Abstract
Human immunodeficiency virus type 1 (HIV-1) infects cells of the monocyte/macrophage lineage. While infection of macrophages by HIV-1 is generally not cytopathic, it does impair macrophage function. In this study, we examined the effect of HIV-1 infection on intracellular signaling in human monocyte-derived macrophages (MDM) stimulated with the growth factor granulocyte-macrophage colony-stimulating factor (GM-CSF). GM-CSF is an important growth factor for cells of both the macrophage and granulocyte lineages and enhances effector functions of these cells via the heterodimeric GM-CSF receptor (GM-CSFR). A major pathway which mediates the effects of GM-CSF on macrophages involves activation of the latent transcription factor STAT5A via a Janus kinase 2 (JAK2)-dependent pathway. We demonstrate that GM-CSF-induced activation of STAT5A is inhibited in MDM after infection in vitro with the laboratory-adapted R5 strain of HIV-1, HIV-1Ba-L, but not after infection with adenovirus. HIV-1 infection of MDM did not decrease the STAT5A or JAK2 mRNA level or STAT5A protein level or result in increased constitutive activation of STAT5A. Surface expression of either the α-chain or common βc-chain of GM-CSFR was also unaffected. We conclude that HIV-1 inhibits GM-CSF activation of STAT5A without affecting expression of the known components of the signaling pathway. These data provide further evidence of disruption of cellular signaling pathways after HIV-1 infection, which may contribute to immune dysfunction and HIV-1 pathogenesis.
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a 22-kDa cytokine that promotes the growth and differentiation of cells of monocyte and granulocyte lineages. GM-CSF also enhances the effector functions of macrophages, including their phagocytic capacity as well as their antiparasitic and antimycobacterial activity (reviewed in reference 3). The binding of GM-CSF to its receptor (GM-CSFR) triggers a biochemical cascade necessary to relay information required for these functions. GM-CSFR comprises a low-affinity GM-CSF-specific α-subunit (Kd of ∼2.7 nM) and a βc-subunit, shared by interleukin-3 (IL-3) and IL-5, which together with the α-subunit confers high-affinity binding (Kd of ∼170 pM) (15). This high-affinity binding between GM-CSF and the βc-chain is dependent on glutamine 21 within the first α-helix of GM-CSF (24).
As the βc-chain lacks intrinsic kinase activity, receptor binding triggers receptor-associated kinases that mediate the phosphorylation of cytoplasmic proteins. Activation of the Janus kinase (JAK) family and the subsequent activation of signal transducers and activators of transcription (STAT) are the most studied pathways activated in response to GM-CSF and involve members of each family that differ according to cell type (1, 2, 7, 8, 29). Janus kinase 2 (JAK2) protein binds constitutively to an intracellular proline-rich motif of the βc-chain of GM-CSFR, termed box 1, and is phosphorylated after engagement and oligomerization of GM-CSFR in response to GM-CSF (27). This phosphorylation is necessary for activation of JAK2 enzyme activity and leads to phosphorylation of eight tyrosine residues on the βc-chain (35). The latent transcription factors STAT5A and STAT5B (STAT5A and STAT5B together make up STAT5) are recruited to the activated receptor complex (32) and phosphorylated on tyrosine residues 694 and 699, respectively. STAT5 then forms dimers between the phosphorylated tyrosine of one molecule and the STAT5 SH2 (Src homology 2) domain of another and dissociates from the receptor. Subsequently, STAT5 translocates to the nucleus and promotes GM-CSF-activated transcription of genes, such as A1 and CIS (11). In primary monocytes and macrophages, STAT5A is the STAT5 isoform predominantly activated in response to GM-CSF (29).
Previous investigations have shown that human immunodeficiency virus type 1 (HIV-1) infection of peripheral blood mononuclear cells (PBMCs) can alter the levels and/or activation of STAT5 (6, 26). Additionally, infection of CD4+ T cells with strain X4 of HIV-1 (HIVNL4.3) inhibits STAT5 activation in response to IL-2 (30). These data suggest that aberrant STAT signaling occurs in HIV-infected T cells, contributing to T-cell dysfunction. It has not been shown whether immediate signaling in response to cytokine stimulation of MDM is disrupted by infection with HIV-1.
Given the importance of GM-CSF to macrophage function and the reported effects of HIV-1 infection on STAT5 activity, we have examined the effect of in vitro HIV-1 infection of human MDM on GM-CSF-induced STAT5A activation in order to determine whether HIV-1 infection may inhibit macrophage function by impairing GM-CSF signaling. Our study shows that GM-CSFR βc-chain-dependent activation of STAT5A is specifically inhibited in HIV-1-infected human MDM. This may contribute to defective macrophage function in HIV-infected individuals.
MATERIALS AND METHODS
Isolation and culture of monocytes.
Human monocytes were isolated from buffy coats of HIV-seronegative blood donors (supplied by the Red Cross Blood Bank, Melbourne, Australia) by Ficoll-Paque density gradient centrifugation and adherence to plastic as previously described (9). Cells were cultured in Iscove's modified Dulbecco medium (Cytosystem, Castle Hill, Australia) supplemented with 10% heat-inactivated human AB+ serum, 2 mM l-glutamine, and 24 μg of gentamicin per ml (supplemented Iscove's medium). Monocytes were cultured adherent to plastic in 10-cm-diameter plates (Costar, Cambridge, Mass.) at a concentration of 5 × 106 cells/plate or, where indicated, in suspension in polytetrafluorethylene (Teflon) pots (Savillex, Minnetonka, Minn.) at a concentration of 1 × 106 cells/ml. Since endotoxin contamination has been shown to alter HIV-1 replication in MDM, culture supernatants and GM-CSF stocks were tested for lipopolysaccharide levels using the Limulus amebocyte lysate assay (Biowhitaker, Walkersville, Md.).
HIV-1 and adenoviral infection of MDM.
The M-tropic strain of HIV-1Ba-L (the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Disease, National Institutes of Health, Bethesda, Md.) was amplified in PBMCs. Briefly, PBMCs stimulated with phytohemagglutinin (PHA) (10 μg/ml; Murex Diagnostics, Dartford, United Kingdom) for 3 days were infected with HIV-1Ba-L and cultured in RPMI 1640 medium containing 10% heat-inactivated human AB+ serum and IL-2 (10 U/ml; Boehringer, Mannheim, Germany). The culture supernatants collected over time were stored at −70°C, and thawed and clarified using 0.2-μm-pore-size filters (Schleicher & Schuell) immediately before use. Monocytes were cultured on tissue culture plates or in Teflon pots and infected at a multiplicity of infection (MOI) of 0.1 to 1 for 4 h as described previously (18). It was observed that infection of cells cultured in Teflon pots generally gave lower levels of HIV-1 replication as measured by reverse transcriptase (RT) analysis. MDM were infected after 4 to 6 days in culture and cultured for 7 days after infection. Under these conditions, HIV-1 infection of MDM was not associated with decreased viability of the cells as assessed by trypan blue exclusion, loss of MDM, or morphological changes. Uninfected MDM prepared simultaneously from monocytes from the same donor were used as controls for each experiment.
