both Kalon and Focus were accurate in detecting established HSV-2 infection, the Kalon gG-based HSV-2 serology was very insensitive for the detection of antibody to early HSV-2 infection. One-half did not seroconvert by 4 months or more, and only 5 of 30 had seroconverted by 2 months after infection, which is far slower than Focus, WB (4), or POCKit (3). If possible, Focus or POCKit should be used to test patients who may have been recently infected; if tested and found negative by Kalon, such patients should be retested by one of these gG-based tests (1).
Acknowledgments
Sera and patient data were collected by Lawrence Corey and Anna Wald.
This work was supported by Public Health Service Grant AI 130731 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Ashley, R. L. 2001. Sorting out the new HSV type specific antibody tests. Sex. Transm. Infect. 77:232-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashley, R. L. 2002. Performance and use of HSV type specific serology test kits. Herpes 9:38-45. [PubMed] [Google Scholar]
- 3.Ashley, R. L., M. Eagleton, and N. Pfeiffer. 1999. Ability of a rapid serology test to detect seroconversion to herpes simplex virus type 2 glycoprotein G soon after infection. J. Clin. Microbiol. 37:1632-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashley, R. L., E. Krantz, and A. Wald. 2003. Time course of seroconversion by HerpeSelect ELISA after acquisition of genital herpes simplex virus type 1 (HSV-1) or HSV-2. Sex. Transm. Dis. 30:310-314. [DOI] [PubMed] [Google Scholar]
- 5.Ashley, R. L., J. Militoni, F. Lee, A. Nahmias, and L. Corey. 1988. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J. Clin. Microbiol. 26:662-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2002. Sexually transmitted disease treatment guidelines 2002. Morb. Mortal. Wkly. Rep. 51:1-77. [Google Scholar]
- 7.Corey, L., H. G. Adams, Z. A. Brown, and K. K. Holmes. 1983. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann. Intern. Med. 98:958-972. [DOI] [PubMed] [Google Scholar]
- 8.Hashido, M., F. K. Lee, S. Inouye, and T. Kawana. 1997. Detection of herpes simplex virus type-specific antibodies by an enzyme-linked immunosorbent assay based on glycoprotein G. J. Med. Virol. 543:319-323. [DOI] [PubMed] [Google Scholar]
- 9.Hogrefe, W., X. Su, J. Song, R. Ashley, and L. Kong. 2002. Detection of herpes simplex virus 2 immunoglobulin G antibodies in African sera by using recombinant gG2, Western blotting, and gG2 inhibition. J. Clin. Microbiol. 40:3635-3640. [DOI] [PMC free article] [PubMed] [Google Scholar]