An adenovirus vector, Ad-HIVΔTARluc, was a kind gift from Jonathan Axelrod (Department of Virology, The Hebrew University-Hadassah Medical School, Jerusalem, Israel) (4). This vector contains the luciferase gene expressed under the control of an HIV-1 long terminal repeat promoter lacking the TAR element such that luciferase expression is driven by endogenous, cellular transcription factors, and acts as a reporter for monitoring the extent of adenoviral infection. Large-scale preparations of adenovirus stocks were performed by Con Sonza (AIDS Pathogenesis Unit, The Burnet Institute, Melbourne, Victoria, Australia). Briefly, 293 T cells were infected with the adenoviral vector at a MOI of ∼1 and incubated until a nearly complete cytopathic effect was obtained. The cells were then centrifuged (2,000 × g for 5 min at room temperature), resuspended in 1 ml of phosphate-buffered saline containing calcium and magnesium (PBS) (Gibco-BRL, Life Technologies, Grand Island, N.Y.) and 10% glycerol. The cells were lysed by three cycles of freezing and thawing, and the resulting extracts containing the virus were clarified by centrifugation at 1,000 × g for 4 min at room temperature. Viral stocks were stored at −70°C until used to infect MDM. MDM were infected with adenovirus at a time (day 10 postisolation) that ensured peak adenovirus infection occurred on the same day that the effect of HIV-1 infection on GM-CSF-induced STAT5A phosphorylation was examined (day 13 postisolation). After 3 days of adenoviral infection, MDM were washed with PBS and stimulated with GM-CSF. MDM were then lysed in radioimmunoprecipitation assay (RIPA) buffer for immunoprecipitation and Western blotting (as described below) or alternatively, in cell culture lysis reagent (CCLB; Promega, Madison, Wis.) for the luciferase assay. Extracts were stored at −70°C until analysis of luciferase activity and STAT5A phosphorylation. Luciferase activity in MDM extracts was measured by the addition of 10 μl of cell lysate to 50 μl of luciferase assay reagent (LAR; Promega), and chemiluminescence was measured using a Triathler multilabel tester (Hidex, Turku, Finland). Uninfected MDM from the same donors were used as controls for each experiment.
Quantification of HIV-1 replication.
Supernatant was collected from MDM cultures on day 7 postinfection, and HIV-1 replication was measured by measuring RT activity via a micro-RT assay. Briefly, 10 μl of culture supernatant was added to 10 μl of 0.3% Nonidet P-40 (NP-40) in a 96-well plate. Forty microliters of an RT mixture was added to give a final concentration per well of 50 mM Tris (pH 7.8), 7.5 mM KCl, 5 mM MgCl2, 2 mM dithiothreitol (Sigma, St. Louis, Mo.), 5 μg of template-primer p.An.dT12-18 (Pharmacia-Biotech) per ml, and 3 μCi of [α-33P]dTTP (Amersham, Amersham Place, United Kingdom). Reaction mixtures were incubated for 4 h at 37°C before spotting 6 μl onto DE81 chromatography paper (Whatman) and air drying. Dry filters were washed six times with 2× SSC buffer (2× SSC is 0.3 M sodium chloride and 34 mM sodium citrate) to remove free radioactive deoxynucleoside triphosphate (dNTP), rinsed twice in 95% ethanol, and dried. Meltilex scintillant (Wallac) was spotted onto the filters, and then bound radioactivity was determined using a micro beta liquid scintillation counter (Wallac). Results are expressed as counts per minute of RT activity per microliter of culture supernatant.
Expression of GM-CSFR.
MDM cultured in Teflon jars were analyzed for expression of the α- and βc-chains of the GM-CSFR using monoclonal antibodies directed against the α-chain (4H1; 3 μg/ml) or βc-chain (1C1; 3 μg/ml), a gift from Angel Lopez (The Hanson Centre for Cancer Research, Adelaide, South Australia, Australia) (31), or an isotype-matched control (MOPC 21) (2 μg/ml) (Bionetics, Charleston, S.C.). After incubation on ice for 30 min and three washes using cold PBS (calcium and magnesium free) and centrifugation at 250 × g, MDM were incubated for 30 min on ice with goat anti-mouse antibody conjugated to fluorescein isothiocyanate. Cells were washed, and α- or βc-chain expression was quantified by flow cytometry using a FacStarPlus. Fluorescence values were converted to molecules of equivalent soluble fluorescence units using a standard curve generated from calibration beads (Quantum25; FCSC, Bangs Laboratories) and the QuickCal program. To determine regulation of GM-CSFR surface expression by HIV-1, monocytes were cultured to MDM in Teflon pots and infected with HIV-1Ba-L 5 days after isolation, and surface GM-CSFR α- and βc-chain expression was analyzed 3 days postinfection.
Stimulation of MDM using wild-type GM-CSF or mutant GM-CSF, E21R.
MDM were exposed to recombinant human GM-CSF (a gift from Angel Lopez) at various concentrations (0 to 10 ng/ml) or to a mutant form of GM-CSF (E21R; BresaGen, Adelaide, South Australia, Australia) (1 μg/ml), in serum-free Iscove's modified Dulbecco medium for the indicated times prior to cell lysis. E21R contains a mutation within the first α-helix resulting in a substitution of arginine for glutamic acid (16, 24). This mutant GM-CSF binds with a low affinity to the α-chain of the GM-CSFR and thus was used at a concentration of at least 100 times that of the wild-type GM-CSF in order to achieve saturation.
Use of AG490.
MDM cultured adherent to 10-cm-diameter tissue culture plates at a concentration of 5 × 106 cells/plate were washed three times with PBS warmed to 37°C. The tyrphostin inhibitor of JAK2, AG490 (Calbiochem, San Diego, Calif.), was resuspended in supplemented Iscove's medium to the appropriate concentration and added to MDM for 4 h. Cells were then washed three times with warmed PBS, and MDM incubated with GM-CSF (10 ng/ml) in supplemented Iscove's medium for the indicated times in a humidified 5% CO2 incubator at 37°C. Cells were then washed three times with calcium- and magnesium-free PBS cooled to 4°C and lysed for Western blotting or electrophoretic mobility shift assay (EMSA) as indicated.
Immunoprecipitation and Western blotting.
For Western blotting, whole-cell lysates were generated at 4°C from cells treated as indicated using RIPA lysis buffer (25 mM Tris-HCl [pH 7.5], 0.14 M NaCl, 1 mM EDTA, 0.1% sodium dodecyl sulfate [SDS]). Phosphatase inhibitors (50 mM NaF, 1 mM sodium orthovanadate [Sigma], 40 mM β-glycerophosphate [Sigma]) and protease inhibitors (1 mM Pefabloc, 1 μM pepstatin, 1 μM leupeptin [Boehringer]) were included in the buffer. After 30 min of lysis on ice, cell lysates were centrifuged at 20,000 × g for 12 min at 4°C. Protein in the resulting lysate was quantified using the DC protein assay (Bio-Rad) according to the manufacturer's instructions. RIPA extracts containing equal amounts of protein were incubated with anti-STAT5A antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.) overnight at 4°C. A slurry of 50% protein G (Amersham Pharmacia Biotech, Uppsala, Sweden) and RIPA buffer was added for a further 45 min with mixing at 4°C. The beads were washed four times with RIPA buffer, before Laemmli sample buffer was added (to a final concentration of 10 mM Tris [pH 8.0], 2 mM EDTA, 1% SDS, 5% β-mercaptoethanol, 10% glycerol). Proteins were resolved by SDS-polyacrylamide gel electrophoresis and subsequently transferred to Hybond nitrocellulose membrane by electroblotting. Membranes were blocked for 1 h with Tris-buffered saline containing Tween 20 and supplemented with 5% nonfat milk. The membranes were then probed with antibody against phosphorylated STAT5 (anti-phospho-STAT5A/B) (0.5 μg/ml; Upstate Biotechnology, Lake Placid, N.Y.). Membranes were extensively washed and incubated with sheep anti-mouse antibody conjugated to horseradish peroxidase (Amersham Life Sciences) before enhanced chemiluminescence (ECL) assay using Western blotting detection reagents according to the manufacturer's instructions (Amersham Pharmacia Biotech). Immunoblots were incubated in a solution consisting of 2% (wt/vol) SDS, 62.5 mM Tris (pH 6.8), and 100 mM β-mercaptoethanol at 60°C for 30 min before reprobing with anti-STAT5A antibody (Santa Cruz Biotechnology) for 2 h. Membranes were again washed and incubated with goat anti-rabbit antibody conjugated to horseradish peroxidase (DAKO Corporation, Carpinteria, Calif.) before ECL assay using Western blotting detection reagents according to the manufacturer's instructions (Amersham Pharmacia Biotech).
Generation of nuclear lysates and EMSA.
Nuclear extracts were prepared by resuspending cell pellets in a solution containing 10 mM Tris-HCl (pH 7.8), 2 mM MgCl2, 5 mM KCl, 10% (vol/vol) glycerol, 1 mM EDTA, 2 mM dithiothreitol (DTT), 2 mM phenylmethylsulfonyl fluoride (PMSF), 4 μg of aprotinin per ml, 1.25 μg of leupeptin per ml, and 1× Complete protease inhibitor (Boehringer Mannheim). NP-40 was subsequently added to a final concentration of 0.5%. After incubation for 5 min at 4°C and centrifugation at 500 × g and 4°C for 5 min, the supernatant was discarded. The nuclear pellet was then resuspended in a solution containing 10 mM Tris-HCl (pH 8), 1 mM EDTA, 2 mM PMSF, 4 μg of aprotinin per ml, and 1× Complete protease inhibitor, and then NaCl was added to a final concentration of 0.5 M. After extraction for 30 min at 4°C and centrifugation at 20,000 x g for 15 min, nuclear lysates were frozen in liquid nitrogen and stored at −70°C until analysis by EMSA. For EMSA, 10 μg of an oligonucleotide derived from the STAT5-binding sequence in the FcγR1 promoter (GTATTTCCCAGAAAAAGGAC) and 10 μg of its antisense sequence (GTCCTTTTTCTGGGAAATAC) (13) were annealed by boiling at 100°C for 5 min in 0.05 M NaCl and cooling very slowly to room temperature. The probe (50 ng) was end labeled with 50 μCi (5 μl) of [32P]ATP, 5 μl of 10× polynucleotide kinase exchange buffer, and 1 μl of T4 polynucleotide kinase in a 50-μl reaction mixture. The reaction mixture was incubated for 45 min at 37°C. The probe was purified over a 1-ml Sephadex G25 spin column, which was equilibrated with 10× EMSA binding mix, by centrifugation at 2,060 × g for 3 min. For the binding reaction, an equal amount of each nuclear lysate was incubated with 1 ng of probe, 1.5 μg of sonicated salmon sperm DNA, and 0.5 mM DTT in a solution consisting of 10 mM HEPES (pH 7.9), 40 mM NaCl, 1 mM EDTA, and 4% glycerol. Where indicated, protein was preincubated on ice with the indicated anti-STAT5A antibody for 30 min. The antibody was either (i) polyclonal anti-STAT5A sc-1081 antibody raised against STAT5A amino acids 774 to 793 (Santa Cruz Biotechnology) or (ii) isoform-specific polyclonal anti-STAT5A against the C-terminal peptides specific for mouse STAT-5A (LDARLSPPAGLFTSARSSLS) (12, 21, 22), a kind gift from Lothar Hennighausen (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md.). After the addition of probe, EMSA reaction mixtures were incubated at room temperature for 15 min. 6× DNA loading dye (6× DNA loading dye is 50% glycerol, 0.25% bromophenol blue, and 0.25% xylene cyanol) was added to the reaction mixture, and reaction mixtures were immediately loaded on a prerun nondenaturing Tris-borate-EDTA-5% acrylamide gel. Protein-DNA complexes were resolved before the gel was dried for 2 h at 80°C and then visualized by autoradiography.
Isolation of mRNA from MDM.
mRNA was extracted from MDM lysates using oligo(dT)25 beads (Dynabeads; Dynal Biotech, Carlton South, Australia), according to the manufacturer's protocol. Briefly, 2 × 106 MDM were lysed in 1 ml of lysis buffer (100 mM Tris-HCl [pH 8.0], 500 mM LiCl, 10 mM EDTA [pH 8.0], 1% lithium dodecyl sulfate [LiDS], 5 mM DTT). Lysates were stored at −70°C until mRNA extraction was performed. They were then thawed at 4°C, and the DNA was sheared by repeated syringing through a 21-gauge needle. Beads were hybridized with cell lysates (106 MDM/25 μl of beads) and mixed at room temperature for 10 min to form Dynabead oligo(dT)25-mRNA complexes. Complexes were concentrated on a magnetic particle separator (Dynal) and washed twice with 1 ml of washing buffer with LiDS (10 mM Tris-HCl [pH 8.0], 150 mM LiCl, 1 mM EDTA [pH 8.0], 0.1% LiDS) and three times with 1 ml of washing buffer without LiDS (10 mM Tris-HCl [pH 8.0], 150 mM LiCl, 1 mM EDTA [pH 8.0]). Beads were transferred to clean Eppendorf tubes and washed three times with 250 μl of RT buffer (10 mM Tris-HCl [pH 8.3], 75 mM KCl). To convert mRNA to cDNA, the washed Dynabead oligo(dT)25-mRNA complexes were resuspended in 25 μl of a solution consisting of 1 mM concentrations (each) of the four dNTPs, 4 mM sodium pyrophosphate, 40 U of RNasin, 25 U of avian myeloblastosis virus RT, and 1× RT buffer. cDNA was synthesized by incubating the RT reaction mixture at 42°C for 1 h. Beads were then resuspended with 100 μl of TE buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA), transferred to new Eppendorf tubes, and placed on the magnetic particle separator (MPS). After the supernatant was removed, the beads were resuspended in 100 μl of TE buffer and heated at 95°C for 1 min to remove the mRNA. Following another separation using the MPS, bead-cDNA complexes were resuspended in 100 μl of TE buffer (pH 8.0) and stored at 4°C until used for PCRs.
PCR of cDNA for actin, JAK2, and STAT5A.
PCR for β-actin was performed using primers to amplify a 225-bp region of human β-actin cDNA in a dilution series of cDNA to confirm equal template levels (14). Reactions were performed in 50-μl reaction mixtures composed of 0.2 mM (each) dNTP, 1.5 mM MgCl2, 0.4 μM (each) primer, 1.1 U of Taq polymerase (Fisher Biotech, Perth, Australia) and 1× reaction buffer (Fisher Biotech). Primers for JAK2 (5′-TTCAGAAGCAGGCAACAGG-3′ and 5′-TCTGTCATCGTAAGGCAGGC-3′) and STAT5A (5′GGTGAGATCCTGAACAACTGC-3′ and 5′-TGAACTTCTCCTCTGTCACGG-3′) were designed using the Geneworks program. PCR for JAK2 and STAT5A was performed on cDNA in a threefold dilution series starting with 20,000 cell equivalents, in a reaction mixture similar to that for β-actin but with 1 mM MgCl2. All samples were treated as follows: (i) an initial denaturation step of 2 min at 94°C; (ii) 30 cycles of PCR amplification, with 1 cycle consisting of 45 s at 94°C, 45 s at 56°C, and 1 min at 72°C, and (iii) a final extension step of 7 min at 72°C. PCR products were analyzed by agarose gel electrophoresis and ethidium bromide staining.
RESULTS
STAT5A activation occurs in response to GM-CSF stimulation of uninfected MDM and is abolished by AG490.
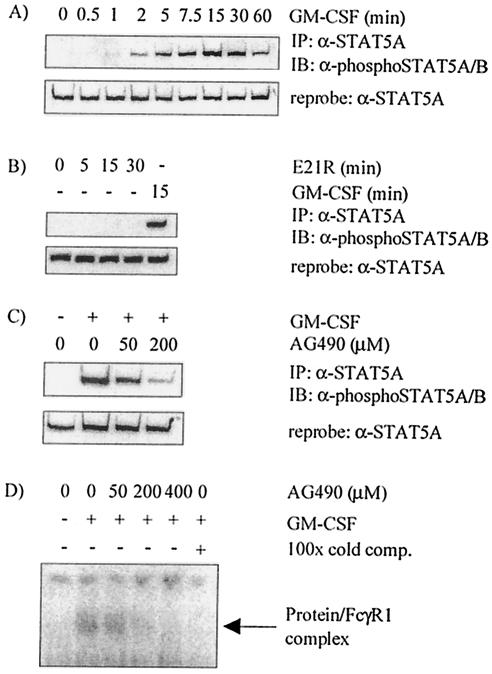
STAT5A was activated in human MDM in response to GM-CSF engagement of its receptor, as previously reported (29). Tyrosine phosphorylation of STAT5A occurred within 30 s of stimulation of MDM with GM-CSF, peaked at 15 min, and gradually diminished after this time (Fig. 1A). As little as 0.1 ng of GM-CSF per ml was sufficient to induce STAT5A tyrosine phosphorylation, and STAT5A tyrosine phosphorylation increased with increasing concentration of GM-CSF, showing 50% saturation between 0.3 and 1 ng of GM-CSF per ml (data not shown). Stimulation of MDM with mutant GM-CSF E21R did not result in tyrosine phosphorylation of STAT5A (Fig. 1B), demonstrating the requirement for binding to GM-CSFR αβc heterodimer for STAT5A phosphorylation in response to GM-CSF.
FIG. 1.
STAT5A is phosphorylated on tyrosine 694 in response to GM-CSF stimulation of MDM, and STAT5A activation is inhibited by the JAK2 inhibitor AG490. Monocytes were cultured for 7 days before the following treatment: stimulation with 10 ng of GM-CSF per ml for 0 to 60 min (A); stimulation with 1 μg of E21R (mutant GM-CSF) per ml or with 10 ng of wild-type GM-CSF per ml for the times shown (B); or preincubation with AG490 for 4 h, stimulation with 20 ng of GM-CSF per ml for 5 min, and lysis with RIPA buffer (C). Immunoprecipitation (IP) of equal amounts of cell protein was performed with an anti-STAT5A antibody (α-STAT5A), and blots were probed with anti-phospho-STAT5A/B antibody (α-phosphoSTAT5A/B). Equivalent protein levels are shown by reprobing (after stripping) the blots with anti-STAT5A antibody. Results shown are representative of four independent experiments using MDM isolated from different donors. IB, immunoblotting. (D) Nuclear lysates were generated from MDM preincubated with AG490 for 4 h before stimulation with 10 ng of GM-CSF (+) per ml for 15 min. EMSA was performed using a 32P-labeled oligonucleotide containing the STAT response element derived from the FcγR1 promoter. In each reaction mixture, the same amount of protein was incubated with labeled oligonucleotide, sonicated salmon sperm DNA, and DTT in a solution consisting of 10 mM HEPES, 40 mM NaCl, 1 mM EDTA, and 4% glycerol for 15 min at room temperature. Extract was preincubated (+) with unlabeled competing probe (cold comp.) for 30 min. The gel was dried, and DNA-protein complexes were visualized via autoradiography. Results are representative of MDM isolated from two donors.
GM-CSF stimulation of MDM induced maximum binding of STAT5A to an oligonucleotide probe containing the STAT response element derived from the FcγR1 promoter element after 15 min, as assessed by EMSA, consistent with kinetics of tyrosine phosphorylation of STAT5A (data not shown). Additionally, mutant GM-CSF E21R did not induce binding of STAT5A to DNA, indicating that signaling via the βc-chain of GM-CSFR was required for STAT5A nuclear localization and DNA-binding activity (data not shown).
The JAK2 inhibitor AG490 inhibited STAT5A phosphorylation in response to GM-CSF in a dose-dependent manner with near-complete inhibition by 200 μM AG490 (Fig. 1C). In addition to GM-CSF-induced STAT5A tyrosine phosphorylation, the effect of AG490 on the ability of STAT5A to bind DNA was examined in an EMSA. The DNA binding of STAT5A was inhibited by 200 μM AG490 to a degree similar to that of STAT5A tyrosine phosphorylation (Fig. 1D).
HIV-1 infection of MDM inhibits GM-CSF-mediated activation of STAT5A.
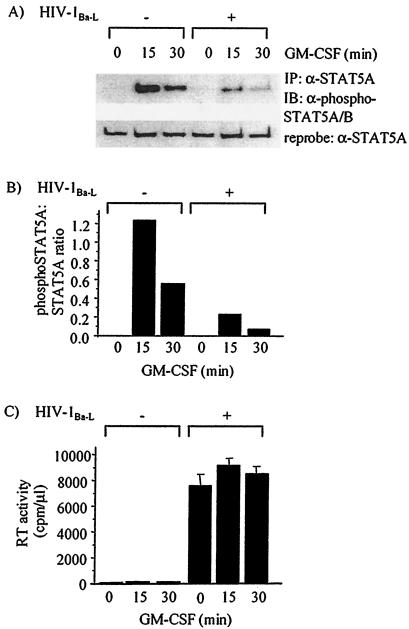
HIV-1 infection markedly inhibited the phosphorylation of STAT5A in response to GM-CSF stimulation, as shown in a representative experiment by immunoprecipitation of STAT5A from MDM lysates and immunoblotting with anti-phospho-STAT5A/B (Fig. 2A). There was no constitutive phosphorylation of STAT5A in either uninfected or infected MDM. Additionally, the total level of STAT5A was not decreased in HIV-1-infected MDM, as shown by reprobing the blot with an anti-STAT5A antibody (Fig. 2A). Densitometry showed that the ratios of phosphorylated STAT5A to total STAT5A in HIV-1-infected MDM stimulated with GM-CSF for 15 and 30 min were five- and sevenfold less than those in uninfected MDM, respectively (Fig. 2B). HIV-1 replication was quantified by RT assay using supernatant taken from each culture prior to GM-CSF stimulation. HIV-1 replication was evident in each HIV-1-infected MDM culture, with no significant difference between each tissue culture plate of MDM (Fig. 2C). The ratio of phospho-STAT5A/B to STAT5 was calculated similarly in seven independent experiments and a mean inhibition of 76% ± 9% (standard error of the mean) was found.
FIG. 2.
HIV-1 infection of MDM inhibits the GM-CSF-induced phosphorylation of STAT5A on tyrosine 694. Monocytes were cultured for 5 days prior to infection with HIV-1Ba-L (+) or mock infection (−). Seven days postinfection, MDM were stimulated with 10 ng of GM-CSF per ml for the indicated times, and cells were lysed. (A) STAT5A was immunoprecipitated (IP) with an anti-STAT5A antibody (α-STAT5A), and phosphorylated STAT5A was immunoblotted (IB) with an anti-phosphorylated STAT5A/B antibody (α-phospho-STAT5A/B). (B) The ratio of phosphorylated STAT5A to the level of STAT5A was evaluated by densitometry. (C) An equivalent level of HIV-1 infection for each MDM culture was demonstrated using RT assay on culture supernatants, taken immediately prior to GM-CSF stimulation. RT activity is given as counts per minute and represents activity in 1 μl of culture supernatant. The immunoblots shown are representative of MDM prepared from seven different donors.
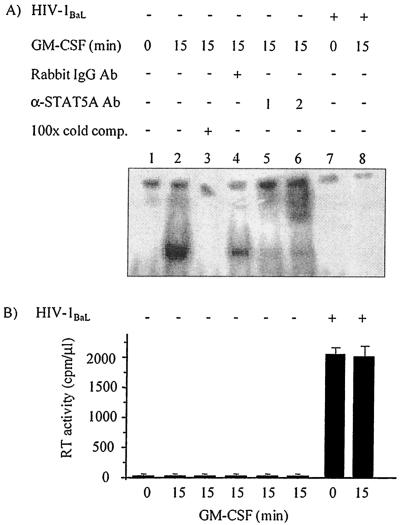
Compared with nuclear lysates generated from uninfected MDM, GM-CSF-induced STAT5A DNA binding in EMSA was inhibited in HIV-1-infected MDM (Fig. 3A, lanes 2 and 8). Specificity of protein bound to the 32P-labeled FcγR1 oligonucleotide was demonstrated using excess competitive unlabeled probe (Fig. 3A, lane 3). The STAT5A-DNA complex was almost completely removed by an antibody generated against the STAT5A C terminus (Santa Cruz Biotechnology) (Fig. 3A, lane 5), a reported modified supershift (5, 34), and shifted by an alternative antibody to a similar epitope of STAT5A (Fig. 3A, lane 6). Preincubation of MDM lysates with a nonspecific antibody did not significantly abolish the formation of the protein-DNA complex (Fig. 3A, lane 4). In accordance with the phosphorylation data, DNA binding of STAT5A was not detected in the absence of GM-CSF stimulation in either uninfected MDM or HIV-1-infected MDM (Fig. 3A, lanes 1 and 7). The level of HIV-1 infection was assessed by RT assay of culture supernatants (Fig. 3B).
FIG. 3.
GM-CSF-induced DNA binding of STAT5A is inhibited in HIV-1-infected MDM. Monocytes were cultured for 5 days prior to infection with HIV-1Ba-L (+) or mock infection (−). After 7 days, uninfected and HIV-1-infected MDM were incubated with 10 ng of GM-CSF per ml for 0 or 15 min and lysed for nuclear extraction. (A) Nuclear lysates were used in EMSA with 32P-end-labeled oligonucleotide containing the STAT response element of the FcγR1 promoter. Where indicated, extract was preincubated with an anti-STAT5A antibody (lanes 5 and 6), a nonspecific rabbit antibody (+) (lane 4), or unlabeled competing (cold comp.) probe (+) (lane 3) for 30 min at 4°C. Extract was preincubated with one of two different anti-STAT5A antibodies in lanes 5 and 6: antibody 1 was a rabbit anti-STAT5A antibody (α-STAT5A Ab) (Santa Cruz Biotechnology) (lane 5), and antibody 2 was a rabbit anti-STAT5A antibody (a gift from Lothar Hennighausen) (lane 6). The gel was dried, and the protein-DNA complexes visualized via autoradiography. IgG; immunoglobulin G. (B) The supernatant was removed from each culture of MDM prior to stimulation with GM-CSF, and the level of HIV-1 infection was assessed by RT assay. RT activity is given as counts per minute and represents activity in 1 μl of culture supernatant. Results are representative of MDM prepared from two donors.
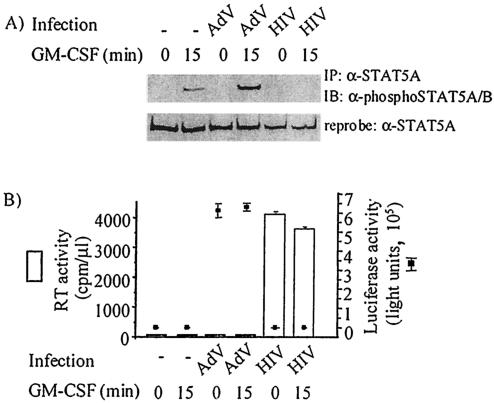
As a control for the specificity of HIV-1 in inhibiting GM-CSF-induced STAT5A activation, MDM were infected with an adenoviral vector constructed with a luciferase gene driven from a long terminal repeat promoter responsive only to cellular transcription factors. After MDM were infected by either adenovirus or HIV-1Ba-L, MDM were stimulated with GM-CSF and lysed for immunoprecipitation of STAT5A and subsequent analysis of phosphorylation of tyrosine 694. In uninfected cells, stimulation for 15 min with GM-CSF resulted in tyrosine phosphorylation of STAT5A (Fig. 4A, second lane from the left). HIV-1Ba-L strongly inhibited GM-CSF-induced STAT5A phosphorylation (Fig. 4A, rightmost lane). In contrast, adenovirus infection did not inhibit STAT5A phosphorylation, but GM-CSF-induced STAT5A phosphorylation was instead enhanced in adenovirus-infected cells (Fig. 4A, fourth lane from the left). The level of adenoviral infection was assessed by using a luciferase assay (Fig. 4B, right y axis), and the level of HIV-1Ba-L infection was assessed by using an RT assay of culture supernatants (Fig. 4B, left y axis).
FIG. 4.
HIV-1, not adenoviral, infection of MDM inhibits GM-CSF-induced STAT5A tyrosine phosphorylation. Monocytes were cultured for 6 days prior to mock infection (−) or HIV-1Ba-L infection or for 10 days prior to infection using the ΔTARluc adenovirus (AdV) construct. After 7 days of HIV infection and 3 days of AdV infection, MDM were stimulated with 10 ng of GM-CSF per ml for 0 to 15 min and lysed. (A) Equivalent amounts of cell lysate were immunoprecipitated (IP) with an anti-STAT5A antibody (α-STAT5A), and the immunoblot (IB) was probed with an anti-phospho-STAT5A/B antibody (α-phosphoSTAT5A/B) and reprobed with an anti-STAT5A antibody. (B) Similar levels of HIV-1 infection in separate MDM cultures were demonstrated by RT activity in culture supernatants (in counts per minute per microliter of culture supernatant) taken prior to GM-CSF stimulation (left y axis; white bars). The level of AdV infection was assessed by lysing MDM which had been infected in parallel cultures to those used for immunoprecipitation and performing the luciferase assay on those lysates (right y axis; solid squares). The results shown are representative of MDM prepared from three donors.
HIV-1 does not inhibit surface expression of GM-CSFR.
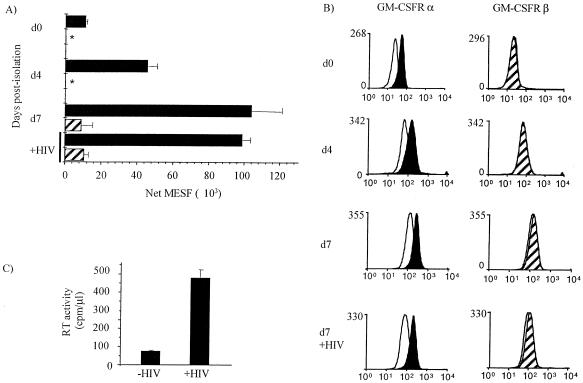
Since STAT5A levels in MDM were not altered by infection with HIV-1, characterization of the point at which GM-CSF-induced STAT5A activation was inhibited by HIV-1 infection required investigation of events upstream in the signaling pathway. Flow cytometric analysis of surface expression of both the α- and βc-chain of GM-CSFR on MDM showed higher α-chain expression than βc-chain expression on freshly isolated monocytes and an increase of α-chain surface expression during culture (Fig. 5A and B). βc-Chain expression was not detected on freshly isolated monocytes, but it increased with differentiation to MDM during 7 days in culture. Surface expression of α- and βc-chain 3 days after HIV-1 infection was not reduced compared to that of uninfected MDM from the same donors (Fig. 5A and B), indicating that postreceptor mechanisms were responsible for the inhibition of GM-CSF-induced STAT5A phosphorylation. The level of HIV-1Ba-L infection was assessed using RT assay of culture supernatants (Fig. 5C).
FIG. 5.
GM-CSFR α- and βc-chain surface expression is not inhibited by HIV-1 infection of MDM. Monocytes were cultured from day 0 in Teflon jars, and 4 days after isolation of monocytes, the cells were mock infected or infected with HIV-1Ba-L. (A and B) Surface expression of GM-CSFR α- and βc-chain was assessed in duplicate on the day of isolation (d0), 4 days after isolation (d4) and 3 days after HIV-1 infection (d7), using monoclonal antibody against the α-chain (4H1; 3 μg/ml; black bars and histograms) and βc-chain (1C1; 3 μg/ml; hatched bars and histograms) or an isotype-matched control IgG1 (MOPC 21; 2 μg/ml; white histograms). All samples were analyzed by flow cytometry. Fluorescence values were converted to molecules of equivalent soluble fluorochrome (MESF) units using a standard curve generated from calibration beads and the QuickCal program. Duplicate test samples were corrected for background fluorescence and averaged. The asterisks indicate that surface expression was not detected. (C) Seven days after HIV-1 infection, supernatants were removed from each MDM culture and assayed for HIV-1 RT activity with results expressed as counts per minute per microliter of culture supernatant. Results are representative of two experiments using MDM prepared from different donors.
HIV-1 does not inhibit STAT5A or JAK2 mRNA.
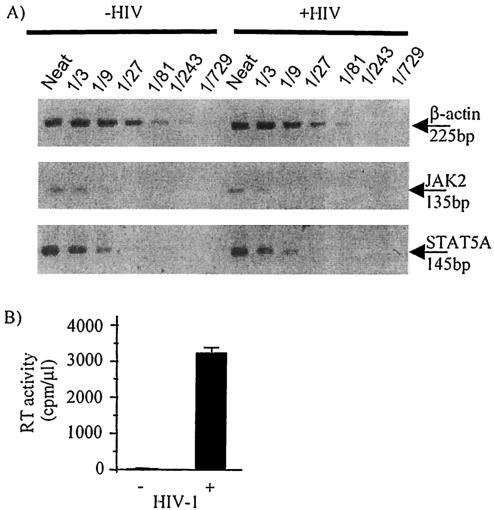
In order to examine expression of STAT5A and JAK2 mRNA, HIV-1-infected and control MDM lysates were analyzed by RT-PCR using primers specific for β-actin, JAK2, and STAT5A. There was no significant reduction in the level of JAK2 mRNA in HIV-1-infected MDM (n = 2) (Fig. 6A). There was also no downregulation of STAT5A mRNA in HIV-1-infected MDM compared to that of uninfected MDM (n = 4) (Fig. 6A). Equal β-actin levels were present in lysates generated from uninfected and HIV-1-infected MDM (Fig. 6A). HIV-1Ba-L-infection of MDM was confirmed by RT assay of the supernatant (Fig. 6B).
FIG. 6.
JAK2 and STAT5A mRNA is not inhibited by HIV-1 infection of MDM. Monocytes were cultured for 5 days before infection with HIV-1Ba-L (+HIV) or mock infection (−HIV). MDM were cultured for another 7 days before extraction of mRNA which was used to synthesize cDNA by reverse transcription. (A) PCR was performed on cDNA using primers specific for β-actin, JAK2, and STAT5A. Levels of cDNA were standardized according to β-actin levels. The first sample (undiluted, or neat) of each dilution series corresponds to 20,000 MDM. (B) The supernatant was removed from each culture of MDM (on day 12 after isolation of the cells), and the level of HIV-1 infection was assessed by RT assay. Results for STAT5A PCR are representative of MDM prepared from four donors, and results for JAK2 PCR are representative of MDM prepared from two donors.
DISCUSSION
We demonstrate for the first time that HIV-1 infection of human MDM in vitro results in the disruption of GM-CSF-induced STAT5A activation. The tyrosine phosphorylation and nuclear DNA binding of STAT5A are dependent on signaling via the αβc heterodimer of the GM-CSFR, as a mutant form of GM-CSF, E21R, which binds only to the α-chain of GM-CSFR (24), failed to stimulate STAT5A activation in uninfected MDM. GM-CSF stimulation of MDM resulted in maximum tyrosine phosphorylation and consequent DNA binding of STAT5A within 15 min of stimulation. Infection of MDM with an R5 strain of HIV-1 (HIV-1Ba-L) inhibited STAT5A phosphorylation in response to GM-CSF and reduced the ability of STAT5A to bind its DNA target, strongly suggesting that STAT5A-promoted transcription is disrupted in HIV-1Ba-L-infected MDM. This HIV-1-induced inhibition of GM-CSF-mediated STAT5A activation was not due to diminished GM-CSFR surface expression or lower levels of STAT5A mRNA, STAT5A protein, or JAK2 mRNA. The effects were HIV-1 specific, as adenoviral infection of MDM did not inhibit STAT5A tyrosine phosphorylation. These data suggest that HIV-1 specifically disrupts signaling in macrophages, and the subsequent dysregulation of macrophage function may contribute to HIV-1 pathogenesis.
Previous investigations have shown that HIV-1 infection of PBMCs can alter the level and/or activation of STAT5 (6, 26). Western blotting has demonstrated decreased expression of STAT5A, STAT5B, and STAT1α in T-cell lysates from HIV-infected individuals and reduced STAT5B expression in PHA blasts infected in vitro with dual-tropic HIV-1BZ167 (26). The reported constitutive activation of STAT in PBMCs from 10 of 14 HIV-infected individuals and in resting PBMCs infected in vitro with HIV-1Ba-L or HIV-1IIIB was accounted for by activation of STAT1α and STAT5Δ, but not full-length STAT5 (6). However, this constitutive activation of STAT was not observed upon infection of human MDM, indicating that a HIV-1 strain can have different effects in different cell types (6). In the current study, constitutive full-length STAT5A tyrosine phosphorylation and DNA binding in HIV-1-infected MDM were also not observed. STAT5A activation was not detected in non-GM-CSF-treated MDM, regardless of HIV-1 infection.
Previous reports have also demonstrated strain specificity of HIV-1 for inhibition of STATs, with HIV-1NL4.3 infection of CD4+ T cells resulting in a decrease in total cellular levels of STAT5A, which was not observed after infection with HIV-1IIIB (30). In comparison, our study shows that infection of MDM with HIV-1Ba-L results in inhibition of GM-CSF-induced STAT5A tyrosine phosphorylation in the absence of decreased STAT5A expression as measured by immunoblotting for protein and PCR for cDNA. This is similar to observations of PBMCs infected with HIV-1Ba-L, in which STAT5A expression was not reduced (6, 26). Adenoviral infection has recently been shown to specifically inhibit gamma interferon-induced STAT1 activation in human tracheobronchial epithelial cells, but not IL-4-induced STAT6 activation (17). In our study, adenovirus infection of MDM did not inhibit GM-CSF-induced STAT5A tyrosine phosphorylation, supporting the specificity of our observation.
Disruption of IL-2-mediated JAK/STAT signaling in HIV-1NL4.3-infected CD4+ T cells was characterized by decreased JAK3 expression and hence decreased activation, as well as decreased expression of STAT5A itself (30). Thus, HIV-1 may act at several points in the JAK/STAT pathway to affect cytokine signaling. As surface expression of cytokine receptors can influence subsequent STAT activation in cells and may be disrupted by viral infection (17, 23), we considered that surface expression of GM-CSFR α- and βc-chains was reduced in HIV-1-infected MDM. However, our study found that this was not the case.
The level of JAK2 protein in primary cells, such as neutrophils and monocytes, is very low (34), and observation of activated JAK2 in response to GM-CSF stimulation of these cells has been limited (2, 20). In one report, a faint band with the expected molecular weight of JAK2 was present after immunoprecipitation of tyrosine-phosphorylated proteins from GM-CSF-treated neutrophils and the intensity of this band was reduced by preincubation of cells with AG490 at an optimum concentration of 200 μM (2). In the current study, using primary human MDM, GM-CSF-induced STAT5A activation was also inhibited at the concentration of 200 μM AG490, indicating dependence on JAK2. Lehtonen et al. have recently detected activation of JAK2 in GM-CSF-stimulated MDM but not monocytes (20). Using the same reagents, we were not able to detect phospho-JAK2 in our system by immunoprecipitation coupled with immunoblotting (data not shown). We also could not detect phosphorylated JAK2 in MDM stimulated with GM-CSF for 0, 5, and 15 min after immunoprecipitation of 1 mg of MDM protein with a variety of anti-JAK2 antibodies (data not shown). The difference may be due to differences in culture conditions: Lehtonen et al. (20) cultured monocytes in medium containing GM-CSF, whereas we did not, since we have shown that GM-CSF impairs HIV-1 replication in MDM (19), and it is possible that GM-CSF upregulates components of the JAK/STAT activation pathway such as the receptor itself. In our study, JAK2 mRNA was measured and did not decrease in HIV-1-infected MDM. Although we were not able to ascertain how HIV-1 disrupts GM-CSF signaling, HIV-1-infected MDM appear hyporesponsive to GM-CSF while possessing apparently normal levels of GM-CSFR, STAT5A, and possibly JAK2. In this, they resemble purified CD34+ progenitor cells which have also been reported to be hyporesponsive to GM-SCF activation of STAT5 despite normal expression of these components (33).
The mechanism by which HIV-1 inhibits GM-CSF-induced STAT5A activation is under investigation. An alternate activator of STAT5A may exist in MDM that acts in response to GM-CSF and is inhibited in HIV-1-infected MDM. Src has been shown to phosphorylate STAT5 in vivo when Src and STAT5 proteins are coexpressed in COS7 cells and directly phosphorylate STAT5A on tyrosine 694 in an in vitro kinase assay (25). The HIV-1 accessory protein Nef has not been shown to directly inhibit activation of the JAK/STAT pathway, but it does contain the proline repeat motif PXXP for binding to proteins with SH3 domains (28). Nef can bind and dysregulate cellular protein kinases which contain this motif, such as the Src family member Hck. Lyn is an SH3 domain-containing tyrosine kinase of the Src family which can associate with the common βc subunit of GM-CSFR (10). Interactions involving GM-CSFR coupling to SH3 domain-containing proteins and subsequent signaling events are not characterized in human MDM; however, it is possible that a kinase of the Src family activates STAT5A in response to GM-CSF and that its activity is disrupted by HIV-1 infection of MDM.
Our data show that HIV-1 infection of MDM disrupts activation of STAT5A in response to GM-CSF. GM-CSF is an important immunomodulator of macrophage function, including phagocytosis and antiparasitic and antimycobacterial activities, suggesting that inhibition of its signaling by HIV-1 could contribute to the immune dysregulation and cell dysfunction which characterize HIV-1 infection.
Acknowledgments
We thank Géza Paukovics for assistance with flow cytometric analysis, Con Sonza and Phillip Ellery for technical advice, and Kathy Tolli for secretarial assistance.
This work was supported in part by grant RA022101 from the Clive and Vera Ramaciotti Foundation and by the National Centre for HIV Virology. Tammra Warby was supported by an Australian Postgraduate Award (APA). Suzanne Crowe is a research fellow with the National Health and Medical Research Council (NHMRC).
REFERENCES
- 1.Al-Shami, A., W. Mahanna, and P. H. Naccache. 1998. Granulocyte-macrophage colony-stimulating factor-activated signaling pathways in human neutrophils. Selective activation of Jak2, Stat3, and Stat5b. J. Biol. Chem. 273:1058-1063. [DOI] [PubMed] [Google Scholar]
- 2.Al-Shami, A., and P. H. Naccache. 1999. Granulocyte-macrophage colony-stimulating factor-activated signaling pathways in human neutrophils. Involvement of Jak2 in the stimulation of phosphatidylinositol 3-kinase. J. Biol. Chem. 274:5333-5338. [DOI] [PubMed] [Google Scholar]
- 3.Armitage, J. O. 1998. Emerging applications of recombinant human granulocyte-macrophage colony-stimulating factor. Blood 92:4491-4508. [PubMed] [Google Scholar]
- 4.Axelrod, J. H., and A. Honigman. 1999. A sensitive and versatile bioluminescence bioassay for HIV type 1 based on adenoviral vectors. AIDS Res. Hum. Retroviruses 15:759-767. [DOI] [PubMed] [Google Scholar]
- 5.Azam, M., H. Erdjument-Bromage, B. L. Kreider, M. Xia, F. Quelle, R. Basu, C. Saris, P. Tempst, J. N. Ihle, and C. Schindler. 1995. Interleukin-3 signals through multiple isoforms of Stat5. EMBO J. 14:1402-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bovolenta, C., L. Camorali, A. L. Lorini, S. Ghezzi, E. Vicenzi, A. Lazzarin, and G. Poli. 1999. Constitutive activation of STATs upon in vivo human immunodeficiency virus infection. Blood 94:4202-4209. [PubMed] [Google Scholar]
- 7.Brizzi, M. F., M. G. Aronica, A. Rosso, G. P. Bagnara, Y. Yarden, and L. Pegoraro. 1996. Granulocyte-macrophage colony-stimulating factor stimulates JAK2 signaling pathway and rapidly activates p93fes, STAT1 p91, and STAT3 p92 in polymorphonuclear leukocytes. J. Biol. Chem. 271:3562-3567. [DOI] [PubMed] [Google Scholar]
- 8.Caldenhoven, E., T. B. van Dijk, J. A. Raaijmakers, J. W. Lammers, L. Koenderman, and R. P. de Groot. 1999. Activation of a functionally distinct 80-kDa STAT5 isoform by IL-5 and GM-CSF in human eosinophils and neutrophils. Mol. Cell. Biol. Res. Commun. 1:95-101. [DOI] [PubMed] [Google Scholar]
- 9.Crowe, S., J. Mills, and M. S. McGrath. 1987. Quantitative immunocytofluorographic analysis of CD4 surface antigen expression and HIV infection of human peripheral blood monocyte/macrophages. AIDS Res. Hum. Retroviruses 3:135-145. [DOI] [PubMed] [Google Scholar]
- 10.Dahl, M. E., K. I. Arai, and S. Watanabe. 2000. Association of Lyn tyrosine kinase to the GM-CSF and IL-3 receptor common βc subunit and role of Src tyrosine kinases in DNA synthesis and anti-apoptosis. Genes Cells 5:143-153. [DOI] [PubMed] [Google Scholar]
- 11.Feldman, G. M., L. A. Rosenthal, X. Liu, M. P. Hayes, A. Wynshaw-Boris, W. J. Leonard, L. Hennighausen, and D. S. Finbloom. 1997. STAT5A-deficient mice demonstrate a defect in granulocyte-macrophage colony-stimulating factor-induced proliferation and gene expression. Blood 90:1768-1776. [PubMed] [Google Scholar]
- 12.Gaffen, S. L., S. Y. Lai, M. Ha, X. Liu, L. Hennighausen, W. C. Greene, and M. A. Goldsmith. 1996. Distinct tyrosine residues within the interleukin-2 receptor beta chain drive signal transduction specificity, redundancy, and diversity. J. Biol. Chem. 271:21381-21390. [DOI] [PubMed] [Google Scholar]
- 13.Gaffen, S. L., S. Y. Lai, W. Xu, F. Gouilleux, B. Groner, M. A. Goldsmith, and W. C. Greene. 1995. Signaling through the interleukin 2 receptor beta chain activates a STAT-5-like DNA-binding activity. Proc. Natl. Acad. Sci. USA 92:7192-7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grassi, G., G. Pozzato, M. Moretti, and M. Giacca. 1995. Quantitative analysis of hepatitis C virus RNA in liver biopsies by competitive reverse transcription and polymerase chain reaction. J. Hepatol. 23:403-411. [DOI] [PubMed] [Google Scholar]
- 15.Hayashida, K., T. Kitamura, D. M. Gorman, K. Arai, T. Yokota, and A. Miyajima. 1990. Molecular cloning of a second subunit of the receptor for human granulocyte-macrophage colony-stimulating factor (GM-CSF): reconstitution of a high-affinity GM-CSF receptor. Proc. Natl. Acad. Sci. USA 87:9655-9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hercus, T. R., B. Cambareri, M. Dottore, J. Woodcock, C. J. Bagley, M. A. Vadas, M. F. Shannon, and A. F. Lopez. 1994. Identification of residues in the first and fourth helices of human granulocyte-macrophage colony-stimulating factor involved in biologic activity and in binding to the alpha- and beta-chains of its receptor. Blood 83:3500-3508. [PubMed] [Google Scholar]
- 17.Joseph, T. D., and D. C. Look. 2001. Specific inhibition of interferon signal transduction pathways by adenoviral infection. J. Biol. Chem. 276:47136-47142. [DOI] [PubMed] [Google Scholar]
- 18.Kedzierska, K., and S. M. Crowe. 2001. Culture of HIV in monocytes and macrophages, p. 12.4.1. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and V. Strober (ed.), Current protocols in immunology, vol. II. Wiley, New York, N.Y. [DOI] [PubMed]
- 19.Kedzierska, K., A. Maerz, T. Warby, A. Jaworowski, H. Chan, J. Mak, S. Sonza, A. Lopez, and S. Crowe. 2000. Granulocyte-macrophage colony-stimulating factor inhibits HIV-1 replication in monocyte-derived macrophages. AIDS 14:1739-1748. [DOI] [PubMed] [Google Scholar]
- 20.Lehtonen, A., S. Matikainen, M. Miettinen, and I. Julkunen. 2002. Granulocyte-macrophage colony-stimulating factor (GM-CSF)-induced STAT5 activation and target-gene expression during human monocyte/macrophage differentiation. J. Leukoc. Biol. 71:511-519. [PubMed] [Google Scholar]
- 21.Liu, X., G. W. Robinson, F. Gouilleux, B. Groner, and L. Hennighausen. 1995. Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc. Natl. Acad. Sci. USA 92:8831-8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, X., G. W. Robinson, and L. Hennighausen. 1996. Activation of Stat5a and Stat5b by tyrosine phosphorylation is tightly linked to mammary gland differentiation. Mol. Endocrinol. 10:1496-1506. [DOI] [PubMed] [Google Scholar]
- 23.Look, D. C., W. T. Roswit, A. G. Frick, Y. Gris-Alevy, D. M. Dickhaus, M. J. Walter, and M. J. Holtzman. 1998. Direct suppression of Stat1 function during adenoviral infection. Immunity 9:871-880. [DOI] [PubMed] [Google Scholar]
- 24.Lopez, A. F., M. F. Shannon, T. Hercus, N. A. Nicola, B. Cambareri, M. Dottore, M. J. Layton, L. Eglinton, and M. A. Vadas. 1992. Residue 21 of human granulocyte-macrophage colony-stimulating factor is critical for biological activity and for high but not low affinity binding. EMBO J. 11:909-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okutani, Y., A. Kitanaka, T. Tanaka, H. Kamano, H. Ohnishi, Y. Kubota, T. Ishida, and J. Takahara. 2001. Src directly tyrosine-phosphorylates STAT5 on its activation site and is involved in erythropoietin-induced signaling pathway. Oncogene 20:6643-6650. [DOI] [PubMed] [Google Scholar]
- 26.Pericle, F., L. A. Pinto, S. Hicks, R. A. Kirken, G. Sconocchia, J. Rusnak, M. J. Dolan, G. M. Shearer, and D. M. Segal. 1998. HIV-1 infection induces a selective reduction in STAT5 protein expression. J. Immunol. 160:28-31. [PubMed] [Google Scholar]
- 27.Quelle, F. W., N. Sato, B. A. Witthuhn, R. C. Inhorn, M. Eder, A. Miyajima, J. D. Griffin, and J. N. Ihle. 1994. JAK2 associates with the beta c chain of the receptor for granulocyte-macrophage colony-stimulating factor, and its activation requires the membrane-proximal region. Mol. Cell. Biol. 14:4335-4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renkema, G. H., and K. Saksela. 2000. Interactions of HIV-1 NEF with cellular signal transducing proteins. Front. Biosci. 5:D268-D283. [DOI] [PubMed]
- 29.Rosen, R. L., K. D. Winestock, G. Chen, X. Liu, L. Hennighausen, and D. S. Finbloom. 1996. Granulocyte-macrophage colony-stimulating factor preferentially activates the 94-kD STAT5A and an 80-kD STAT5A isoform in human peripheral blood monocytes. Blood 88:1206-1214. [PubMed] [Google Scholar]
- 30.Selliah, N., and T. H. Finkel. 2001. HIV-1 NL4-3, but not IIIB, inhibits JAK3/STAT5 activation in CD4+ T cells. Virology 286:412-421. [DOI] [PubMed] [Google Scholar]
- 31.Stomski, F. C., J. M. Woodcock, B. Zacharakis, C. J. Bagley, Q. Sun, and A. F. Lopez. 1998. Identification of a Cys motif in the common beta chain of the interleukin 3, granulocyte-macrophage colony-stimulating factor, and interleukin 5 receptors essential for disulfide-linked receptor heterodimerization and activation of all three receptors. J. Biol. Chem. 273:1192-1199. [DOI] [PubMed] [Google Scholar]
- 32.van Dijk, T. B., E. Caldenhoven, J. A. Raaijmakers, J. W. Lammers, L. Koenderman, and R. P. de Groot. 1997. Multiple tyrosine residues in the intracellular domain of the common beta subunit of the interleukin 5 receptor are involved in activation of STAT5. FEBS Lett. 412:161-164. [DOI] [PubMed] [Google Scholar]
- 33.Wheadon, H., P. J. Roberts, M. J. Watts, and D. C. Linch. 1999. Changes in signal transduction downstream from the granulocyte-macrophage colony-stimulating factor receptor during differentiation of primary hemopoietic cells. Exp. Hematol. 27:1077-1086. [DOI] [PubMed] [Google Scholar]
- 34.Yagisawa, M., K. Saeki, E. Okuma, T. Kitamura, S. Kitagawa, H. Hirai, Y. Yazaki, F. Takaku, and A. Yuo. 1999. Signal transduction pathways in normal human monocytes stimulated by cytokines and mediators: comparative study with normal human neutrophils or transformed cells and the putative roles in functionality and cell biology. Exp. Hematol. 27:1063-1076. [DOI] [PubMed] [Google Scholar]
- 35.Zhao, Y., F. Wagner, S. J. Frank, and A. S. Kraft. 1995. The amino-terminal portion of the JAK2 protein kinase is necessary for binding and phosphorylation of the granulocyte-macrophage colony-stimulating factor receptor beta c chain. J. Biol. Chem. 270:13814-13818. [DOI] [PubMed] [Google Scholar]